 Found 1466 hits Enz. Inhib. hit(s) with Target = 'Protein kinase C beta type'
Found 1466 hits Enz. Inhib. hit(s) with Target = 'Protein kinase C beta type' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286349
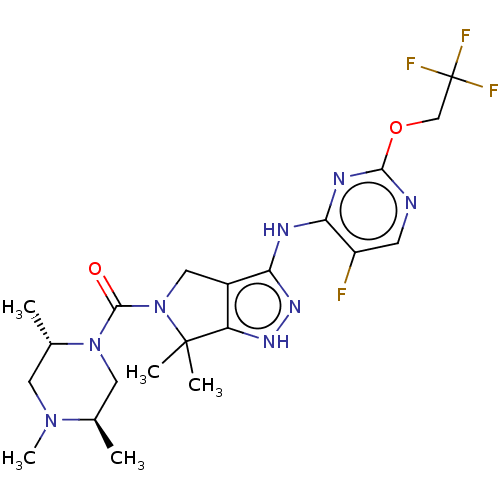
(US11220518, Ex. No. K7 | US11780853, Example K7 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C21H28F4N8O2/c1-11-8-32(12(2)7-31(11)5)19(34)33-9-13-15(20(33,3)4)29-30-16(13)27-17-14(22)6-26-18(28-17)35-10-21(23,24)25/h6,11-12H,7-10H2,1-5H3,(H2,26,27,28,29,30)/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286349

(US11220518, Ex. No. K7 | US11780853, Example K7 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C21H28F4N8O2/c1-11-8-32(12(2)7-31(11)5)19(34)33-9-13-15(20(33,3)4)29-30-16(13)27-17-14(22)6-26-18(28-17)35-10-21(23,24)25/h6,11-12H,7-10H2,1-5H3,(H2,26,27,28,29,30)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50391386

(CHEMBL2148106)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cccc(O)c1 |r| Show InChI InChI=1S/C32H48O10/c1-18(11-12-24(38-7)22-9-8-10-23(34)13-22)29-20(3)26-16-32(41-29)30(5,6)15-19(2)31(37,42-32)17-28(36)39-25(21(4)33)14-27(35)40-26/h8-10,13,18-21,24-26,29,33-34,37H,11-12,14-17H2,1-7H3/t18-,19+,20-,21+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKCbeta C1A domain |
J Med Chem 55: 5614-26 (2012)
Article DOI: 10.1021/jm300566h
BindingDB Entry DOI: 10.7270/Q200036J |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286350

(US11220518, Ex. No. K8 | US11780853, Example K8 | ...)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C22H30F4N8O2/c1-12-8-32(6)20(2,3)10-33(12)19(35)34-9-13-15(21(34,4)5)30-31-16(13)28-17-14(23)7-27-18(29-17)36-11-22(24,25)26/h7,12H,8-11H2,1-6H3,(H2,27,28,29,30,31)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286350

(US11220518, Ex. No. K8 | US11780853, Example K8 | ...)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C22H30F4N8O2/c1-12-8-32(6)20(2,3)10-33(12)19(35)34-9-13-15(21(34,4)5)30-31-16(13)28-17-14(23)7-27-18(29-17)36-11-22(24,25)26/h7,12H,8-11H2,1-6H3,(H2,27,28,29,30,31)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286348
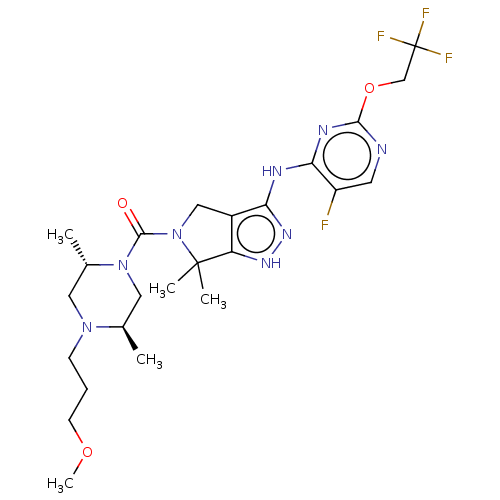
(US11220518, Ex. No. K6 | US11780853, Example K6 | ...)Show SMILES COCCCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C24H34F4N8O3/c1-14-11-35(15(2)10-34(14)7-6-8-38-5)22(37)36-12-16-18(23(36,3)4)32-33-19(16)30-20-17(25)9-29-21(31-20)39-13-24(26,27)28/h9,14-15H,6-8,10-13H2,1-5H3,(H2,29,30,31,32,33)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286348

(US11220518, Ex. No. K6 | US11780853, Example K6 | ...)Show SMILES COCCCN1C[C@H](C)N(C[C@H]1C)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C24H34F4N8O3/c1-14-11-35(15(2)10-34(14)7-6-8-38-5)22(37)36-12-16-18(23(36,3)4)32-33-19(16)30-20-17(25)9-29-21(31-20)39-13-24(26,27)28/h9,14-15H,6-8,10-13H2,1-5H3,(H2,29,30,31,32,33)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50057514
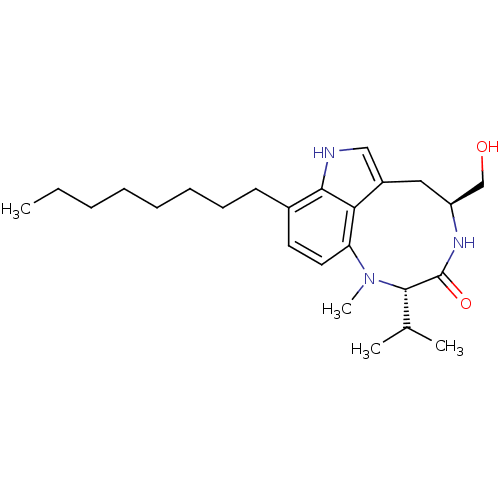
((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...)Show SMILES CCCCCCCCc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30)/t20-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]- PDBu from recombinant PKC beta expressed in baculovirus |
J Med Chem 40: 1316-26 (1997)
Article DOI: 10.1021/jm960875h
BindingDB Entry DOI: 10.7270/Q2319TZS |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50327943
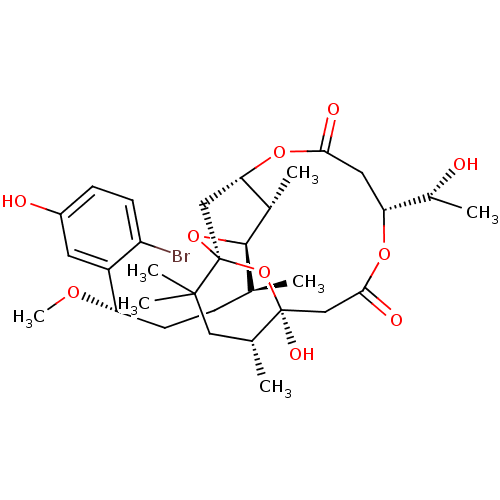
(Aplysiatoxin | CHEMBL1256416)Show SMILES CO[C@@H](CC[C@H](C)[C@H]1O[C@@]23C[C@H](OC(=O)C[C@@H](OC(=O)C[C@](O)(O2)[C@H](C)CC3(C)C)[C@@H](C)O)[C@@H]1C)c1cc(O)ccc1Br |r| Show InChI InChI=1S/C32H47BrO10/c1-17(8-11-24(39-7)22-12-21(35)9-10-23(22)33)29-19(3)26-15-32(42-29)30(5,6)14-18(2)31(38,43-32)16-28(37)40-25(20(4)34)13-27(36)41-26/h9-10,12,17-20,24-26,29,34-35,38H,8,11,13-16H2,1-7H3/t17-,18+,19-,20+,24-,25+,26?,29+,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to PKC beta C1A peptide |
Bioorg Med Chem Lett 20: 6064-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.051
BindingDB Entry DOI: 10.7270/Q2FJ2H04 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of PKCbeta1 (unknown origin) |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286347

(US11220518, Ex. No. K5 | US11780853, Example K5 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O3/c1-7-36-22-26-11-18(25)21(28-22)27-20-17-14-33(24(4,5)19(17)29-30-20)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286347

(US11220518, Ex. No. K5 | US11780853, Example K5 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O3/c1-7-36-22-26-11-18(25)21(28-22)27-20-17-14-33(24(4,5)19(17)29-30-20)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286345
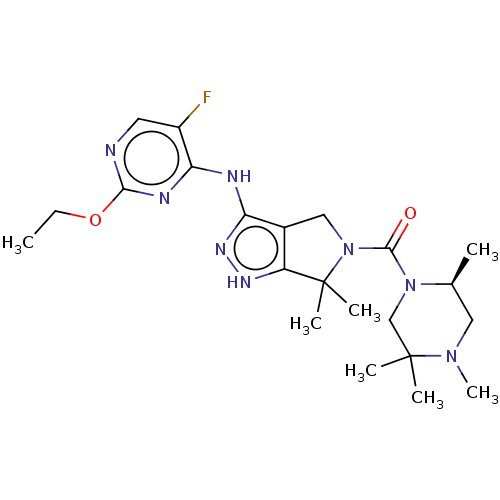
(US11220518, Ex. No. K3 | US11780853, Example K3 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286345

(US11220518, Ex. No. K3 | US11780853, Example K3 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286246

(5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286246

(5-{[(8S)-6,8-dimethyl-6,9-diazaspiro[4.5]dec-9-yl]...)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533472

(US11220518, Ex. No. K1 | US11780853, Example K1)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533472

(US11220518, Ex. No. K1 | US11780853, Example K1)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533459

(US11220518, Ex. No. J6 | US11780853, Example J6)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533459

(US11220518, Ex. No. J6 | US11780853, Example J6)Show SMILES C[C@H]1CN(C)C(C)(C)CN1C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50133236
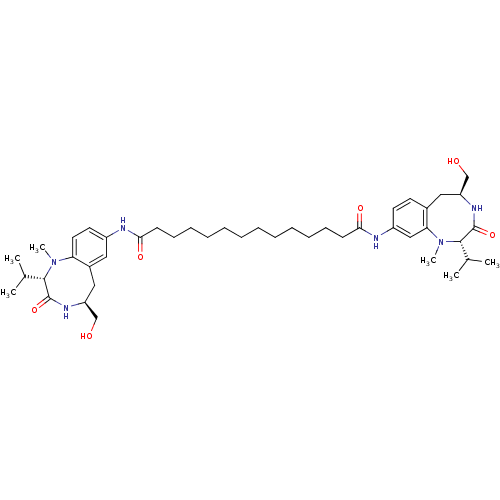
(CHEMBL337834 | Tetradecanedioic acid ((S)-5-hydrox...)Show SMILES CC(C)[C@@H]1N(C)c2ccc(NC(=O)CCCCCCCCCCCCC(=O)Nc3ccc4C[C@@H](CO)NC(=O)[C@H](C(C)C)N(C)c4c3)cc2C[C@@H](CO)NC1=O Show InChI InChI=1S/C44H68N6O6/c1-29(2)41-43(55)48-36(28-52)25-32-24-33(21-22-37(32)49(41)5)45-39(53)17-15-13-11-9-7-8-10-12-14-16-18-40(54)46-34-20-19-31-23-35(27-51)47-44(56)42(30(3)4)50(6)38(31)26-34/h19-22,24,26,29-30,35-36,41-42,51-52H,7-18,23,25,27-28H2,1-6H3,(H,45,53)(H,46,54)(H,47,56)(H,48,55)/t35-,36-,41-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of human PKC beta-1 |
J Med Chem 46: 4196-204 (2003)
Article DOI: 10.1021/jm0302041
BindingDB Entry DOI: 10.7270/Q2GF0SX2 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50493217

(DEMETHOXYDEBROMOAPLYSIATOXIN)Show SMILES [H][C@]12C[C@]3(O[C@]([H])([C@@H](C)CCCc4cccc(O)c4)[C@H]1C)O[C@@](O)(CC(=O)O[C@]([H])(CC(=O)O2)[C@@H](C)O)[C@H](C)CC3(C)C |r| Show InChI InChI=1S/C31H46O9/c1-18(9-7-10-22-11-8-12-23(33)13-22)28-20(3)25-16-31(39-28)29(5,6)15-19(2)30(36,40-31)17-27(35)37-24(21(4)32)14-26(34)38-25/h8,11-13,18-21,24-25,28,32-33,36H,7,9-10,14-17H2,1-6H3/t18-,19+,20-,21+,24+,25-,28+,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from PKCbeta C1A domain (unknown origin) |
Bioorg Med Chem Lett 23: 4319-23 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.096
BindingDB Entry DOI: 10.7270/Q2VD72CX |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286270
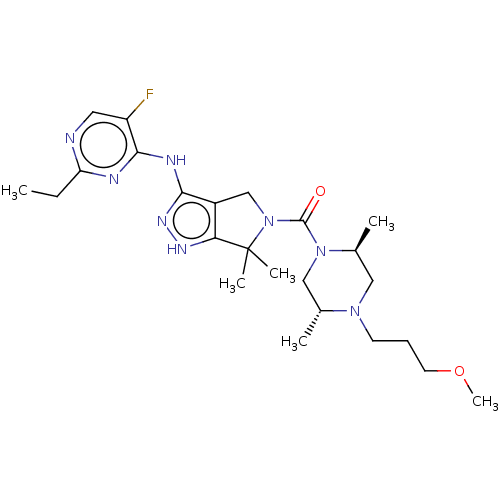
(US11220518, Ex. No. E2 | US11780853, Example E2 | ...)Show SMILES CCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O2/c1-7-19-26-11-18(25)22(27-19)28-21-17-14-33(24(4,5)20(17)29-30-21)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286270

(US11220518, Ex. No. E2 | US11780853, Example E2 | ...)Show SMILES CCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C24H37FN8O2/c1-7-19-26-11-18(25)22(27-19)28-21-17-14-33(24(4,5)20(17)29-30-21)23(34)32-13-15(2)31(12-16(32)3)9-8-10-35-6/h11,15-16H,7-10,12-14H2,1-6H3,(H2,26,27,28,29,30)/t15-,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM153901
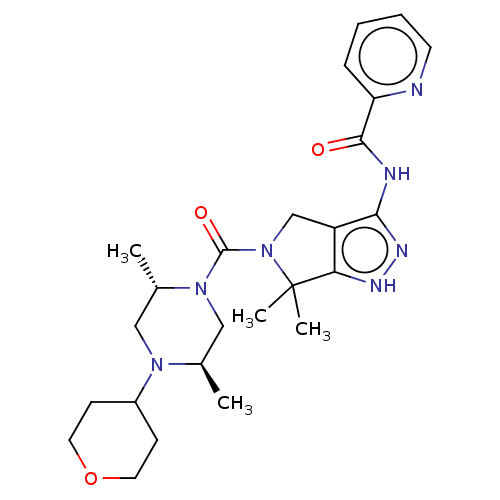
(US8999981, A146)Show SMILES C[C@@H]1CN([C@@H](C)CN1C1CCOCC1)C(=O)N1Cc2c(NC(=O)c3ccccn3)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer Inc.; Pfizer Products Inc.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US8999981 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X0K |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286377
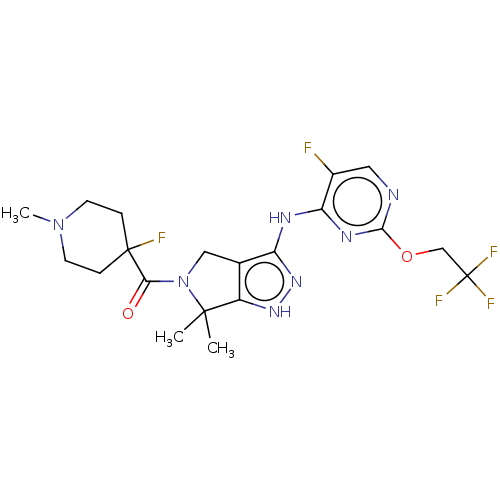
(US11220518, Ex. No. L4 | US11780853, Example L4 | ...)Show SMILES CN1CCC(F)(CC1)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C Show InChI InChI=1S/C20H24F5N7O2/c1-18(2)13-11(9-32(18)16(33)19(22)4-6-31(3)7-5-19)14(30-29-13)27-15-12(21)8-26-17(28-15)34-10-20(23,24)25/h8H,4-7,9-10H2,1-3H3,(H2,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286377

(US11220518, Ex. No. L4 | US11780853, Example L4 | ...)Show SMILES CN1CCC(F)(CC1)C(=O)N1Cc2c(Nc3nc(OCC(F)(F)F)ncc3F)n[nH]c2C1(C)C Show InChI InChI=1S/C20H24F5N7O2/c1-18(2)13-11(9-32(18)16(33)19(22)4-6-31(3)7-5-19)14(30-29-13)27-15-12(21)8-26-17(28-15)34-10-20(23,24)25/h8H,4-7,9-10H2,1-3H3,(H2,26,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533452

(US11220518, Ex. No. I1 | US11780853, Example I1)Show SMILES CCCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533452

(US11220518, Ex. No. I1 | US11780853, Example I1)Show SMILES CCCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099066
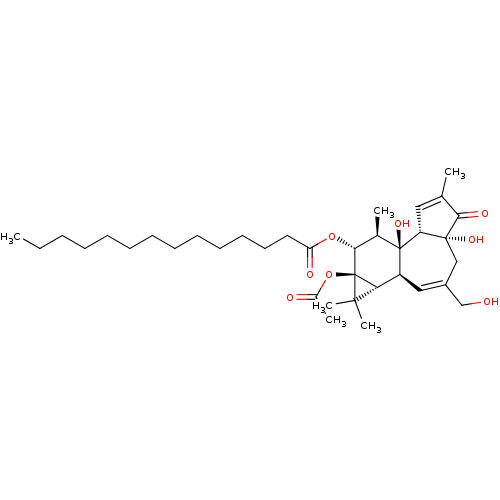
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50057512
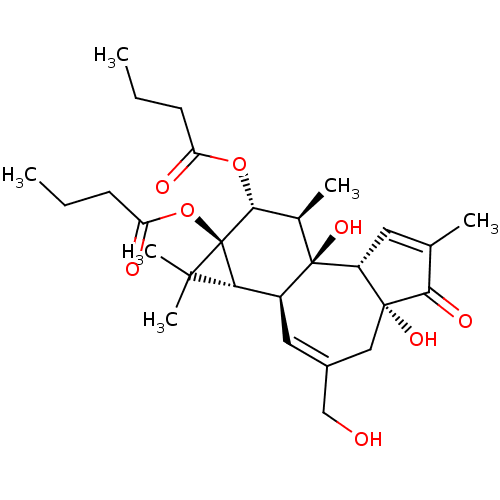
((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of 3[H]PDBu from Protein kinase C epsilon C1a domain |
Bioorg Med Chem Lett 11: 719-22 (2001)
BindingDB Entry DOI: 10.7270/Q2VM4BHZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM153898
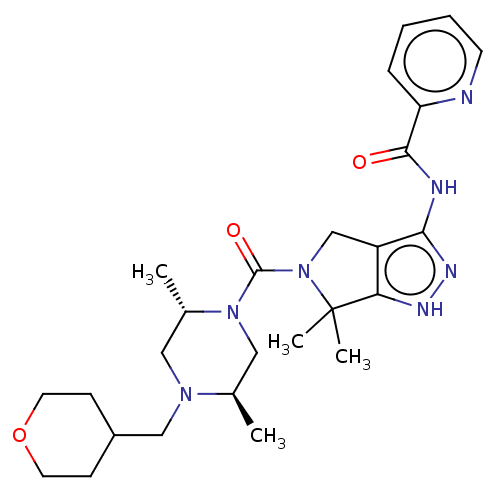
(US8999981, A143)Show SMILES C[C@@H]1CN([C@@H](C)CN1CC1CCOCC1)C(=O)N1Cc2c(NC(=O)c3ccccn3)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.90 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer Inc.; Pfizer Products Inc.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US8999981 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X0K |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50057512

((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of 3[H]PDBu from Protein kinase C beta C1a domain |
Bioorg Med Chem Lett 11: 719-22 (2001)
BindingDB Entry DOI: 10.7270/Q2VM4BHZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286260

(US11220518, Ex. No. B3 | US11780853, Example B3 | ...)Show SMILES CCNc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C21H32FN9O/c1-7-23-19-24-8-15(22)18(26-19)25-17-14-11-31(21(4,5)16(14)27-28-17)20(32)30-10-12(2)29(6)9-13(30)3/h8,12-13H,7,9-11H2,1-6H3,(H3,23,24,25,26,27,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286260

(US11220518, Ex. No. B3 | US11780853, Example B3 | ...)Show SMILES CCNc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(C)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C21H32FN9O/c1-7-23-19-24-8-15(22)18(26-19)25-17-14-11-31(21(4,5)16(14)27-28-17)20(32)30-10-12(2)29(6)9-13(30)3/h8,12-13H,7,9-11H2,1-6H3,(H3,23,24,25,26,27,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533462

(US11220518, Ex. No. J9 | US11780853, Example J9)Show SMILES COCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533462

(US11220518, Ex. No. J9 | US11780853, Example J9)Show SMILES COCc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286344
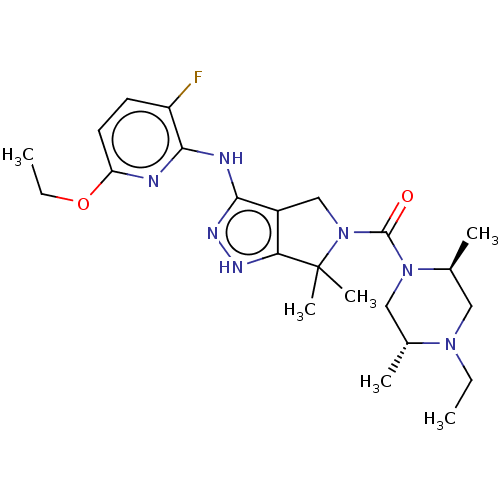
(US11220518, Ex. No. K2 | US11780853, Example K2 | ...)Show SMILES CCOc1ccc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CC)C[C@@H]2C)C3(C)C)n1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286344

(US11220518, Ex. No. K2 | US11780853, Example K2 | ...)Show SMILES CCOc1ccc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CC)C[C@@H]2C)C3(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50097750
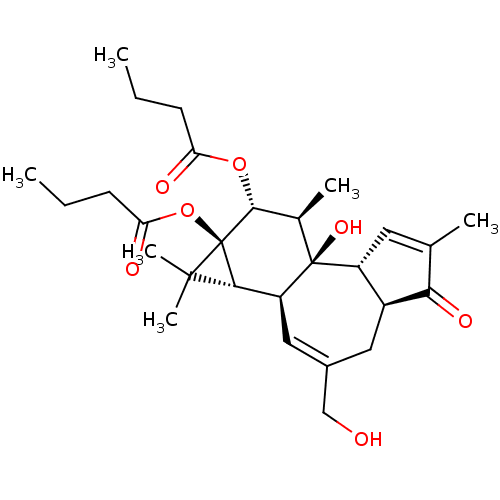
(Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@H]3CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:22,t:12| Show InChI InChI=1S/C28H40O7/c1-7-9-21(30)34-25-16(4)27(33)19-11-15(3)23(32)18(19)12-17(14-29)13-20(27)24-26(5,6)28(24,25)35-22(31)10-8-2/h11,13,16,18-20,24-25,29,33H,7-10,12,14H2,1-6H3/t16-,18+,19-,20+,24?,25-,27+,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of 3[H]PDBu from Protein kinase C beta C1a domain |
Bioorg Med Chem Lett 11: 719-22 (2001)
BindingDB Entry DOI: 10.7270/Q2VM4BHZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286264
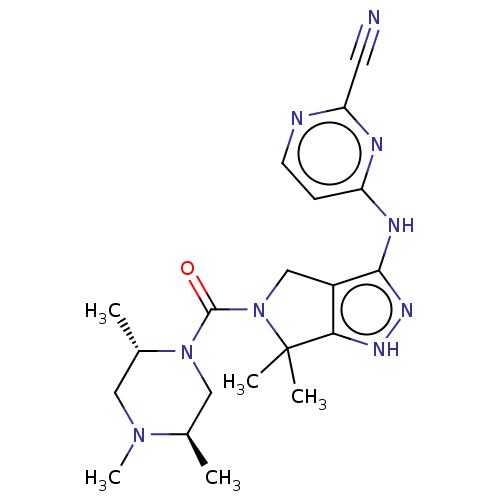
(US11220518, Ex. No. B6 | US11780853, Example B6 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3ccnc(n3)C#N)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C20H27N9O/c1-12-10-28(13(2)9-27(12)5)19(30)29-11-14-17(20(29,3)4)25-26-18(14)24-15-6-7-22-16(8-21)23-15/h6-7,12-13H,9-11H2,1-5H3,(H2,22,23,24,25,26)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286264

(US11220518, Ex. No. B6 | US11780853, Example B6 | ...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3ccnc(n3)C#N)n[nH]c2C1(C)C |r| Show InChI InChI=1S/C20H27N9O/c1-12-10-28(13(2)9-27(12)5)19(30)29-11-14-17(20(29,3)4)25-26-18(14)24-15-6-7-22-16(8-21)23-15/h6-7,12-13H,9-11H2,1-5H3,(H2,22,23,24,25,26)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM533441

(US11220518, Ex. No. H2 | US11780853, Example J3)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(C)ncc3F)n[nH]c2C1(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM533456

(US11220518, Ex. No. J3)Show SMILES C[C@@H]1CN([C@@H](C)CN1C)C(=O)N1Cc2c(Nc3nc(C)ccc3F)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286227
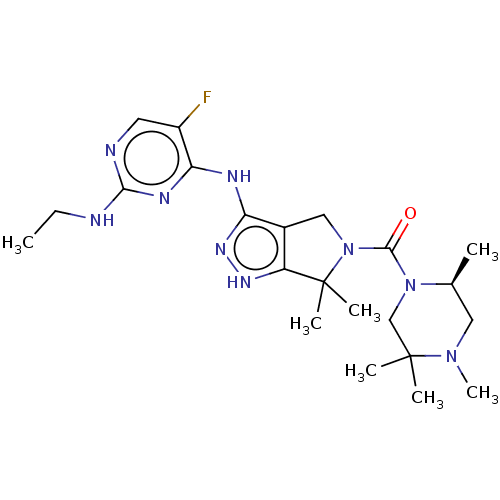
(N4-(6,6-dimethyl-5-{[(2S)-2,4,5,5-tetramethylpiper...)Show SMILES CCNc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C22H34FN9O/c1-8-24-19-25-9-15(23)18(27-19)26-17-14-11-32(22(5,6)16(14)28-29-17)20(33)31-12-21(3,4)30(7)10-13(31)2/h9,13H,8,10-12H2,1-7H3,(H3,24,25,26,27,28,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286227

(N4-(6,6-dimethyl-5-{[(2S)-2,4,5,5-tetramethylpiper...)Show SMILES CCNc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2CC(C)(C)N(C)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C22H34FN9O/c1-8-24-19-25-9-15(23)18(27-19)26-17-14-11-32(22(5,6)16(14)28-29-17)20(33)31-12-21(3,4)30(7)10-13(31)2/h9,13H,8,10-12H2,1-7H3,(H3,24,25,26,27,28,29)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50097750

(Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@H]3CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:22,t:12| Show InChI InChI=1S/C28H40O7/c1-7-9-21(30)34-25-16(4)27(33)19-11-15(3)23(32)18(19)12-17(14-29)13-20(27)24-26(5,6)28(24,25)35-22(31)10-8-2/h11,13,16,18-20,24-25,29,33H,7-10,12,14H2,1-6H3/t16-,18+,19-,20+,24?,25-,27+,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of 3[H]PDBu from Protein kinase C gamma C1b domain |
Bioorg Med Chem Lett 11: 719-22 (2001)
BindingDB Entry DOI: 10.7270/Q2VM4BHZ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM153757

(US8999981, A2)Show SMILES C[C@H]1CN(C)C2(CCCC2)CN1C(=O)N1Cc2c(NC(=O)c3ccccn3)n[nH]c2C1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.05 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer Inc.; Pfizer Products Inc.
US Patent
| Assay Description
Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate pe... |
US Patent US8999981 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X0K |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type Isoform 2
(Homo sapiens (Human)) | BDBM286346

(US11220518, Ex. No. K4 | US11780853, Example K4 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C23H35FN8O3/c1-7-35-21-25-10-17(24)20(27-21)26-19-16-13-32(23(4,5)18(16)28-29-19)22(33)31-12-14(2)30(8-9-34-6)11-15(31)3/h10,14-15H,7-9,11-13H2,1-6H3,(H2,25,26,27,28,29)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM286346

(US11220518, Ex. No. K4 | US11780853, Example K4 | ...)Show SMILES CCOc1ncc(F)c(Nc2n[nH]c3c2CN(C(=O)N2C[C@@H](C)N(CCOC)C[C@@H]2C)C3(C)C)n1 |r| Show InChI InChI=1S/C23H35FN8O3/c1-7-35-21-25-10-17(24)20(27-21)26-19-16-13-32(23(4,5)18(16)28-29-19)22(33)31-12-14(2)30(8-9-34-6)11-15(31)3/h10,14-15H,7-9,11-13H2,1-6H3,(H2,25,26,27,28,29)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q28S4T3T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data