

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50583973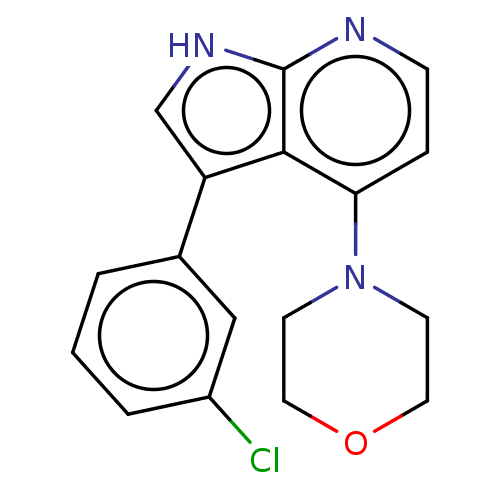 (CHEMBL5093114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50059345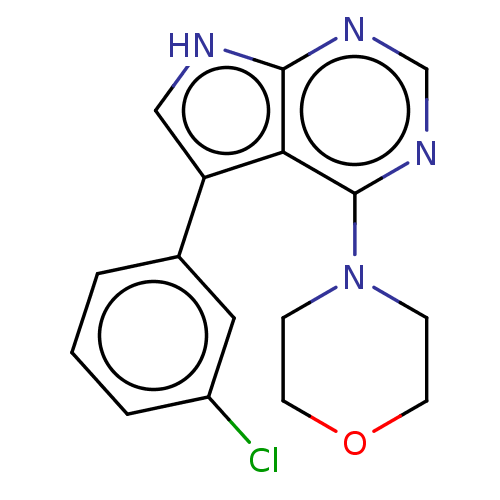 (CHEMBL3393355 | US9156845, 83) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50059345 (CHEMBL3393355 | US9156845, 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM185556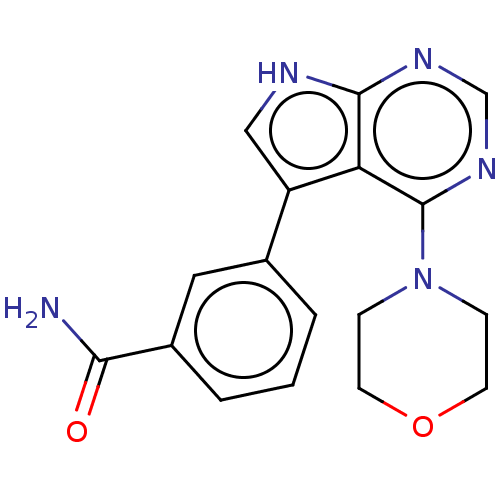 (US9156845, 215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM185556 (US9156845, 215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50583973 (CHEMBL5093114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50341519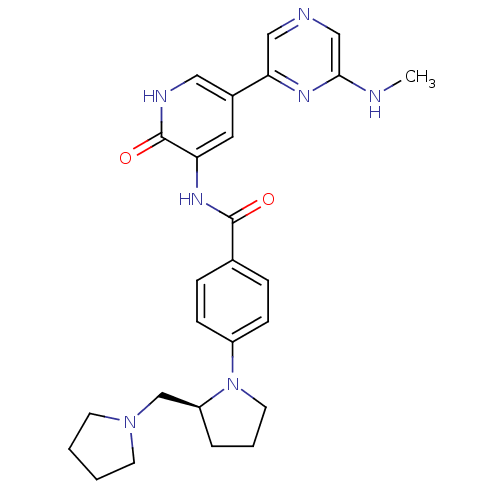 ((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of MST2 | J Med Chem 54: 2341-50 (2011) Article DOI: 10.1021/jm101499u BindingDB Entry DOI: 10.7270/Q2KH0NPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 31 (Homo sapiens (Human)) | BDBM50224883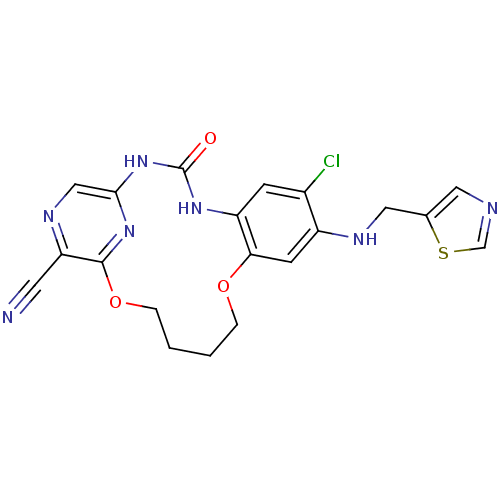 (7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of STK31 | Bioorg Med Chem Lett 17: 6593-601 (2007) Article DOI: 10.1016/j.bmcl.2007.09.063 BindingDB Entry DOI: 10.7270/Q2X067WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50224883 (7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of STK33 | Bioorg Med Chem Lett 17: 6593-601 (2007) Article DOI: 10.1016/j.bmcl.2007.09.063 BindingDB Entry DOI: 10.7270/Q2X067WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MST1 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50463483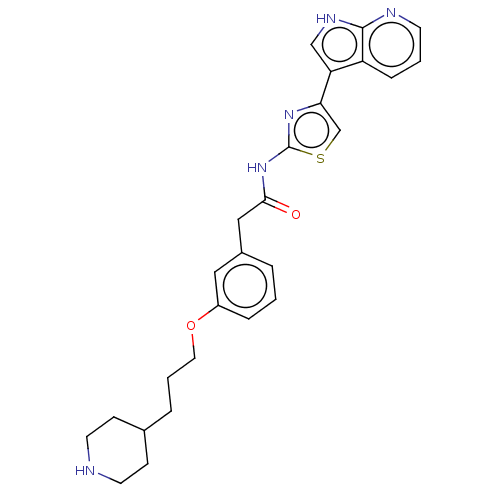 (CHEMBL4245242) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MST1 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50402020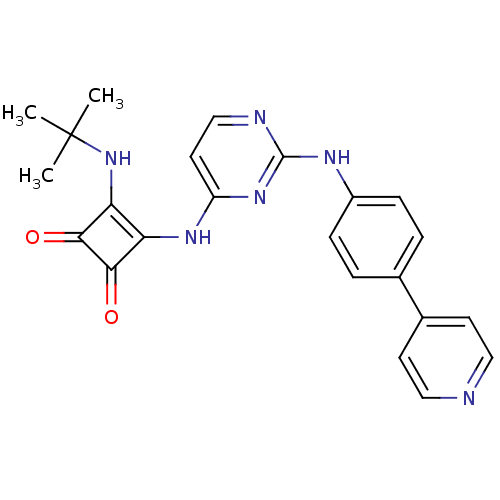 (CHEMBL2205426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant MST2 after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP | Bioorg Med Chem Lett 22: 7615-22 (2012) Article DOI: 10.1016/j.bmcl.2012.10.009 BindingDB Entry DOI: 10.7270/Q2XK8GQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MST2 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50463484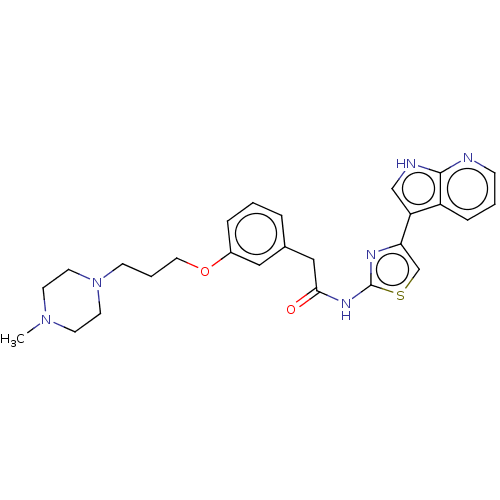 (CHEMBL4248525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MST2 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of MST2 | J Med Chem 52: 3191-204 (2009) Article DOI: 10.1021/jm800861c BindingDB Entry DOI: 10.7270/Q23J3DWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 38 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.628 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human STK38 using KKRNRRLSVA as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 38-like (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.831 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human STK38L using KKRNRRLSVA as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MST1 using KKSRGDYMTMQIG as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 38-like (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human STK38L using KKRNRRLSVA as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human MST1 using KKSRGDYMTMQIG as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MST2 using MBP as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST) Curated by ChEMBL | Assay Description Inhibition of STK3 (unknown origin) | Eur J Med Chem 141: 657-675 (2017) Article DOI: 10.1016/j.ejmech.2017.10.003 BindingDB Entry DOI: 10.7270/Q2CV4M86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human MST2 using MBP as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 38 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human STK38 using KKRNRRLSVA as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400690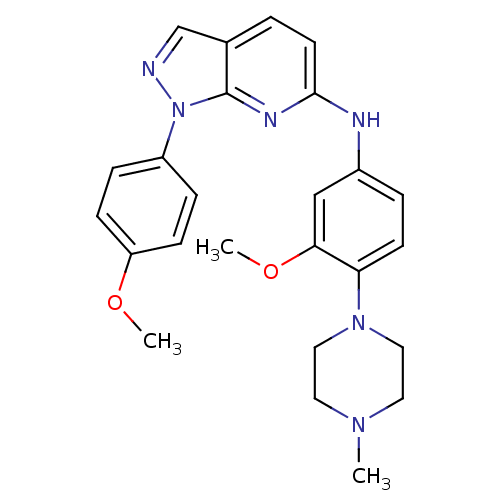 (CHEMBL2203524) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400689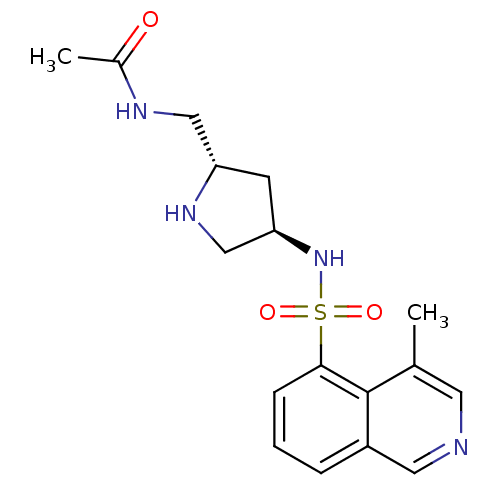 (CHEMBL2203525) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400664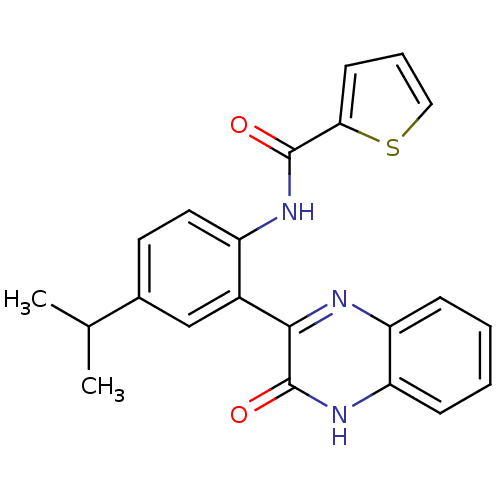 (CHEMBL2204239) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114085 BindingDB Entry DOI: 10.7270/Q2XW4PS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM50400664 (CHEMBL2204239) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged full length human recombinant STK33 using myelin basic protein as substrate incubated for 15 mins before initiat... | ACS Med Chem Lett 3: 1034-1038 (2012) Article DOI: 10.1021/ml300246r BindingDB Entry DOI: 10.7270/Q2PK0H9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STE20/SPS1-related proline-alanine-rich protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human STK39 using MBP as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STE20/SPS1-related proline-alanine-rich protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human STK39 using MBP as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50379186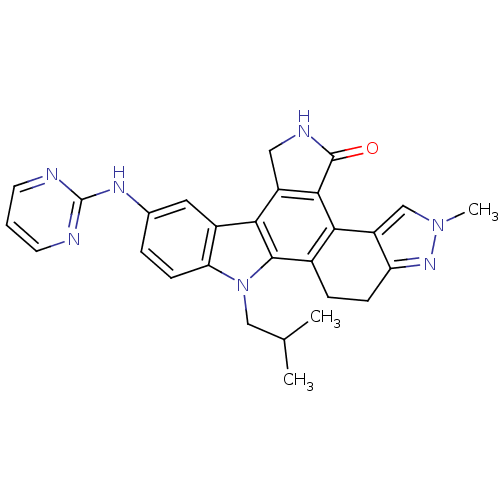 (CEP-11981 | CHEMBL2010872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc. Curated by ChEMBL | Assay Description Inhibition of human MST2 using ATP as substrate | J Med Chem 55: 903-13 (2012) Article DOI: 10.1021/jm201449n BindingDB Entry DOI: 10.7270/Q2ZS2XHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50059277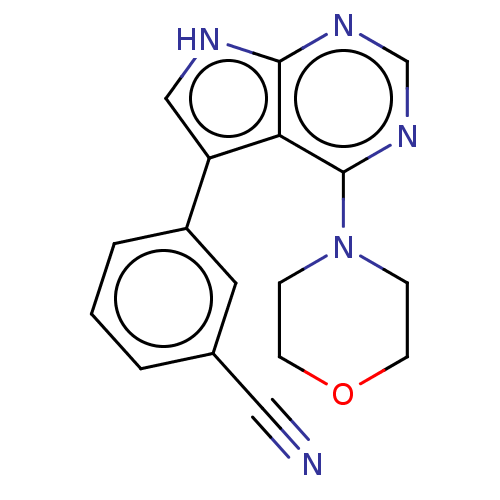 (CHEMBL3393348 | US9156845, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50599287 (CHEMBL5183592) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00926 BindingDB Entry DOI: 10.7270/Q27D306R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 32B (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human STK32B using MBP as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50583984 (CHEMBL5089935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli using myelin basic protein as substrat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM185456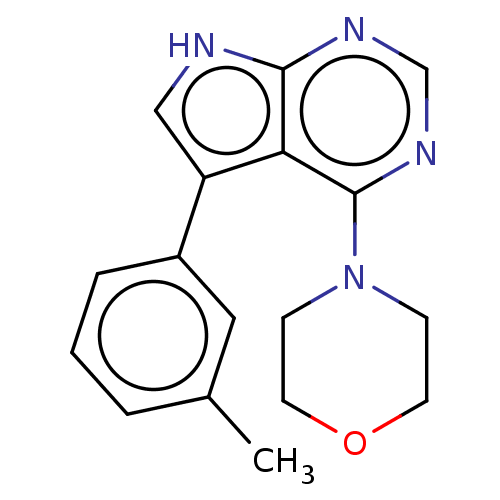 (US9156845, 107) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli using myelin basic protein as substrat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50059345 (CHEMBL3393355 | US9156845, 83) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli using myelin basic protein as substrat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50583984 (CHEMBL5089935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli using myelin basic protein as substrat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human STK33 using MBP as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM50583991 (CHEMBL5092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli using myelin basic protein as substrat... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00804 BindingDB Entry DOI: 10.7270/Q2Q52TH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 33 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human STK33 using MBP as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451711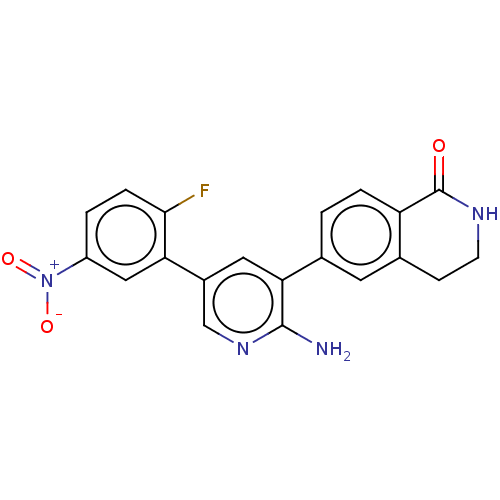 (6-(2-amino-5-(2-fluoro-5- nitrophenyl)pyridin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451712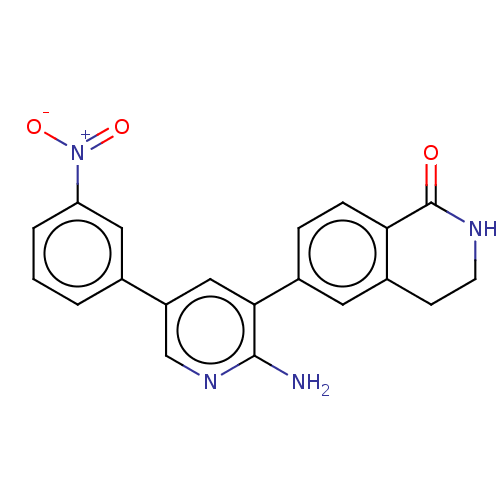 (6-(2-amino-5-(3- nitrophenyl)pyridin-3-yl)-3,4- di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451747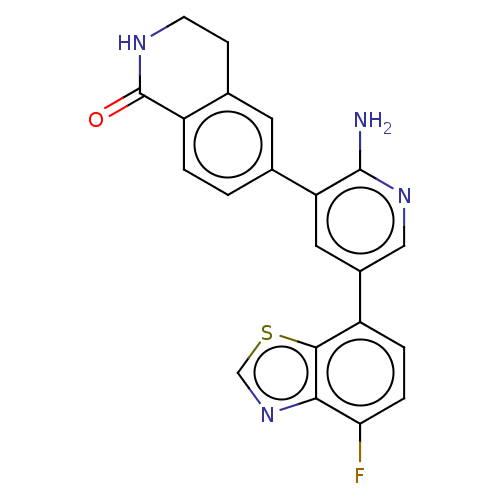 (6-(2-amino-5-(4- fluorobenzo[d]thiazol-7- yl)pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451748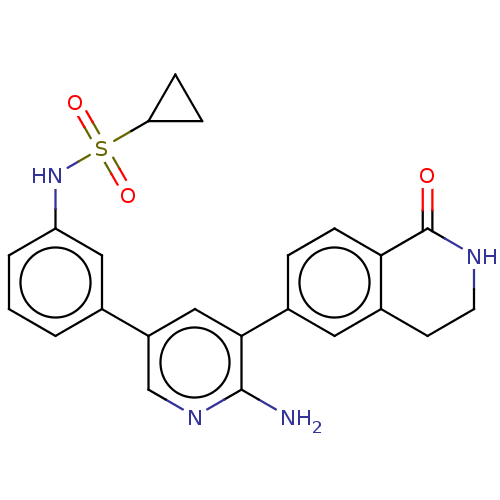 (N-(3-(6-amino-5-(1-oxo- 1,2,3,4-tetrahydroisoquino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451749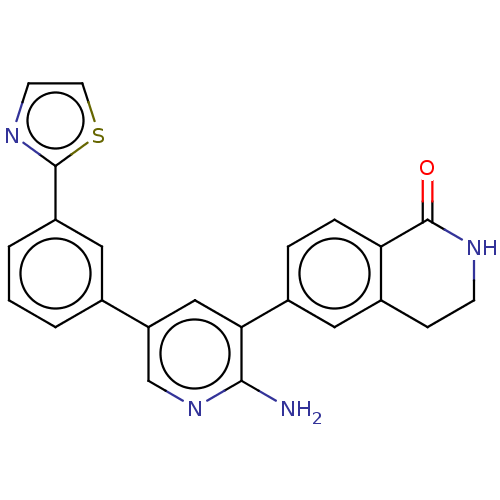 (6-(2-amino-5-(3-(thiazol-2- yl)phenyl)pyridin-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451751 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451752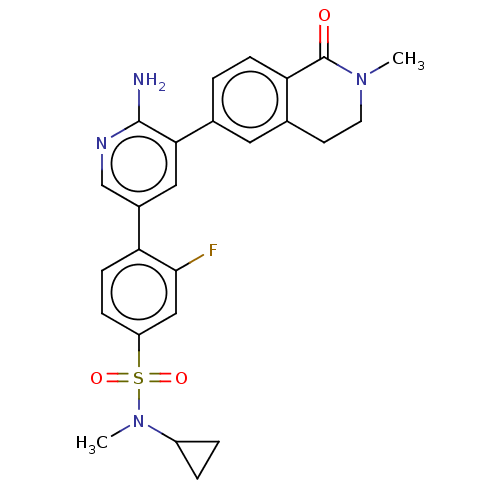 (4-(6-amino-5-(2-methyl-1- oxo-1,2,3,4- tetrahydroi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451753 (4-(6-amino-5-(4-oxo-3,4- dihydroquinazolin-7- yl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 4 (Homo sapiens (Human)) | BDBM451755 (N-(4-(6-amino-5-(1-oxo- 1,2,3,4-tetrahydroisoquino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2313 total ) | Next | Last >> |