 Found 37 hits Enz. Inhib. hit(s) with Target = 'Transmembrane protease serine 2'
Found 37 hits Enz. Inhib. hit(s) with Target = 'Transmembrane protease serine 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606675
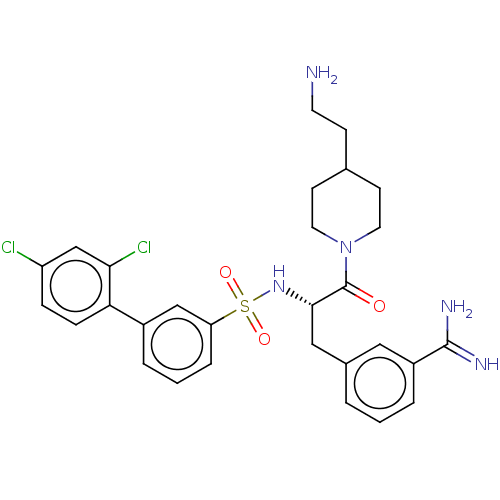
(CHEMBL3219079)Show SMILES NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(Cl)cc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606676
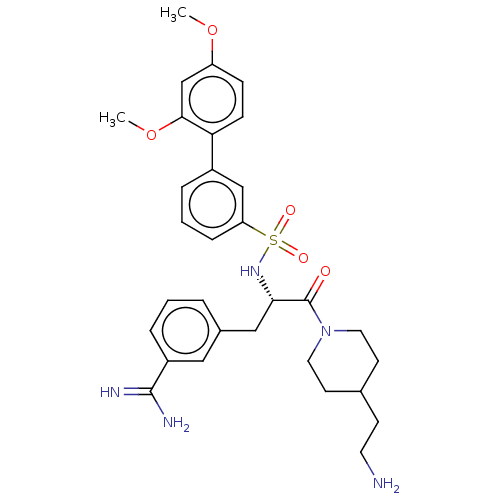
(CHEMBL3219087)Show SMILES COc1ccc(c(OC)c1)-c1cccc(c1)S(=O)(=O)N[C@@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCC(CCN)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606674
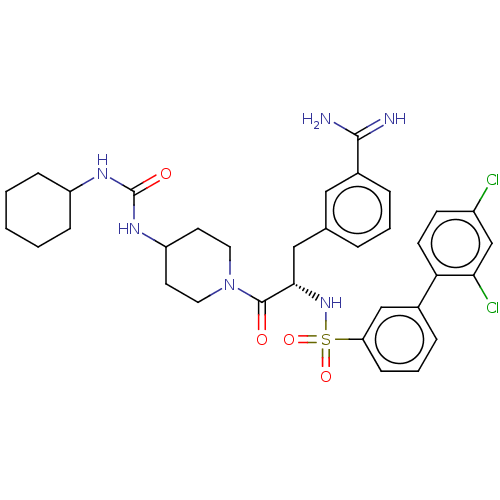
(CHEMBL3219103)Show SMILES NC(=N)c1cccc(C[C@H](NS(=O)(=O)c2cccc(c2)-c2ccc(Cl)cc2Cl)C(=O)N2CCC(CC2)NC(=O)NC2CCCCC2)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606673
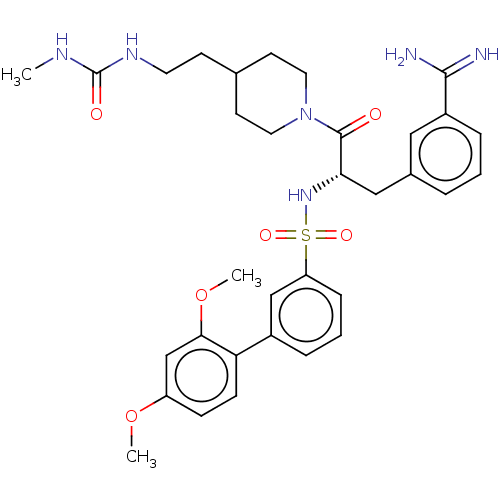
(CHEMBL3219100)Show SMILES CNC(=O)NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(OC)cc1OC |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606672
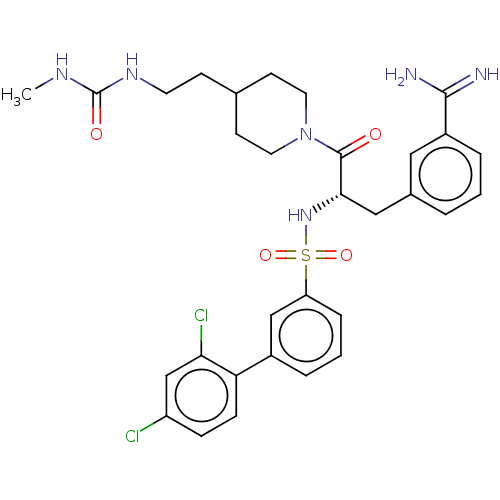
(CHEMBL3219101)Show SMILES CNC(=O)NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(Cl)cc1Cl |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50606671

(CHEMBL3219102)Show SMILES COc1ccc(c(OC)c1)-c1cccc(c1)S(=O)(=O)N[C@@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCC(CC1)NC(=O)NC1CCCCC1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50324475

(Benzylsulfonyl-D-argininyl-proline-(4-amidinobenzy...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H36N8O4S/c27-23(28)20-12-10-18(11-13-20)16-32-24(35)22-9-5-15-34(22)25(36)21(8-4-14-31-26(29)30)33-39(37,38)17-19-6-2-1-3-7-19/h1-3,6-7,10-13,21-22,33H,4-5,8-9,14-17H2,(H3,27,28)(H,32,35)(H4,29,30,31)/t21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50349344

(CHEMBL1809250)Show SMILES [#6]-[#6]-[#6](-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7])-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]S(=O)(=O)[#6]-c1ccccc1 |r| Show InChI InChI=1S/C24H35N7O3S/c1-2-20(30-15-17-10-12-19(13-11-17)23(25)26)22(32)21(9-6-14-29-24(27)28)31-35(33,34)16-18-7-4-3-5-8-18/h3-5,7-8,10-13,20-21,30-31H,2,6,9,14-16H2,1H3,(H3,25,26)(H4,27,28,29)/t20?,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50349345
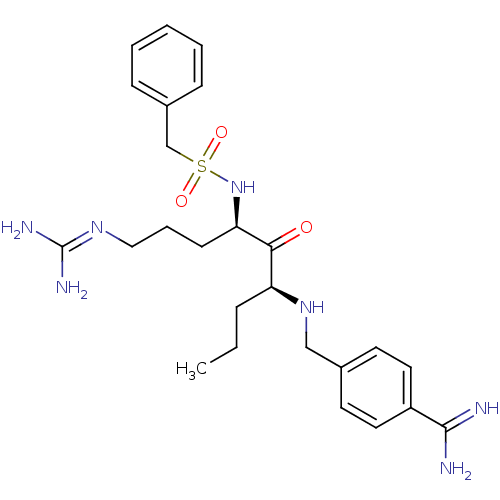
(CHEMBL1809251)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7])-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]S(=O)(=O)[#6]-c1ccccc1 |r| Show InChI InChI=1S/C25H37N7O3S/c1-2-7-21(31-16-18-11-13-20(14-12-18)24(26)27)23(33)22(10-6-15-30-25(28)29)32-36(34,35)17-19-8-4-3-5-9-19/h3-5,8-9,11-14,21-22,31-32H,2,6-7,10,15-17H2,1H3,(H3,26,27)(H4,28,29,30)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant catalytic domain of TMPRSS2 expressed in Escherichia coli using Dcyclohexylalanine- Pro-Arg-AMC as substrate by fluorescenc... |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM525152
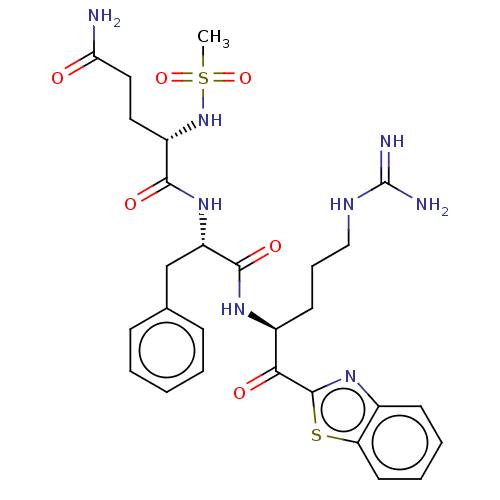
(Ms-QFR-kbt | N-0385 | US10988505, Example 2)Show SMILES CS(=O)(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50032698
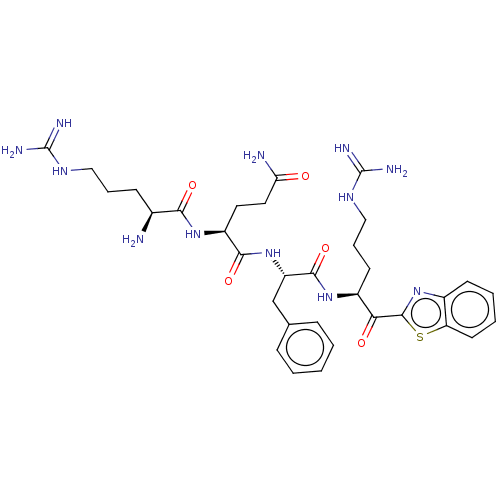
(CHEMBL3354676 | N-0130)Show SMILES N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |r| Show InChI InChI=1S/C33H46N12O5S/c34-20(10-6-16-40-32(36)37)28(48)43-23(14-15-26(35)46)29(49)44-24(18-19-8-2-1-3-9-19)30(50)42-22(12-7-17-41-33(38)39)27(47)31-45-21-11-4-5-13-25(21)51-31/h1-5,8-9,11,13,20,22-24H,6-7,10,12,14-18,34H2,(H2,35,46)(H,42,50)(H,43,48)(H,44,49)(H4,36,37,40)(H4,38,39,41)/t20-,22-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM525153
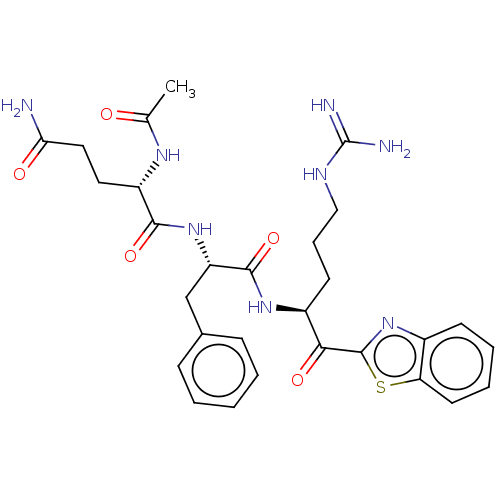
(Ac-QFR-kbt | N-0386 | US10988505, Example 3)Show SMILES CC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM525256
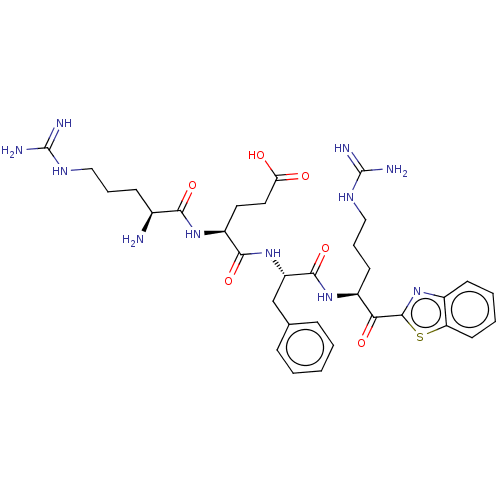
((H)Arg-Glu-Phe-Arg-kbt | N-0438)Show SMILES N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50031706
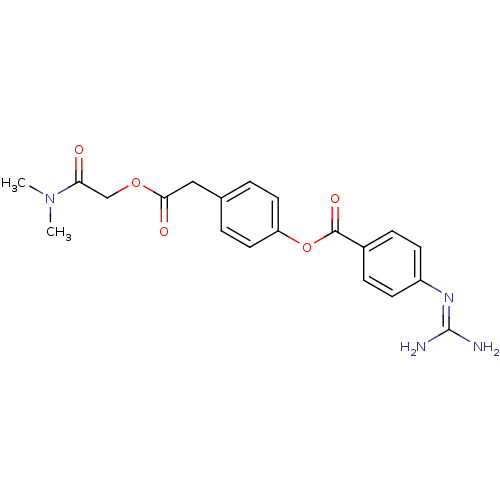
(4-Guanidino-benzoic acid 4-dimethylcarbamoylmethox...)Show SMILES [#6]-[#7](-[#6])-[#6](=O)-[#6]-[#8]-[#6](=O)-[#6]-c1ccc(-[#8]-[#6](=O)-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C20H22N4O5/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22/h3-10H,11-12H2,1-2H3,(H4,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50239965

(Bromhexine | CHEBI:77032)Show InChI InChI=1S/C14H20Br2N2/c1-18(12-5-3-2-4-6-12)9-10-7-11(15)8-13(16)14(10)17/h7-8,12H,2-6,9,17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center
| Assay Description
To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... |
Cancer Discov 4: 1310-25 (2014)
Article DOI: 10.1158/2159-8290.CD-13-1010
BindingDB Entry DOI: 10.7270/Q2HD7Z13 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM420291

(0591-5329 | 4-(Methoxycarbonyl)phenyl thiophene-2-...)Show InChI InChI=1S/C13H10O4S/c1-16-12(14)9-4-6-10(7-5-9)17-13(15)11-3-2-8-18-11/h2-8H,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center
| Assay Description
To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... |
Cancer Discov 4: 1310-25 (2014)
Article DOI: 10.1158/2159-8290.CD-13-1010
BindingDB Entry DOI: 10.7270/Q2HD7Z13 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM45440
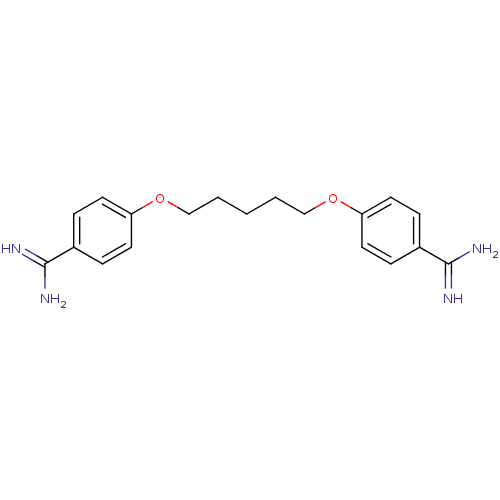
(4-[5-(4-amidinophenoxy)pentoxy]benzamidine;2-hydro...)Show InChI InChI=1S/C19H24N4O2/c20-18(21)14-4-8-16(9-5-14)24-12-2-1-3-13-25-17-10-6-15(7-11-17)19(22)23/h4-11H,1-3,12-13H2,(H3,20,21)(H3,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50022172

((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid meth...)Show InChI InChI=1S/C12H10O5/c1-16-11(14)4-7-5-12(15)17-10-6-8(13)2-3-9(7)10/h2-3,5-6,13H,4H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center
| Assay Description
To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... |
Cancer Discov 4: 1310-25 (2014)
Article DOI: 10.1158/2159-8290.CD-13-1010
BindingDB Entry DOI: 10.7270/Q2HD7Z13 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM420294
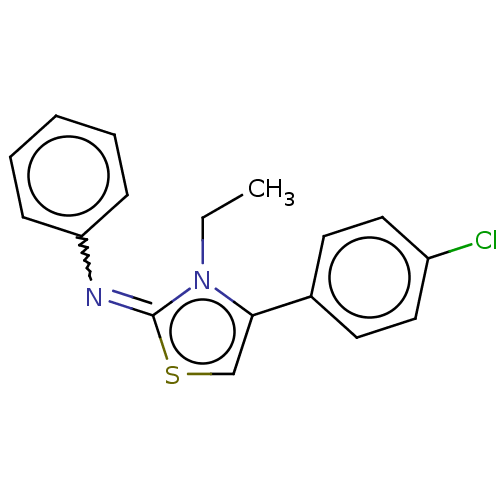
(8008-1235 | N-[4-(4-chlorophenyl)-3-ethyl-1,3-thia...)Show InChI InChI=1S/C17H15ClN2S/c1-2-20-16(13-8-10-14(18)11-9-13)12-21-17(20)19-15-6-4-3-5-7-15/h3-12H,2H2,1H3/b19-17+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
UniChem
| Article
PubMed
| n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center
| Assay Description
To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... |
Cancer Discov 4: 1310-25 (2014)
Article DOI: 10.1158/2159-8290.CD-13-1010
BindingDB Entry DOI: 10.7270/Q2HD7Z13 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM420292

(4401-0077 | Antidepressant agent 1)Show InChI InChI=1S/C16H19BrN2/c1-2-18-8-9-19-14-7-6-11(17)10-13(14)12-4-3-5-15(18)16(12)19/h6-7,10,15H,2-5,8-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fred Hutchinson Cancer Research Center
| Assay Description
To identify inhibitors of TMPRSS2 that may be used directly in clinical studies or as lead compounds to develop targeted drugs, we screened several c... |
Cancer Discov 4: 1310-25 (2014)
Article DOI: 10.1158/2159-8290.CD-13-1010
BindingDB Entry DOI: 10.7270/Q2HD7Z13 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5285196

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5266850

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5270157

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5271456

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50368092
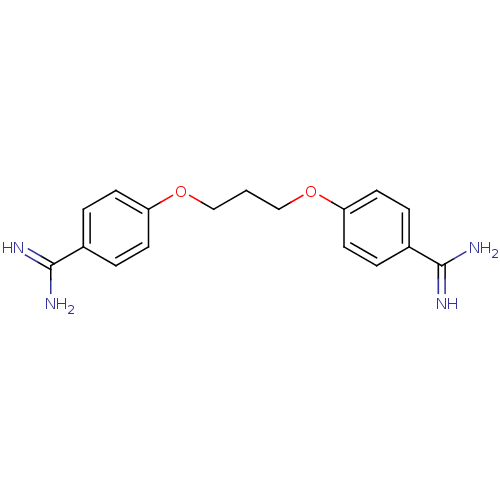
(GNF-PF-3839 | PROPAMIDINE CHLORIDE)Show InChI InChI=1S/C17H20N4O2/c18-16(19)12-2-6-14(7-3-12)22-10-1-11-23-15-8-4-13(5-9-15)17(20)21/h2-9H,1,10-11H2,(H3,18,19)(H3,20,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM525259
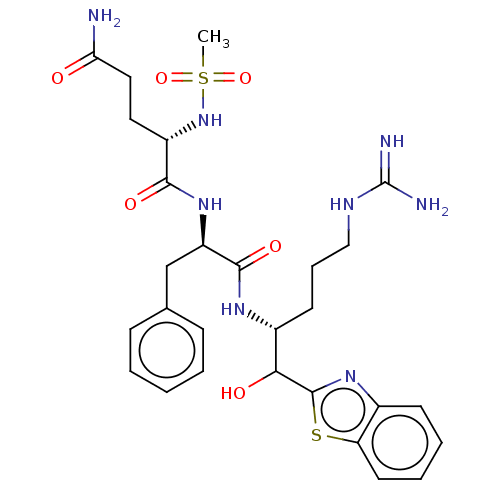
(Ms-Gln-Phe-Arg-kbt(OH) | N-0385(OH))Show SMILES CS(=O)(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(O)c1nc2ccccc2s1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Vero E6 cells were transfected with mock (pcDNA3.1), TMPRSS2 (pcDNA3.1/TMPRSS2 Uniprot: O15393-1), or TMPRSS2-S441A (pcDNA3.1/TMPRSS2-S441A) using Li... |
Citation and Details
Article DOI: 10.1038/s41586-022-04661-w
BindingDB Entry DOI: 10.7270/Q2NP27M4 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5287275
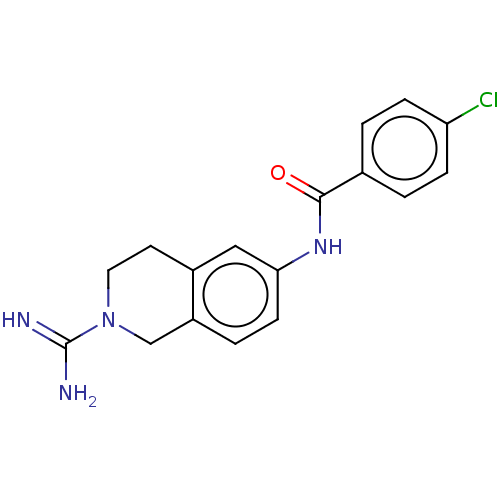
| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5275059

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5275526

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | CHEMBL5270536

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50122613
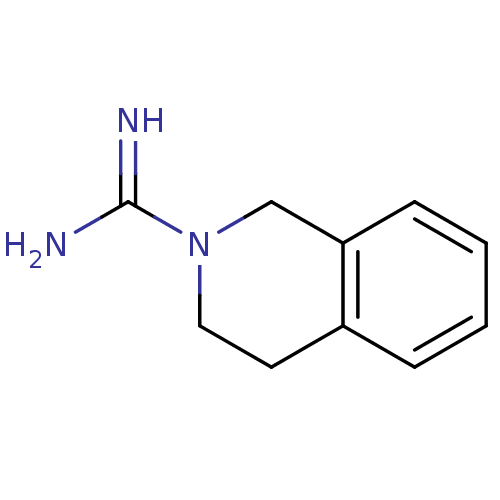
(3,4-dihydroisoquinoline-2(1H)-carboximidamide | CH...)Show InChI InChI=1S/C10H13N3/c11-10(12)13-6-5-8-3-1-2-4-9(8)7-13/h1-4H,5-7H2,(H3,11,12) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50122613

(3,4-dihydroisoquinoline-2(1H)-carboximidamide | CH...)Show InChI InChI=1S/C10H13N3/c11-10(12)13-6-5-8-3-1-2-4-9(8)7-13/h1-4H,5-7H2,(H3,11,12) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM533518
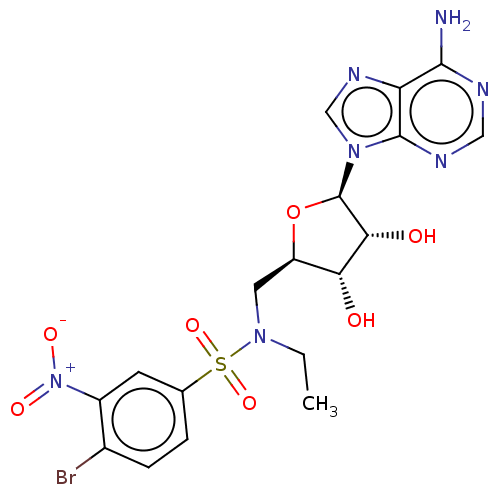
(jm2c00120, Compound 17)Show SMILES CCN(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)S(=O)(=O)c1ccc(Br)c(c1)[N+]([O-])=O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Ec50 of SARS-CoV-infected VeroE6 cells. |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00120
BindingDB Entry DOI: 10.7270/Q21839PH |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50031706

(4-Guanidino-benzoic acid 4-dimethylcarbamoylmethox...)Show SMILES [#6]-[#7](-[#6])-[#6](=O)-[#6]-[#8]-[#6](=O)-[#6]-c1ccc(-[#8]-[#6](=O)-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C20H22N4O5/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22/h3-10H,11-12H2,1-2H3,(H4,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM513873

(bioRxiv20220126.477782, Screening Hit 2)Show SMILES CNC(=O)Cn1c(=O)[nH]\c(=N/c2cc3cn(C)nc3cc2Cl)n(Cc2cc(F)c(F)c(F)c2)c1=O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antiviral activity against SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV-229E was assessed by monitoring cell viability; that against HCoV-OC43 was assesse... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7MFK |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM513874

(bioRxiv20220126.477782, S-217622 | bioRxiv20220126...)Show SMILES Cn1cnc(Cn2c(=O)[nH]\c(=N/c3cc4cn(C)nc4cc3Cl)n(Cc3cc(F)c(F)cc3F)c2=O)n1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antiviral activity against SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV-229E was assessed by monitoring cell viability; that against HCoV-OC43 was assesse... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QJ7MFK |
More data for this
Ligand-Target Pair | |
Transmembrane protease serine 2
(Homo sapiens (Human)) | BDBM50424712
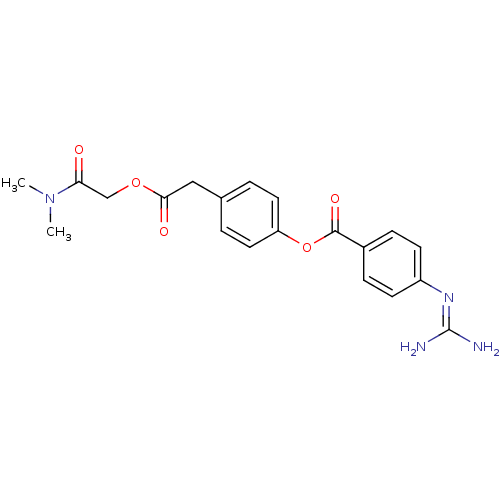
(CAMOSTAT | Camostat Mesilate | Foipan)Show SMILES [#6]-[#7](-[#6])-[#6](=O)-[#6]-[#8]-[#6](=O)-[#6]-c1ccc(-[#8]-[#6](=O)-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C20H22N4O5/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22/h3-10H,11-12H2,1-2H3,(H4,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TMPRSS2-mediated SARS-CoV-2 entry into human Caco-2 cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00626
BindingDB Entry DOI: 10.7270/Q2K35ZBW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data