

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272493 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 156 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272400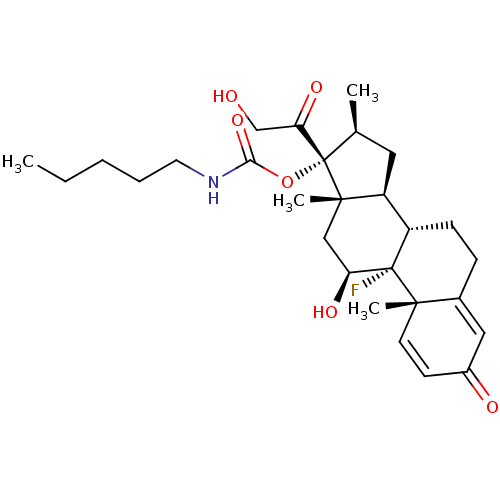 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272434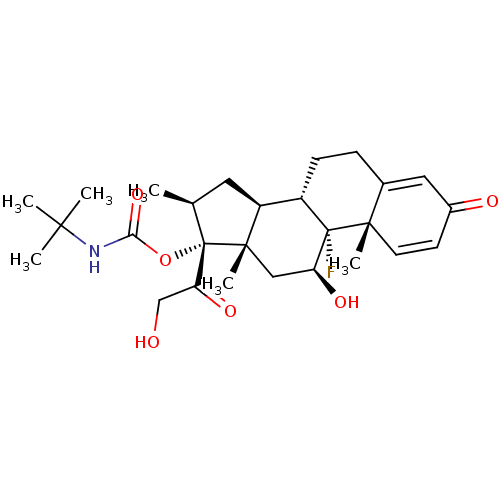 ((11beta,16beta)-9-Fluoro-11,21-dihydroxy-16-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272435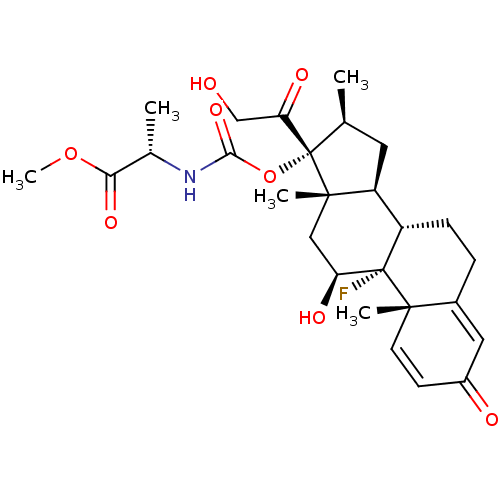 ((11beta,16beta)-9-Fluoro-11,21-dihydroxy-16-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 211 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272492 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 646 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272523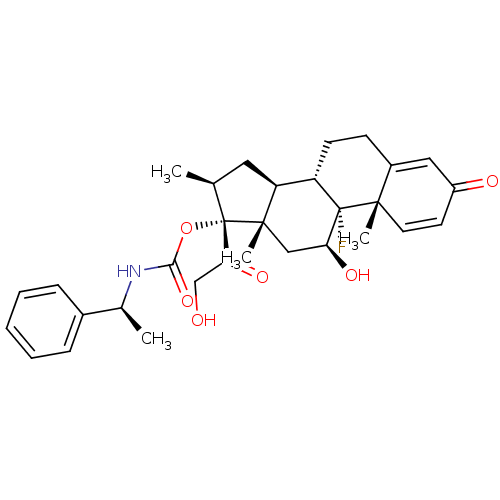 ((11beta,16beta)-9-Fluoro-11,21-dihydroxy-16-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272522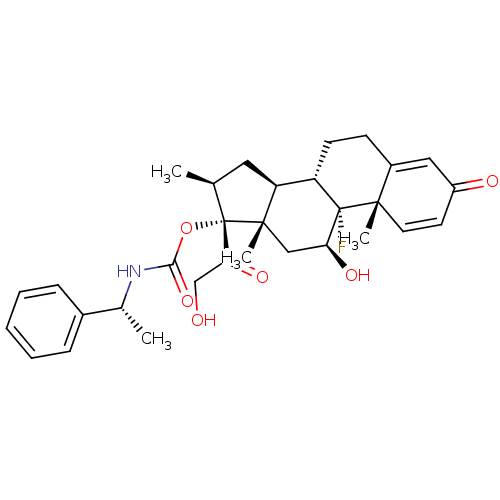 ((11beta,16beta)-9-Fluoro-11,21-dihydroxy-16-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 70.3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272521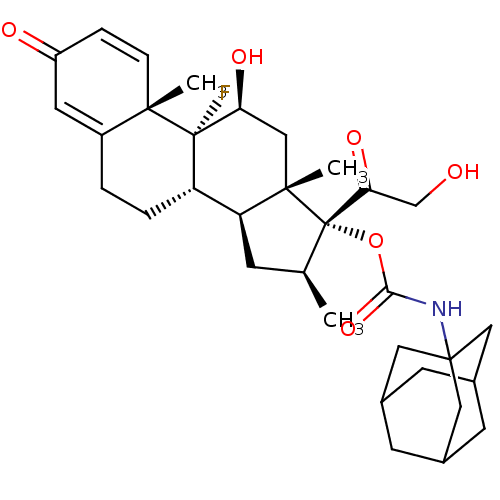 (Adamantan-1-yl-carbamic acid (8S,9R,10S,11S,13S,14...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272520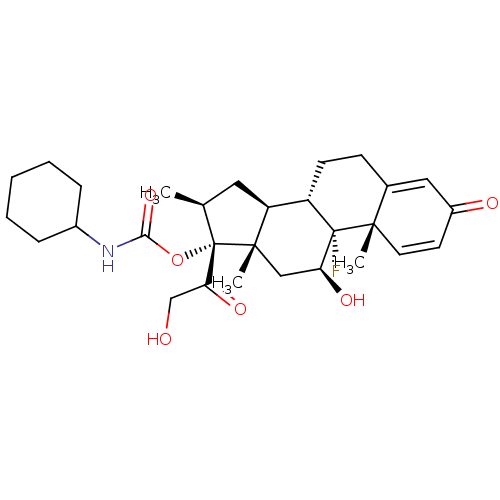 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine aminotransferase (Rattus norvegicus) | BDBM50272469 ((8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 705 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity of tyrosine amino transferase in rat H4 cells | Bioorg Med Chem 16: 7535-42 (2008) Article DOI: 10.1016/j.bmc.2008.07.037 BindingDB Entry DOI: 10.7270/Q2CC10HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||