

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM50526737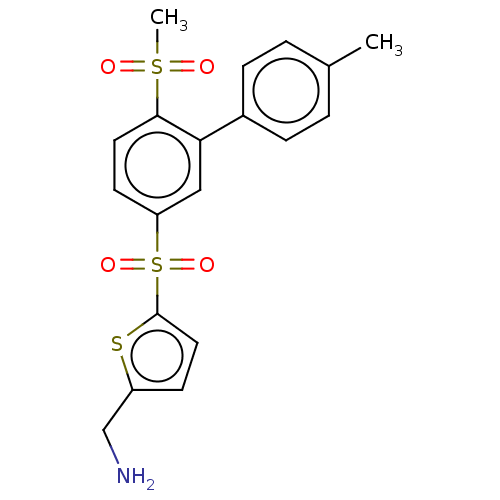 (CHEMBL4528500 | US10807974, Example 82 | US1099508...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <180 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description Cell Culture and TransfectionAll cell lines used in this study was purchased from American Type Culture Collection (ATCC). Mycoplasma contamination w... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Mus musculus) | BDBM50526737 (CHEMBL4528500 | US10807974, Example 82 | US1099508...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <180 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description To produce MDCK cysts, cells were cultured on Matrigel (Corning) with 2% Matrigel supplemented in DMEM with 10% FBS. Cysts were allowed to form for 1... | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM50526721 (CHEMBL4444163 | US10807974, Example 111 | US109950...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description Cell Culture and TransfectionAll cell lines used in this study was purchased from American Type Culture Collection (ATCC). Mycoplasma contamination w... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Mus musculus) | BDBM50526721 (CHEMBL4444163 | US10807974, Example 111 | US109950...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description To produce MDCK cysts, cells were cultured on Matrigel (Corning) with 2% Matrigel supplemented in DMEM with 10% FBS. Cysts were allowed to form for 1... | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469007 (US10807974, Example 16 | US10995088, Example 16 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469007 (US10807974, Example 16 | US10995088, Example 16 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633180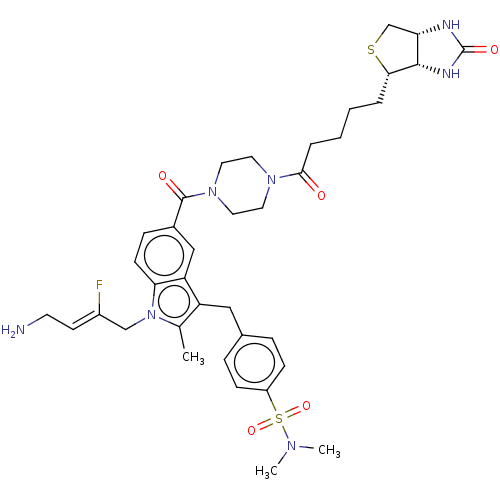 (4-((1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-2-methyl...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633181 (1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-3-(3-(N,N-di...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633197 (N,N′-((((((((((5-(1-((Z)-4-amino-2-fluorobut...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633202 (N,N′-((((5-(1-((Z)-4-amino-2-fluorobut-2-en-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633203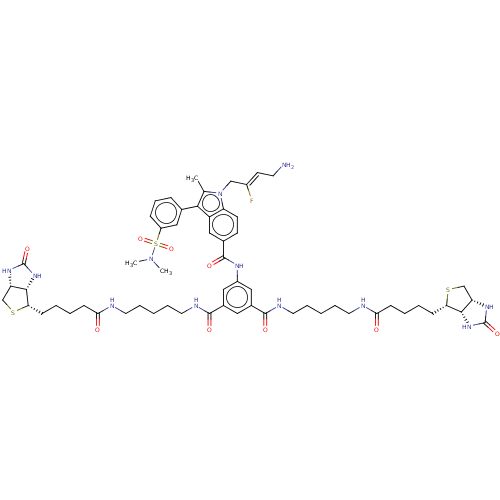 (5-(1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-3-(3-(N,N...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633204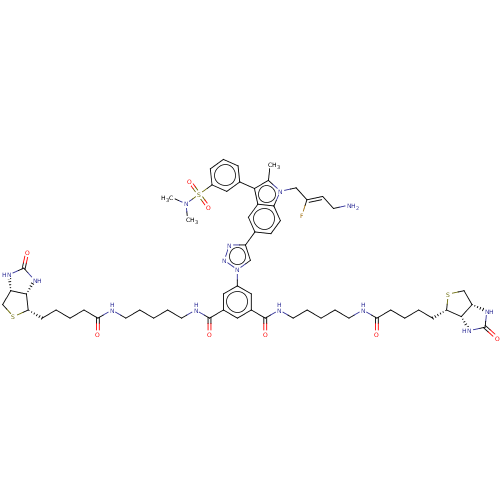 (5-(4-(1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-3-(3-(...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633224 (4-(((Z)-4-amino-2-fluorobut-2-en-1-yl)sulfonyl)-N-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633227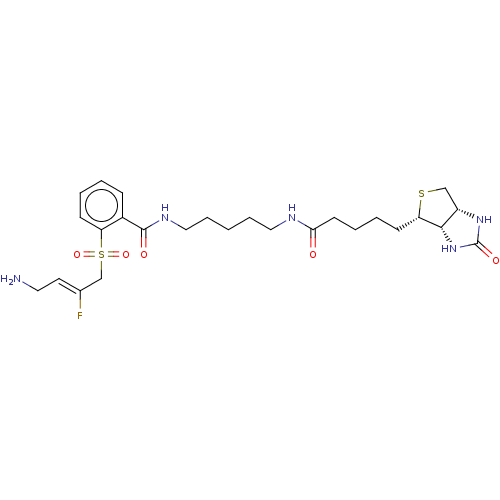 (2-(((Z)-4-amino-2-fluorobut-2-en-1-yl)sulfonyl)-N-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633233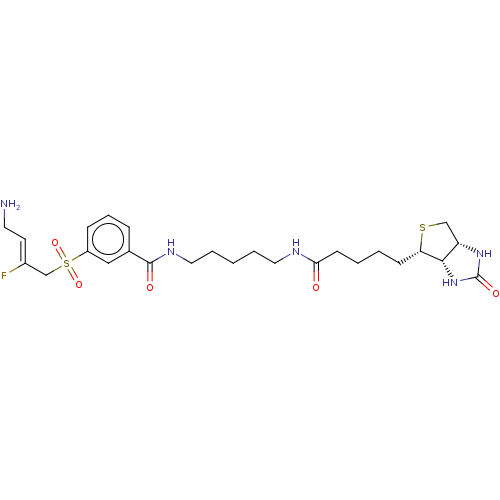 (3-(((Z)-4-amino-2-fluorobut-2-en-1-yl)sulfonyl)-N-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633244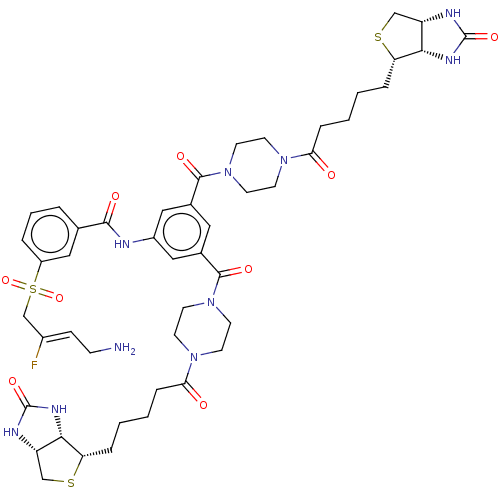 (3-(((Z)-4-amino-2-fluorobut-2-en-1-yl)sulfonyl)-N-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633245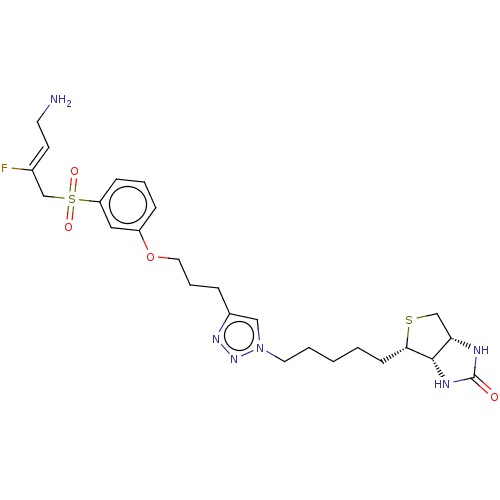 ((3aS,4S,6aR)-4-(5-(4-(3-(3-(((Z)-4-amino-2-fluorob...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633246 ((3aS,3a'S,4S,4'S,6aR,6a′R)-4,4′-(((((5...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633247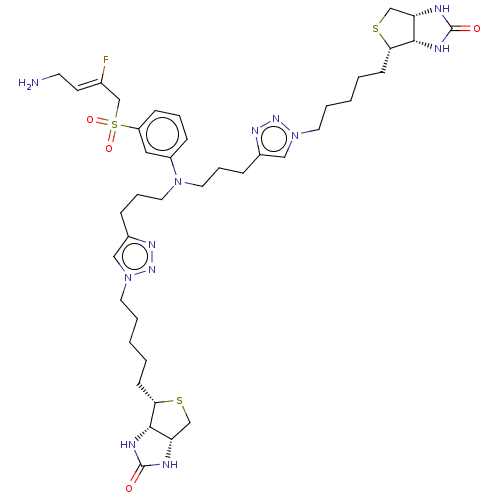 ((3aS,3a'S,4S,4'S,6aR,6a′R)-4,4′-(((((3...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633248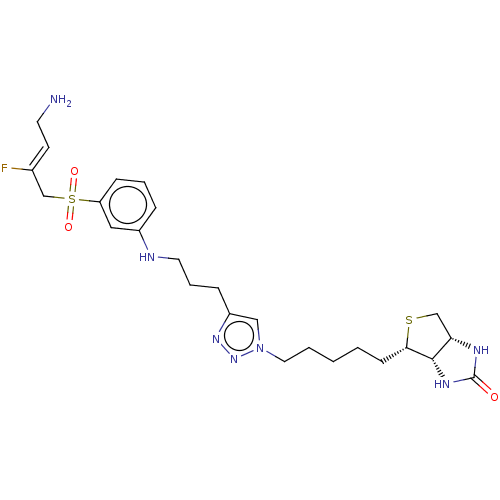 ((3aS,4S,6aR)-4-(4-(4-(3-((3-(((Z)-4-amino-2-fluoro...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633249 (N-((1-(4-(((1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM633250 (N-((1-(4-(((1-((Z)-4-amino-2-fluorobut-2-en-1-yl)-...) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM50058655 (1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human LOX expressed in HEK293 cells using diaminopentane as substrate preincubated for 30 mins followed by substrate additi... | Bioorg Med Chem Lett 28: 3113-3118 (2018) Article DOI: 10.1016/j.bmcl.2018.07.001 BindingDB Entry DOI: 10.7270/Q28P6366 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469005 (US10807974, Example 9 | US10995088, Example 9 | US...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469005 (US10807974, Example 9 | US10995088, Example 9 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM50505633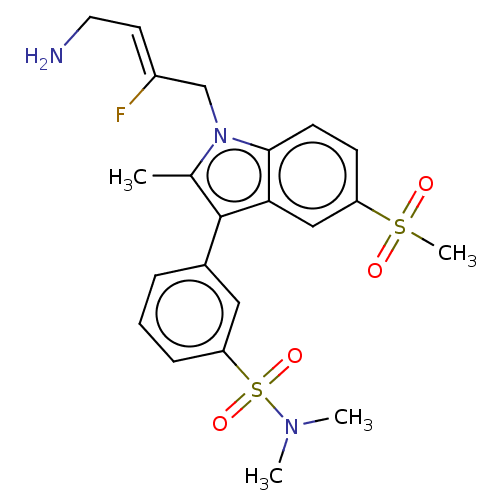 (CHEMBL4448713) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd Curated by ChEMBL | Assay Description Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever... | J Med Chem 62: 9874-9889 (2019) Article DOI: 10.1021/acs.jmedchem.9b01283 BindingDB Entry DOI: 10.7270/Q2VM4GJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM50505649 (CHEMBL4439437) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd Curated by ChEMBL | Assay Description Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever... | J Med Chem 62: 9874-9889 (2019) Article DOI: 10.1021/acs.jmedchem.9b01283 BindingDB Entry DOI: 10.7270/Q2VM4GJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469011 (US10807974, Example 27 | US10995088, Example 27 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469011 (US10807974, Example 27 | US10995088, Example 27 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469032 (US10807974, Example 43 | US10995088, Example 43 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469032 (US10807974, Example 43 | US10995088, Example 43 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469023 (US10807974, Example 39 | US10995088, Example 39 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469023 (US10807974, Example 39 | US10995088, Example 39 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469019 (US10807974, Example 35 | US10995088, Example 35 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469008 (US10807974, Example 17 | US10995088, Example 17 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469008 (US10807974, Example 17 | US10995088, Example 17 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469019 (US10807974, Example 35 | US10995088, Example 35 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469024 (US10807974, Example 40 | US10995088, Example 40 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469024 (US10807974, Example 40 | US10995088, Example 40 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469004 (US10807974, Example 5 | US10995088, Example 5 | US...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469004 (US10807974, Example 5 | US10995088, Example 5 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM50505657 (CHEMBL4467234) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd Curated by ChEMBL | Assay Description Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever... | J Med Chem 62: 9874-9889 (2019) Article DOI: 10.1021/acs.jmedchem.9b01283 BindingDB Entry DOI: 10.7270/Q2VM4GJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM50505659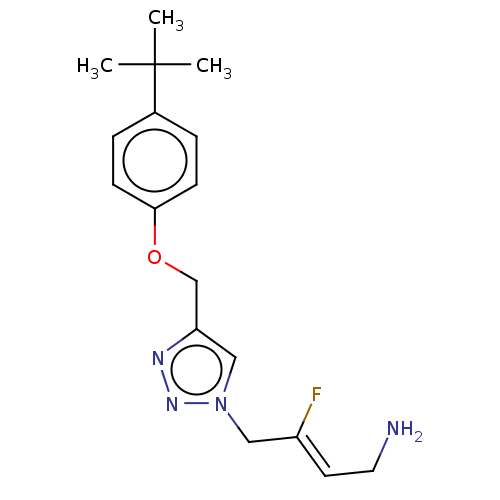 (CHEMBL4459524) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd Curated by ChEMBL | Assay Description Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever... | J Med Chem 62: 9874-9889 (2019) Article DOI: 10.1021/acs.jmedchem.9b01283 BindingDB Entry DOI: 10.7270/Q2VM4GJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Bos taurus) | BDBM50505655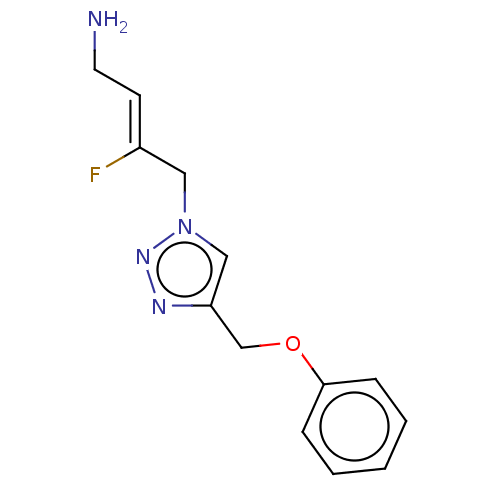 (CHEMBL4441299) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaxis Ltd Curated by ChEMBL | Assay Description Inhibition of LOX in bovine aorta using putrescine as substrate preincubated with enzyme for 30 mins followed by substrate addition and measured ever... | J Med Chem 62: 9874-9889 (2019) Article DOI: 10.1021/acs.jmedchem.9b01283 BindingDB Entry DOI: 10.7270/Q2VM4GJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469027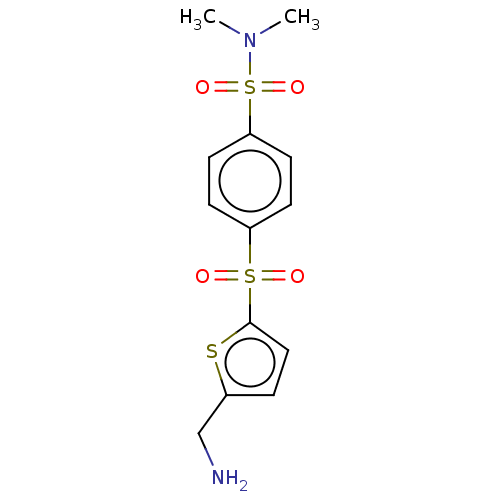 (US10807974, Example 12 | US10995088, Example 12 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469027 (US10807974, Example 12 | US10995088, Example 12 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Sus scrofa) | BDBM469018 (US10807974, Example 34 | US10995088, Example 34 | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOX enzyme was obtained from pig skin by the method of Shackleton et al 1990. | US Patent US10807974 (2020) BindingDB Entry DOI: 10.7270/Q2TH8QS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469018 (US10807974, Example 34 | US10995088, Example 34 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM50232678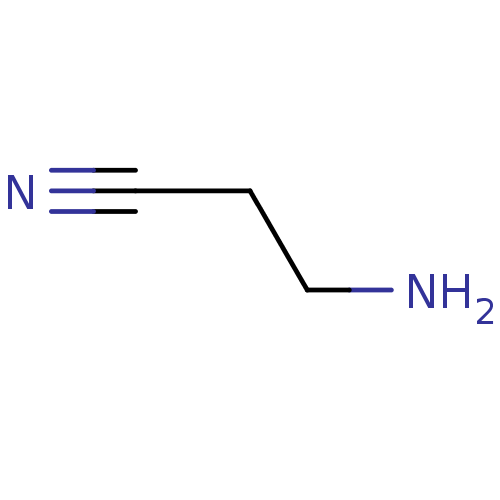 (CHEBI:27413 | CHEMBL1618272 | US10717734, Compound...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmAkea Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human LOX expressed in HEK cells assessed as reduction in H2O2 production using DAP as substrate preincubated f... | ACS Med Chem Lett 8: 423-427 (2017) Article DOI: 10.1021/acsmedchemlett.7b00014 BindingDB Entry DOI: 10.7270/Q2542QV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-lysine 6-oxidase (Homo sapiens (Human)) | BDBM469010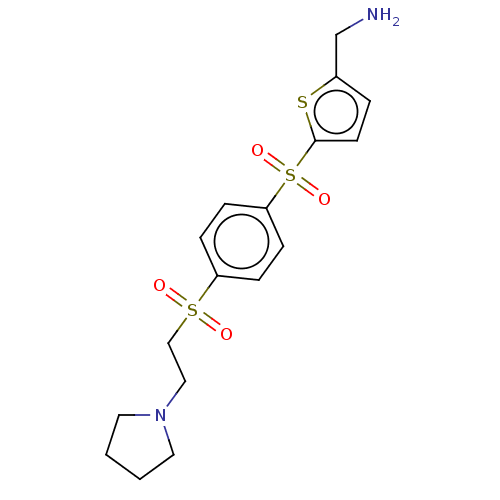 (US10807974, Example 26 | US10995088, Example 26 | ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description The following reagents were prepared:Reagents500 mM CHES, pH 9 (with NaOH), filtered (MW=207.29)20.7 g in 200 ml in dH2O10% Pluronic F-127 (store at ... | US Patent US10995088 (2021) BindingDB Entry DOI: 10.7270/Q2125WSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 325 total ) | Next | Last >> |