

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21726 (2-Trifluoromethyltetradecanoyl-CoA, 3) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 900 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21729 (((2R,3S)-3-Fluoro-2-methylhexadecanoyl-CoA, 5) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21730 ((2S,3R)-3-fluoro-2-methylhexadecanoyl-CoA, 6) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21731 (THC-CoA Analogue, 7) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21732 ((R)-ibuprofenoyl-CoA, 20) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.40E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21733 ((S)-ibuprofenoyl-CoA, 20) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21728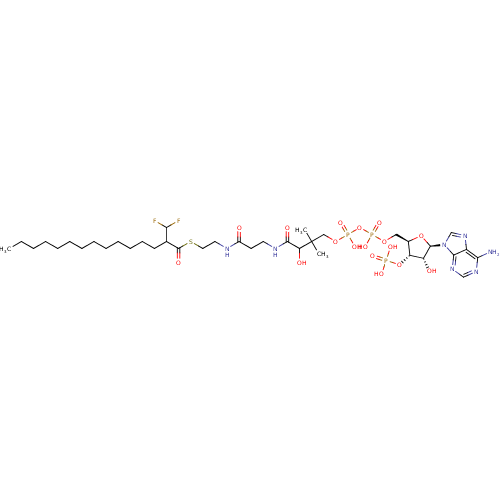 (2-Difluoromethylpentadecanoyl-CoA, 4) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21735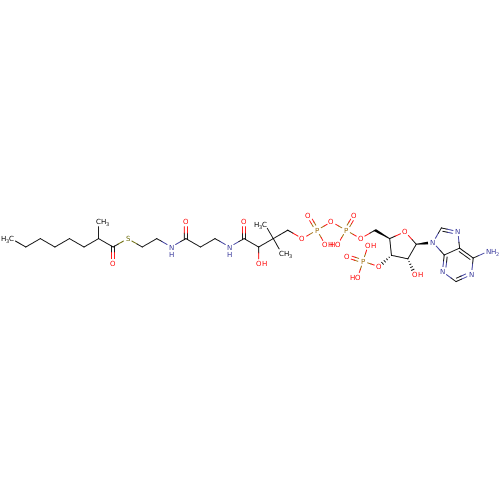 (2-methyloctanoyl CoA, 21 | {[(2R,3S,4R,5R)-5-(6-am...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21734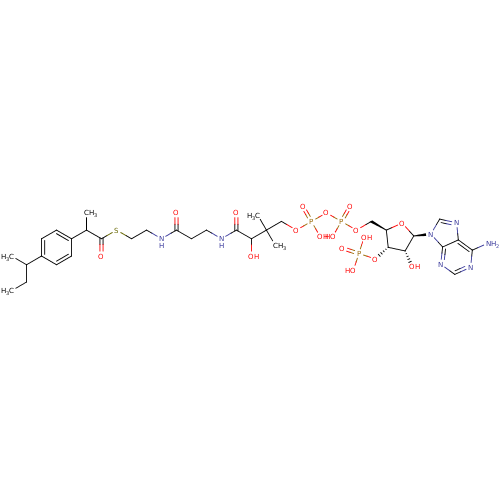 ((Rac)-ibuprofenoyl-CoA, 20 | racemic mixture) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | -25.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-methylacyl-CoA racemase (Rattus norvegicus (rat)) | BDBM21736 (2-methylmyristoyl CoA, 22) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.37E+5 | -22.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Liverpool | Assay Description AMACR activity was determined by monitoring the interconversion of the (25R/S)-isomer of THC-CoA. Reaction product was analyzed by resolution of (25S... | J Med Chem 50: 2700-7 (2007) Article DOI: 10.1021/jm0702377 BindingDB Entry DOI: 10.7270/Q2X065BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||