

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330426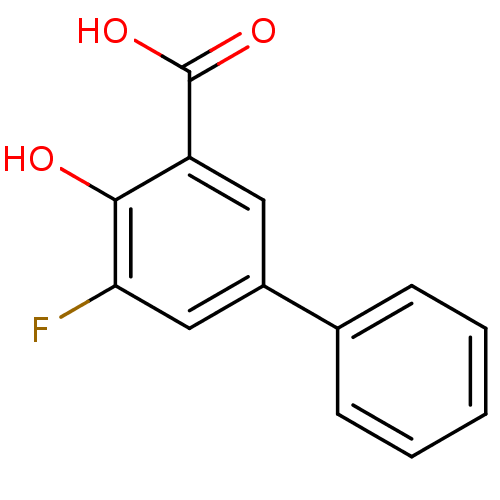 (5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330431 (4'-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330428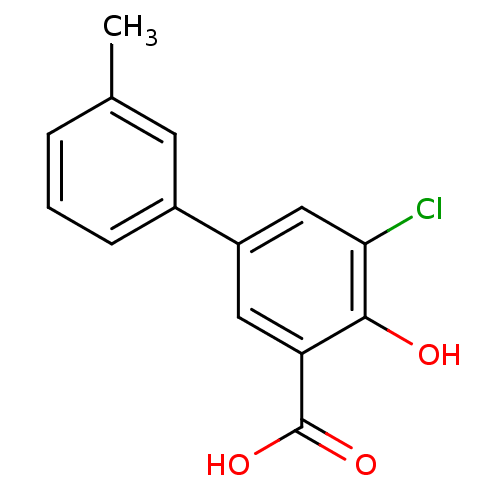 (5-Chloro-4-hydroxy-3'-methylbiphenyl-3-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330427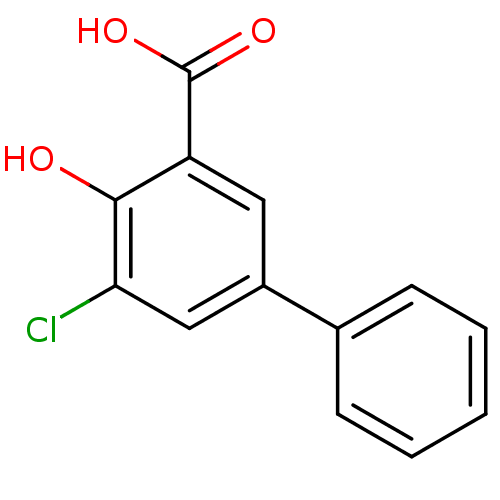 (5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330424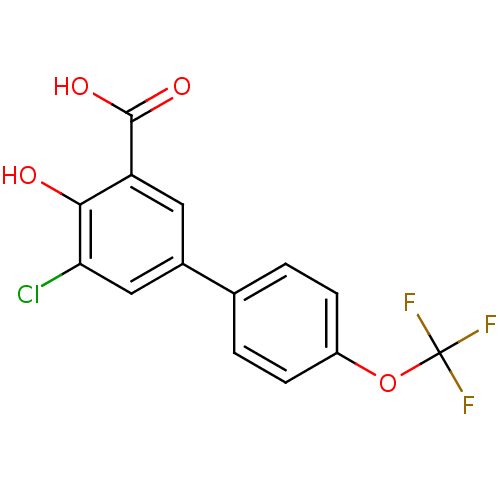 (5-Chloro-4-hydroxy-4'-(trifluoromethoxy)biphenyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330432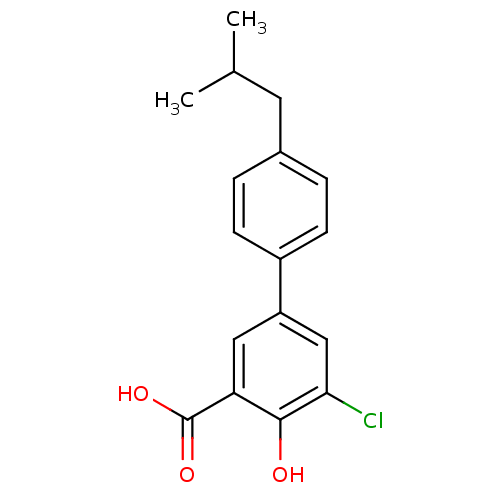 (5-Chloro-4-hydroxy-4'-isobutylbiphenyl-3-carboxyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330429 (5-Chloro-4-hydroxy-4'-methylbiphenyl-3-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM26269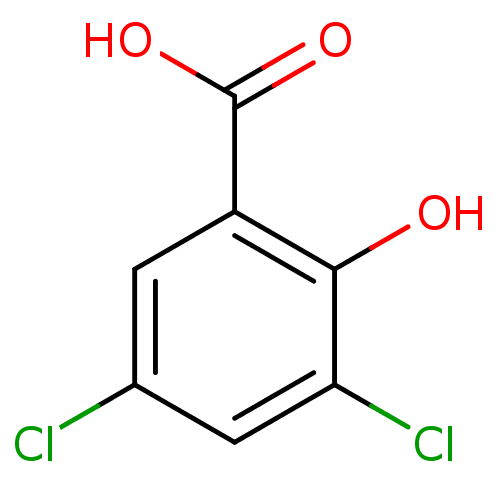 (3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 70 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Monash University | Assay Description The activity was assayed by measuring the rate of change in NADPH fluorescence (at 455 nm with an excitation wavelength of 340 nm) at 298 K. When the... | J Med Chem 51: 4844-8 (2008) Article DOI: 10.1021/jm8003575 BindingDB Entry DOI: 10.7270/Q2MG7MTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM26269 (3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50249792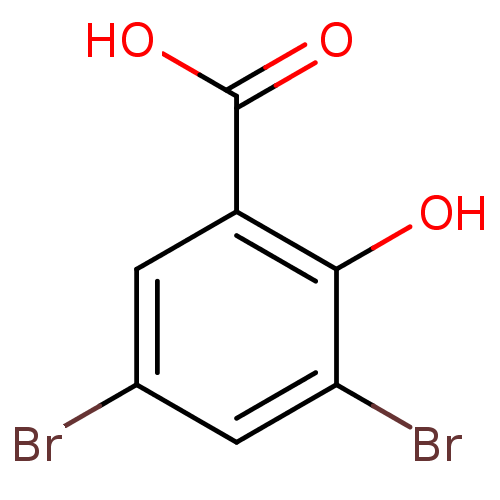 (3,5-Dibromosalicylic acid | CHEMBL447448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 3 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50219490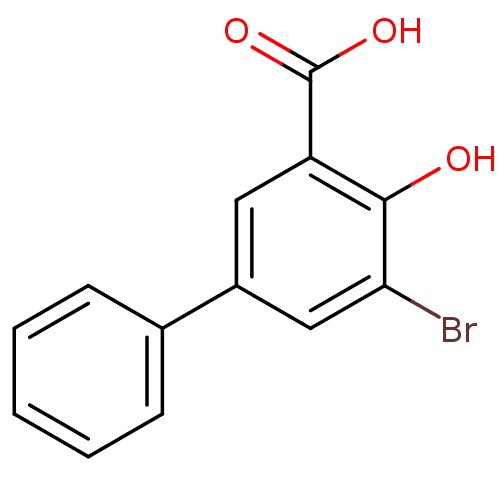 (3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 3 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50219490 (3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330425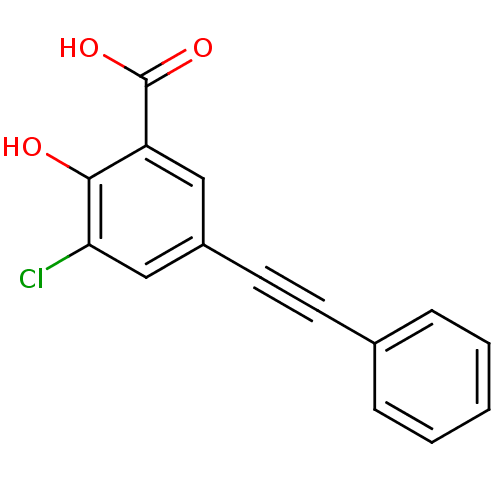 (3-chloro-2-hydroxy-5-(phenylethynyl)benzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50134036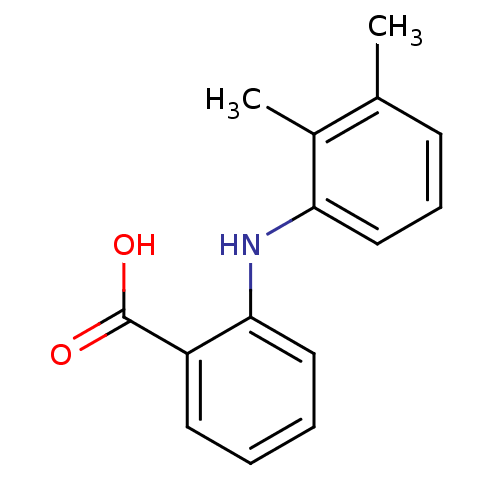 (2-(2,3-Dimethyl-phenylamino)-benzoic acid | 2-(2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330423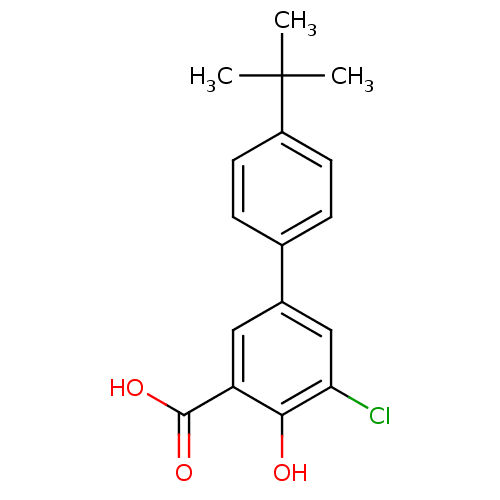 (4'-tert-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM220117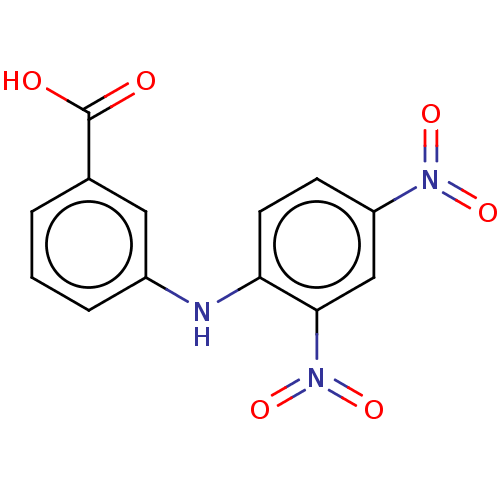 (US9271961, 4-Carboxy-2',4'-dinitrodiphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | US Patent | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50240769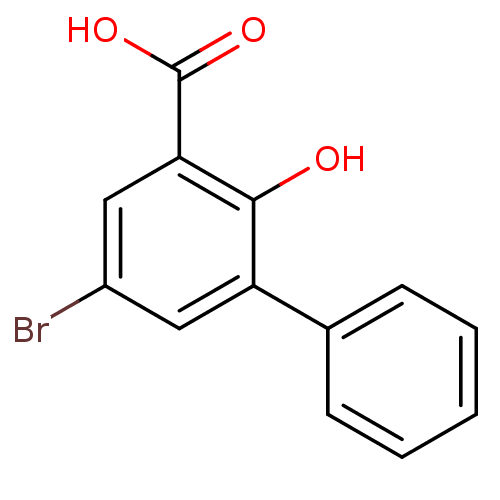 (3-Phenyl-5-bromosalicylic acid | 5-Bromo-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant type 3 3-alpha-HSD expressed in Escherichia coli JM109 | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50330430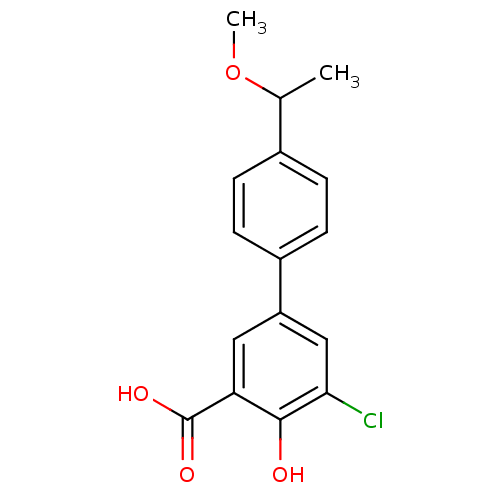 (5-Chloro-4-hydroxy-4'-(1-methoxyethyl)biphenyl-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM220121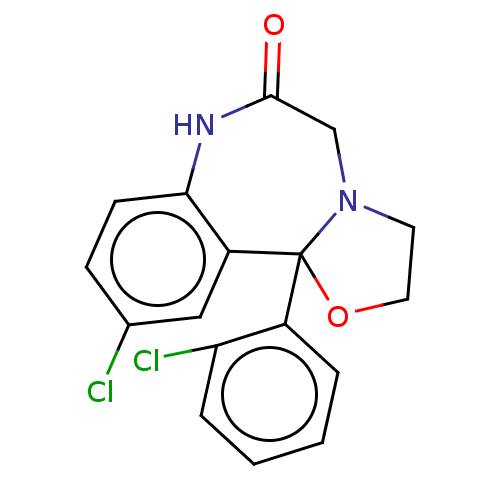 (US9271961, Cloxazolam) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem | US Patent | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C21 (Mus musculus) | BDBM50038843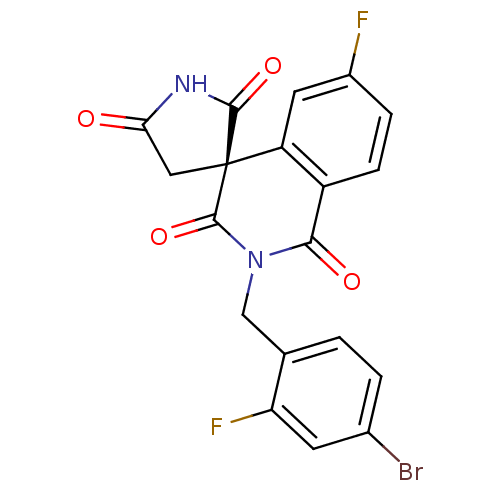 ((4R)-2-(4-bromo-2-fluorobenzyl)-6-fluoro-1H,2'H,5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant AKR1C21 | Bioorg Med Chem 16: 3245-54 (2008) Article DOI: 10.1016/j.bmc.2007.12.016 BindingDB Entry DOI: 10.7270/Q2PZ59P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C21 (Mus musculus) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant AKR1C21 | Bioorg Med Chem 16: 3245-54 (2008) Article DOI: 10.1016/j.bmc.2007.12.016 BindingDB Entry DOI: 10.7270/Q2PZ59P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | US Patent | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396743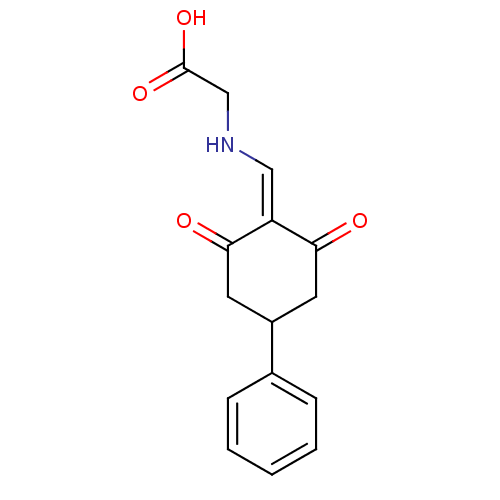 (CHEMBL2172255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396731 (CHEMBL2172254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C21 (Mus musculus) | BDBM16314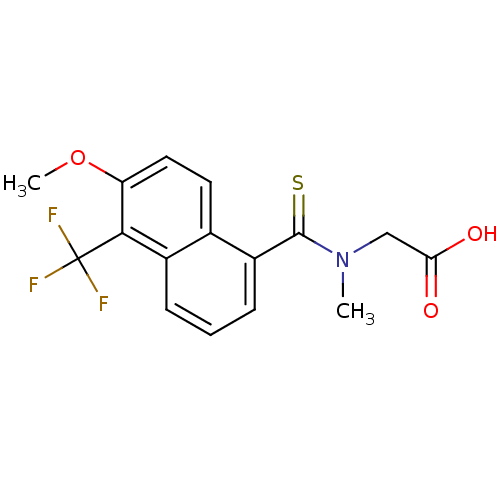 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant AKR1C21 | Bioorg Med Chem 16: 3245-54 (2008) Article DOI: 10.1016/j.bmc.2007.12.016 BindingDB Entry DOI: 10.7270/Q2PZ59P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM34643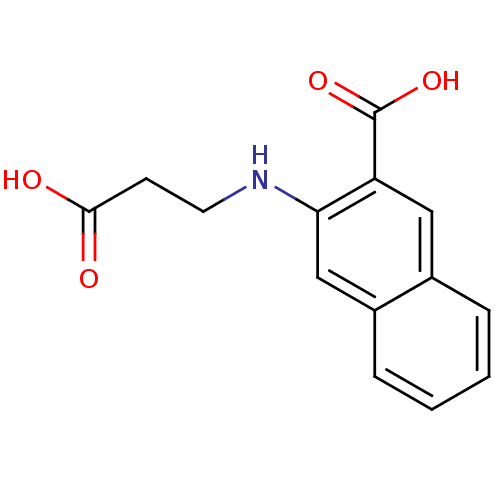 (3-(2-carboxyethylamino)-2-naphthalenecarboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396733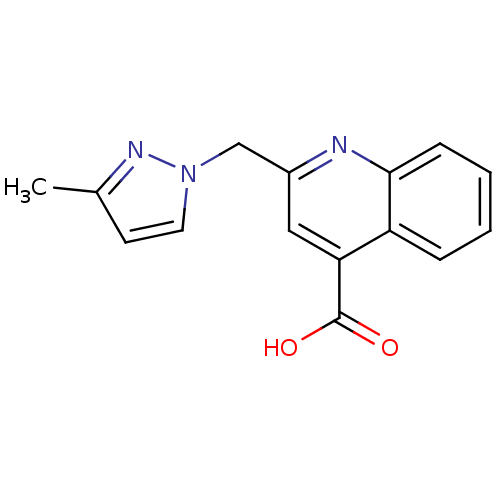 (CHEMBL2172251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396748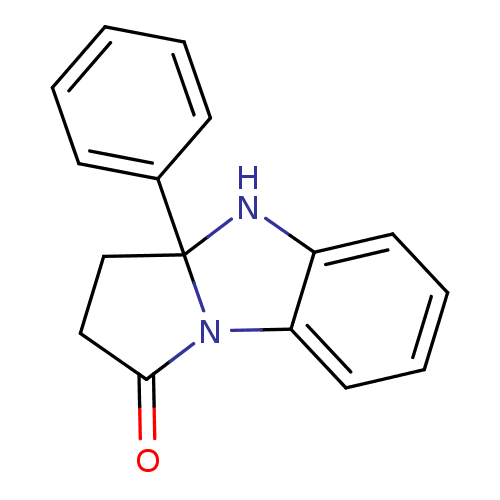 (CHEMBL366350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C2 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysis | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396730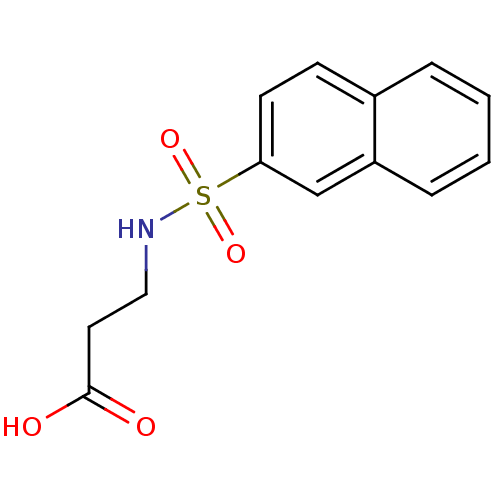 (CHEMBL1328030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 5.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396739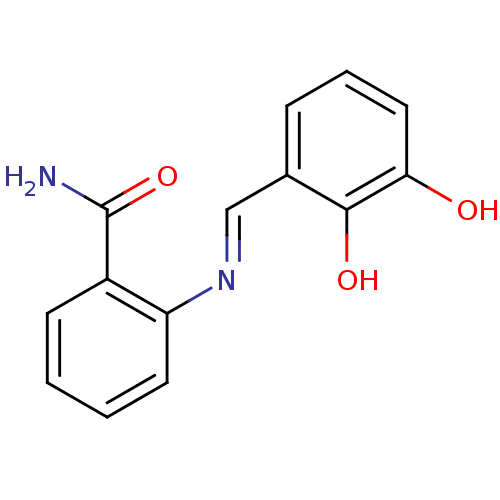 (CHEMBL2172243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396742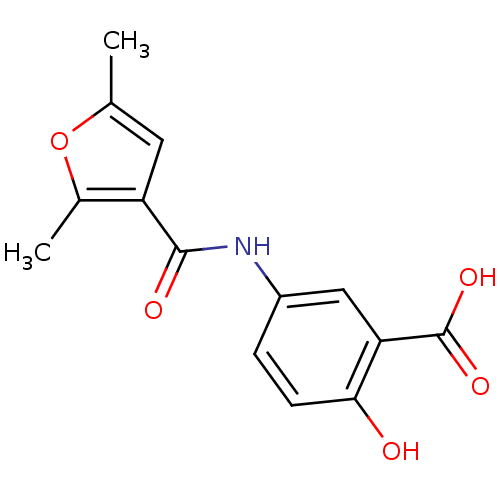 (CHEMBL1373742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM220116 (US9271961, CBM) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396749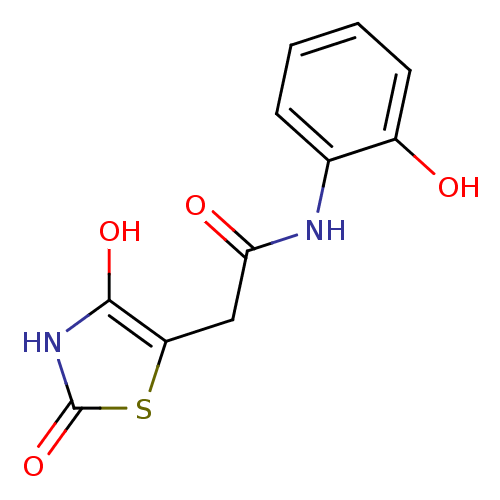 (CHEMBL2172258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AKR1C2 assessed as S-tetralol oxidation by Cheng-Prusoff equation analysis | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396734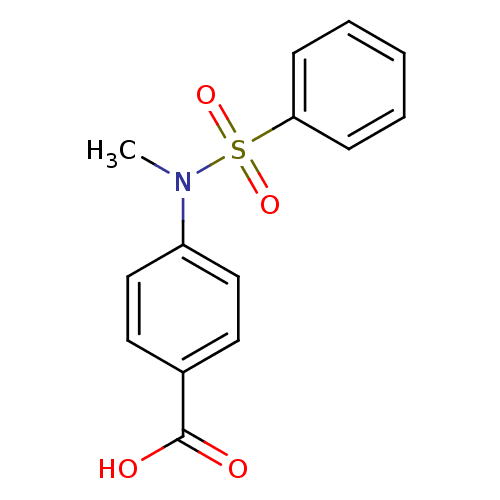 (CHEMBL1896308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396732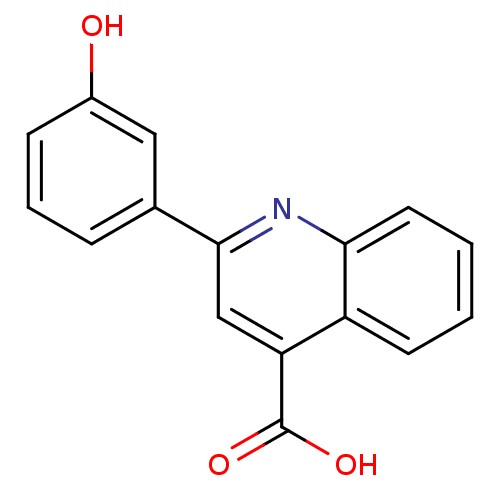 (CHEMBL2172252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396729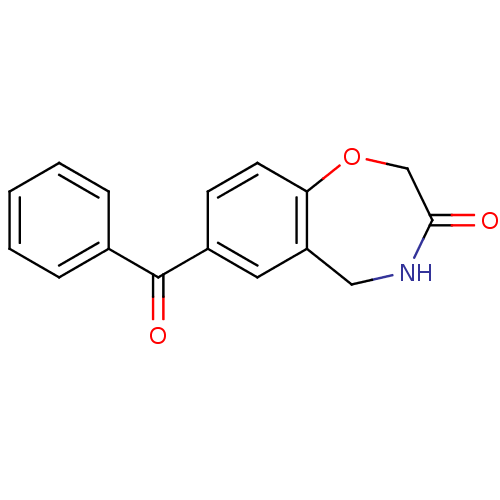 (CHEMBL1414132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 assessed as 1-acenaphthenol oxidation by spectrophotometry | J Med Chem 55: 7417-24 (2012) Article DOI: 10.1021/jm300841n BindingDB Entry DOI: 10.7270/Q2125TR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427620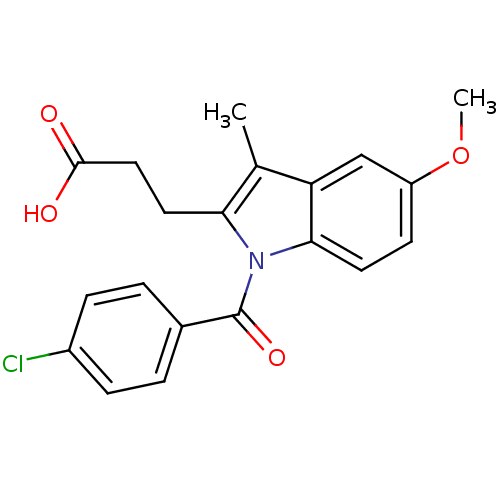 (CHEMBL2323507 | US9346803, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427627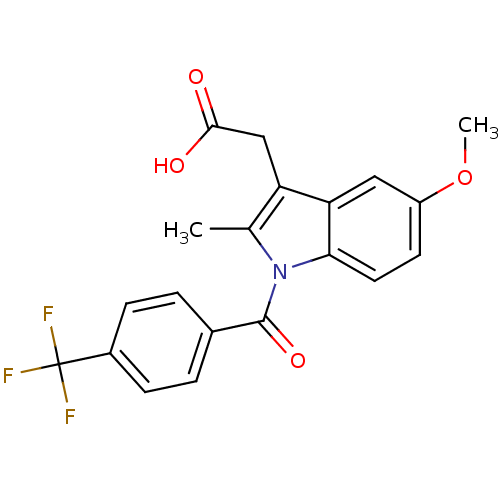 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427628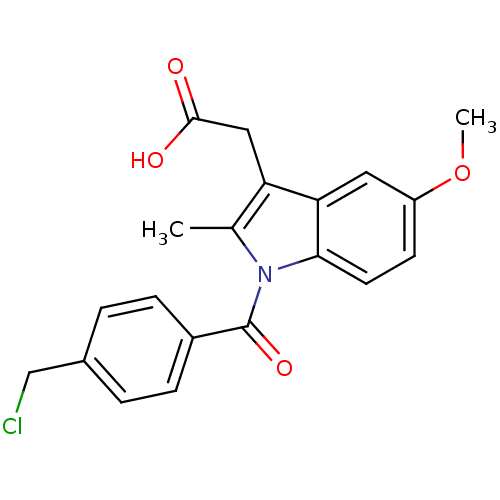 (CHEMBL2323472 | US9346803, Table 2, Compound 8: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427622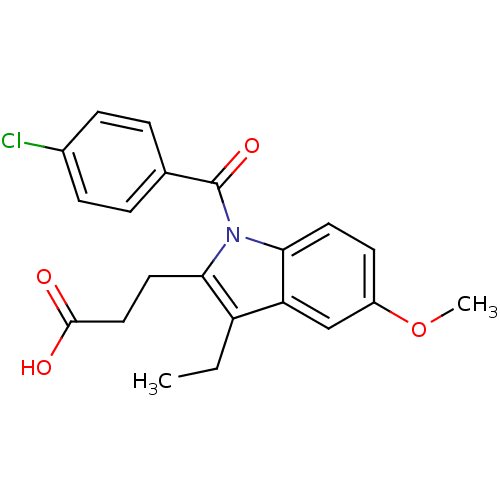 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427621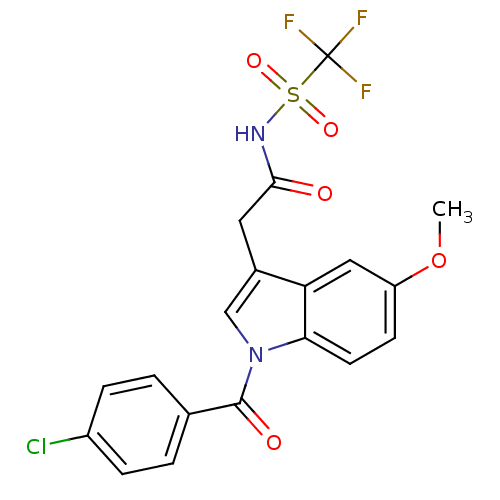 (CHEMBL2323490 | US9346803, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427624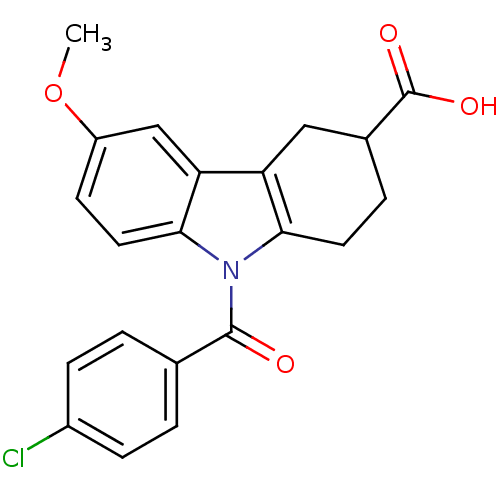 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 53.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427629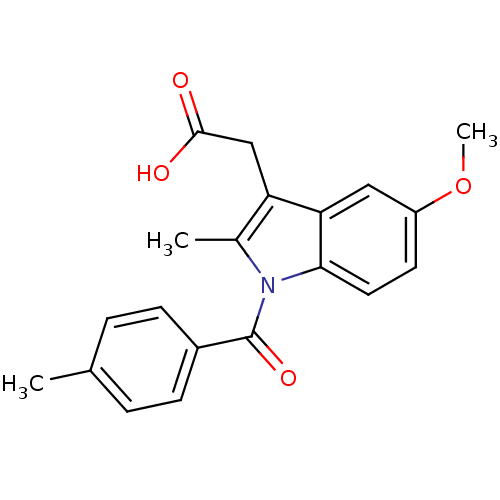 (CHEMBL179587 | US9346803, Table 2, Compound 7: 2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427625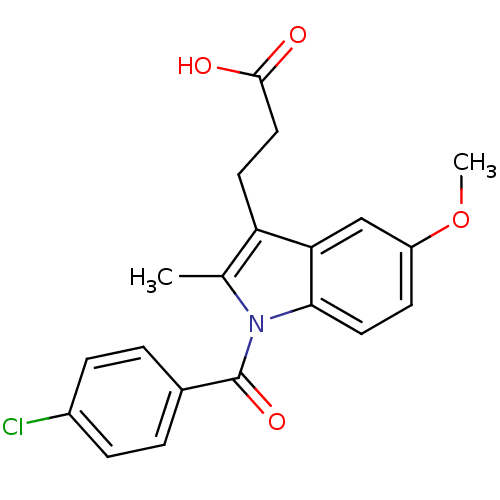 (CHEMBL178687 | US9346803, Table 2, Compound 6: 3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427626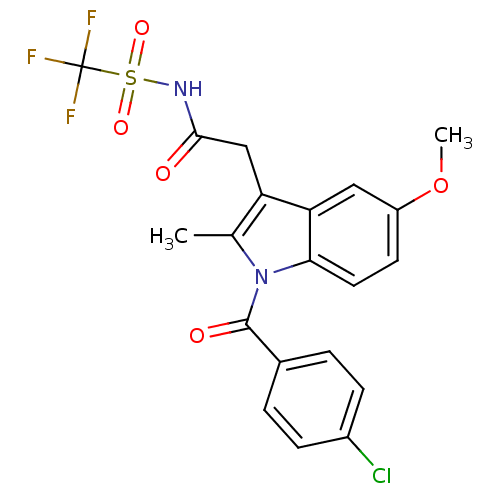 (CHEMBL2323481 | US9346803, Table 2, Compound 5: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427619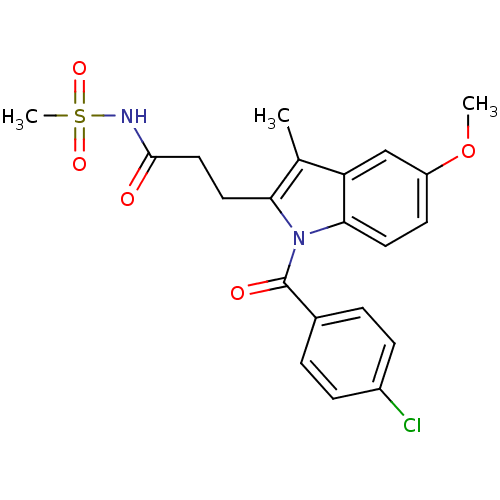 (CHEMBL2323511 | US9346803, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50293598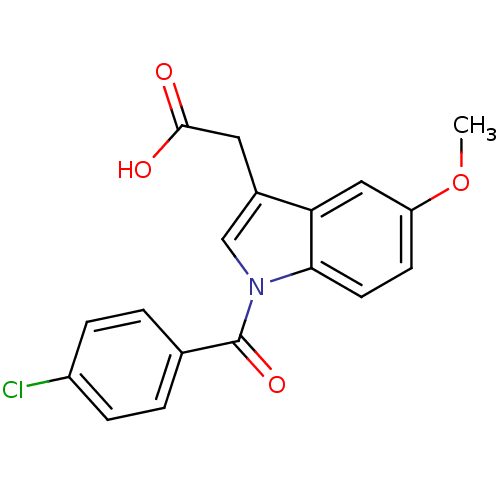 (2'-des-methyl indomethacin | CHEMBL503179 | US9346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50385711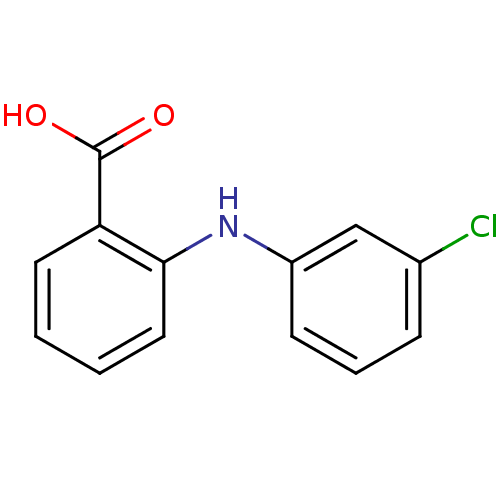 (CHEMBL22815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assay | J Med Chem 55: 2311-23 (2012) Article DOI: 10.1021/jm201547v BindingDB Entry DOI: 10.7270/Q2C24XGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 537 total ) | Next | Last >> |