 Found 2345 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 1B'
Found 2345 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 1B' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50280055
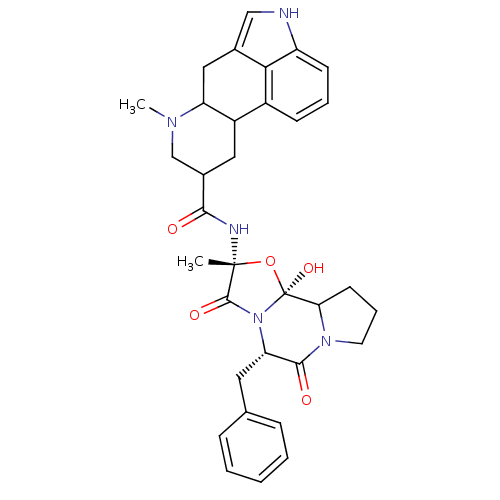
(50285557 | 7-Methyl-4,6,6a,7,8,9,10,10a-octahydro-...)Show SMILES CN1CC(CC2C1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)C3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O Show InChI InChI=1S/C33H37N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39)/t21?,23?,25?,26-,27?,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 184: 752-9 (1992)
Article DOI: 10.1016/0006-291x(92)90654-4
BindingDB Entry DOI: 10.7270/Q2ST7NB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50280055

(50285557 | 7-Methyl-4,6,6a,7,8,9,10,10a-octahydro-...)Show SMILES CN1CC(CC2C1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)C3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O Show InChI InChI=1S/C33H37N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39)/t21?,23?,25?,26-,27?,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(GUINEA PIG) | BDBM50590649
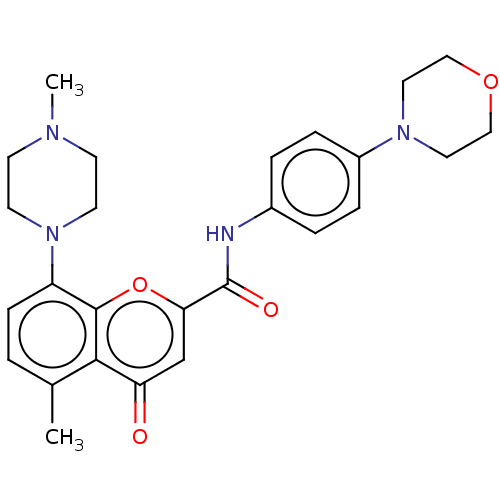
(CHEMBL5209320)Show SMILES Cc1ccc(N2CCN([11CH3])CC2)c2oc(cc(=O)c12)C(=O)Nc1ccc(cc1)N1CCOCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50280055

(50285557 | 7-Methyl-4,6,6a,7,8,9,10,10a-octahydro-...)Show SMILES CN1CC(CC2C1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)C3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O Show InChI InChI=1S/C33H37N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39)/t21?,23?,25?,26-,27?,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50027065
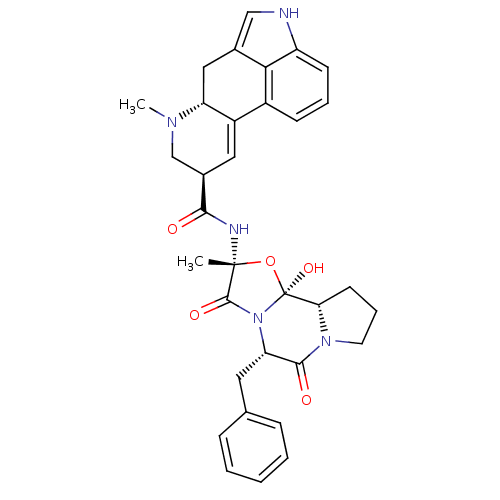
((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM81497
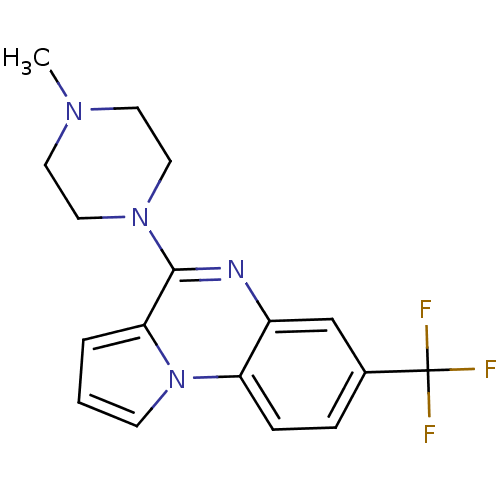
(4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...)Show InChI InChI=1S/C17H17F3N4/c1-22-7-9-23(10-8-22)16-15-3-2-6-24(15)14-5-4-12(17(18,19)20)11-13(14)21-16/h2-6,11H,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 184: 752-9 (1992)
Article DOI: 10.1016/0006-291x(92)90654-4
BindingDB Entry DOI: 10.7270/Q2ST7NB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM81497

(4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...)Show InChI InChI=1S/C17H17F3N4/c1-22-7-9-23(10-8-22)16-15-3-2-6-24(15)14-5-4-12(17(18,19)20)11-13(14)21-16/h2-6,11H,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50271131
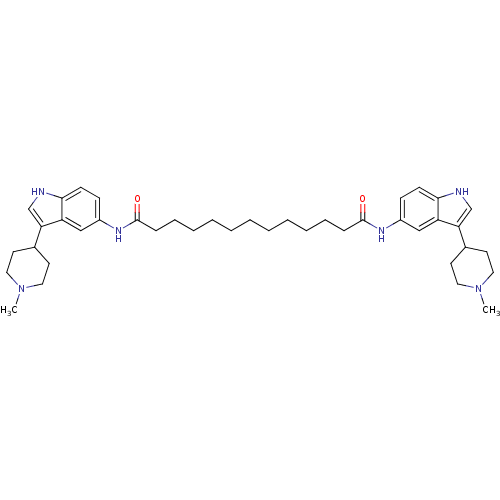
(CHEMBL374973 | N1,N13-bis(3-(1-methylpiperidin-4-y...)Show SMILES CN1CCC(CC1)c1c[nH]c2ccc(NC(=O)CCCCCCCCCCCC(=O)Nc3ccc4[nH]cc(C5CCN(C)CC5)c4c3)cc12 Show InChI InChI=1S/C41H58N6O2/c1-46-22-18-30(19-23-46)36-28-42-38-16-14-32(26-34(36)38)44-40(48)12-10-8-6-4-3-5-7-9-11-13-41(49)45-33-15-17-39-35(27-33)37(29-43-39)31-20-24-47(2)25-21-31/h14-17,26-31,42-43H,3-13,18-25H2,1-2H3,(H,44,48)(H,45,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting |
J Med Chem 51: 3609-16 (2008)
Article DOI: 10.1021/jm7011722
BindingDB Entry DOI: 10.7270/Q26T0MDK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50175484
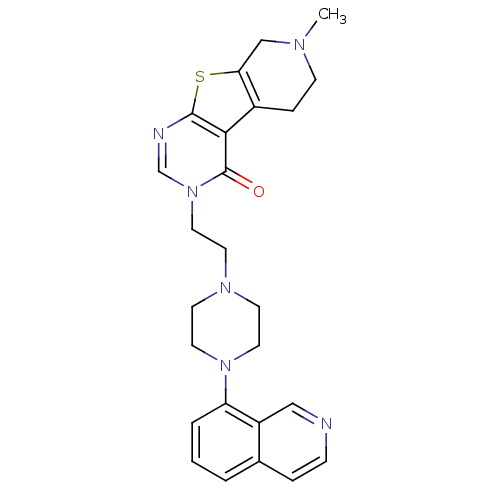
(3-[2-(4-isoquinolin-8-yl-piperazin-1-yl)-ethyl]-7-...)Show SMILES CN1CCc2c(C1)sc1ncn(CCN3CCN(CC3)c3cccc4ccncc34)c(=O)c21 Show InChI InChI=1S/C25H28N6OS/c1-28-8-6-19-22(16-28)33-24-23(19)25(32)31(17-27-24)14-11-29-9-12-30(13-10-29)21-4-2-3-18-5-7-26-15-20(18)21/h2-5,7,15,17H,6,8-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR |
Bioorg Med Chem Lett 15: 5567-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.077
BindingDB Entry DOI: 10.7270/Q2CR5SX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054974
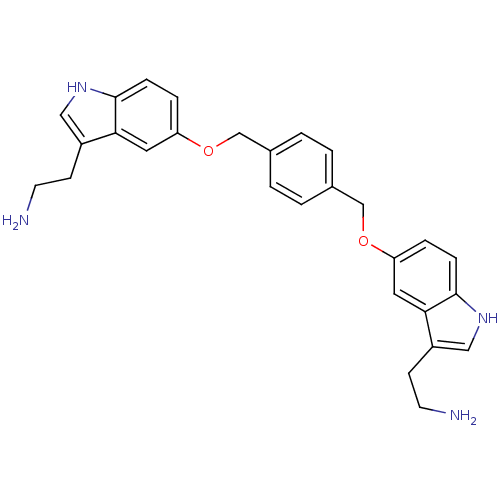
(2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...)Show SMILES NCCc1c[nH]c2ccc(OCc3ccc(COc4ccc5[nH]cc(CCN)c5c4)cc3)cc12 Show InChI InChI=1S/C28H30N4O2/c29-11-9-21-15-31-27-7-5-23(13-25(21)27)33-17-19-1-2-20(4-3-19)18-34-24-6-8-28-26(14-24)22(10-12-30)16-32-28/h1-8,13-16,31-32H,9-12,17-18,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1B receptor expressed in CHOK1 cells by liquid scintillation counting |
J Med Chem 51: 3609-16 (2008)
Article DOI: 10.1021/jm7011722
BindingDB Entry DOI: 10.7270/Q26T0MDK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054974

(2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...)Show SMILES NCCc1c[nH]c2ccc(OCc3ccc(COc4ccc5[nH]cc(CCN)c5c4)cc3)cc12 Show InChI InChI=1S/C28H30N4O2/c29-11-9-21-15-31-27-7-5-23(13-25(21)27)33-17-19-1-2-20(4-3-19)18-34-24-6-8-28-26(14-24)22(10-12-30)16-32-28/h1-8,13-16,31-32H,9-12,17-18,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054987
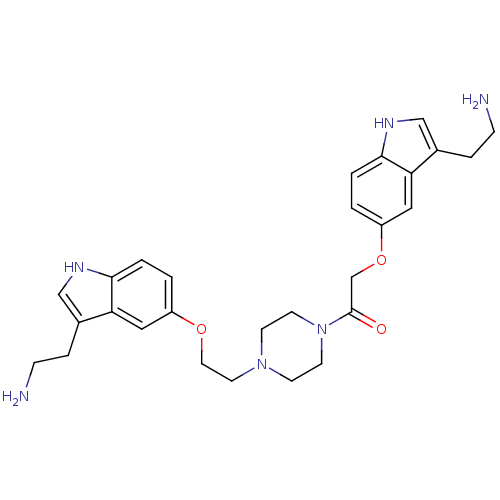
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...)Show SMILES NCCc1c[nH]c2ccc(OCCN3CCN(CC3)C(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C28H36N6O3/c29-7-5-20-17-31-26-3-1-22(15-24(20)26)36-14-13-33-9-11-34(12-10-33)28(35)19-37-23-2-4-27-25(16-23)21(6-8-30)18-32-27/h1-4,15-18,31-32H,5-14,19,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50061303
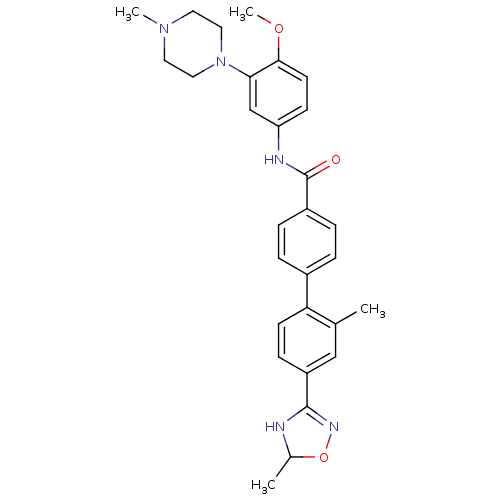
(2'-Methyl-4'-(5-methyl-4,5-dihydro-[1,2,4]oxadiazo...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)C2=NOC(C)N2)cc1N1CCN(C)CC1 |t:24| Show InChI InChI=1S/C29H33N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18,20H,13-16H2,1-4H3,(H,30,32)(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE
Curated by ChEMBL
| Assay Description
Receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor in Cos-7 cells |
J Med Chem 40: 3974-8 (1998)
Article DOI: 10.1021/jm9703552
BindingDB Entry DOI: 10.7270/Q2FT8K57 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054988
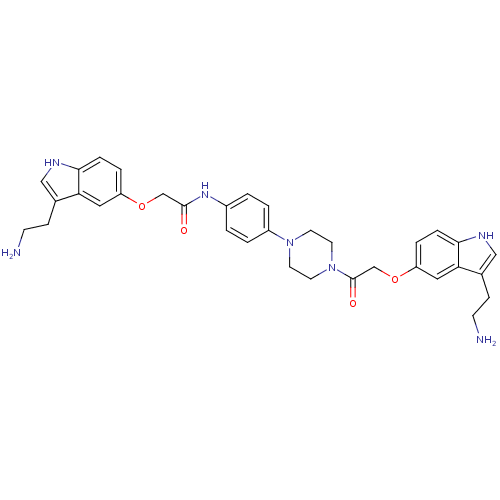
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-[4-(4-{2-...)Show SMILES NCCc1c[nH]c2ccc(OCC(=O)Nc3ccc(cc3)N3CCN(CC3)C(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C34H39N7O4/c35-11-9-23-19-37-31-7-5-27(17-29(23)31)44-21-33(42)39-25-1-3-26(4-2-25)40-13-15-41(16-14-40)34(43)22-45-28-6-8-32-30(18-28)24(10-12-36)20-38-32/h1-8,17-20,37-38H,9-16,21-22,35-36H2,(H,39,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Affinity for 5-hydroxytryptamine 1B receptor subtype |
Bioorg Med Chem Lett 7: 3183-3188 (1997)
Article DOI: 10.1016/S0960-894X(97)10164-0
BindingDB Entry DOI: 10.7270/Q23R0SW9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM79215

(CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)-c2ccc(cc2C)-c2noc(C)n2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C29H31N5O3/c1-19-17-23(28-30-20(2)37-32-28)9-11-25(19)21-5-7-22(8-6-21)29(35)31-24-10-12-27(36-4)26(18-24)34-15-13-33(3)14-16-34/h5-12,17-18H,13-16H2,1-4H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons
Curated by ChEMBL
| Assay Description
Affinity towards cloned human 5-hydroxytryptamine 1B receptor |
Bioorg Med Chem Lett 15: 4786-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.024
BindingDB Entry DOI: 10.7270/Q2NP23ZZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054976

(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-{2-[3-(2-...)Show SMILES NCCc1c[nH]c2ccc(OCCNC(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C24H29N5O3/c25-7-5-16-13-28-22-3-1-18(11-20(16)22)31-10-9-27-24(30)15-32-19-2-4-23-21(12-19)17(6-8-26)14-29-23/h1-4,11-14,28-29H,5-10,15,25-26H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50412441
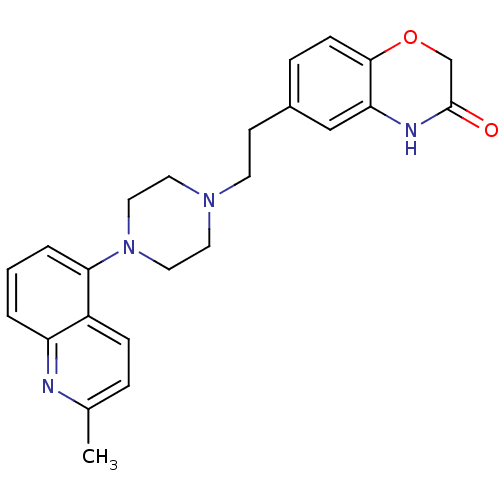
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054983
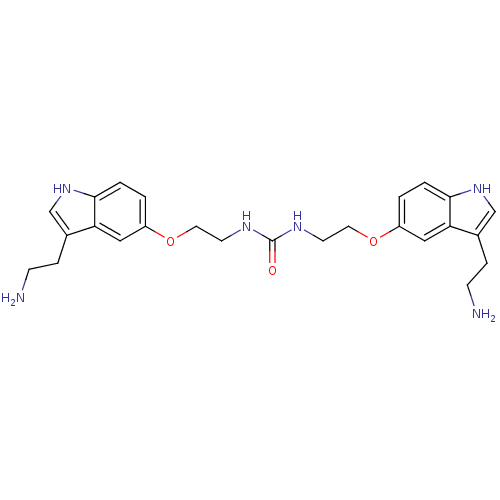
(1,3-Bis-{2-[3-(2-amino-ethyl)-1H-indol-5-yloxy]-et...)Show SMILES NCCc1c[nH]c2ccc(OCCNC(=O)NCCOc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C25H32N6O3/c26-7-5-17-15-30-23-3-1-19(13-21(17)23)33-11-9-28-25(32)29-10-12-34-20-2-4-24-22(14-20)18(6-8-27)16-31-24/h1-4,13-16,30-31H,5-12,26-27H2,(H2,28,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 184: 752-9 (1992)
Article DOI: 10.1016/0006-291x(92)90654-4
BindingDB Entry DOI: 10.7270/Q2ST7NB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054764
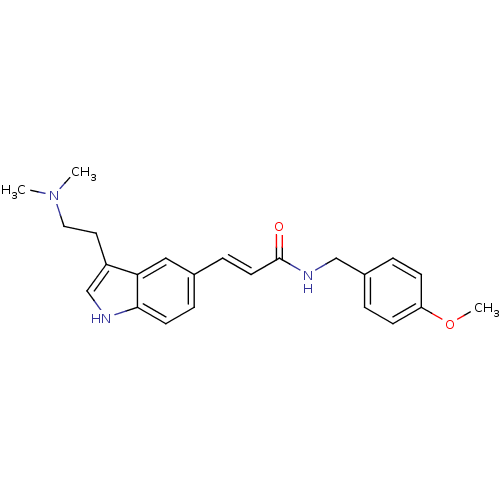
((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...)Show SMILES COc1ccc(CNC(=O)\C=C\c2ccc3[nH]cc(CCN(C)C)c3c2)cc1 Show InChI InChI=1S/C23H27N3O2/c1-26(2)13-12-19-16-24-22-10-6-17(14-21(19)22)7-11-23(27)25-15-18-4-8-20(28-3)9-5-18/h4-11,14,16,24H,12-13,15H2,1-3H3,(H,25,27)/b11-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50454686

(CHEMBL2112662)Show SMILES CN1CCN(CC1)c1cccc2ccc(OCC(=O)N3CCN(Cc4ccccc4C)CC3)cc12 Show InChI InChI=1S/C29H36N4O2/c1-23-6-3-4-7-25(23)21-31-14-18-33(19-15-31)29(34)22-35-26-11-10-24-8-5-9-28(27(24)20-26)32-16-12-30(2)13-17-32/h3-11,20H,12-19,21-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE
Curated by ChEMBL
| Assay Description
Receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor in Cos-7 cells |
J Med Chem 40: 3974-8 (1998)
Article DOI: 10.1021/jm9703552
BindingDB Entry DOI: 10.7270/Q2FT8K57 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054980
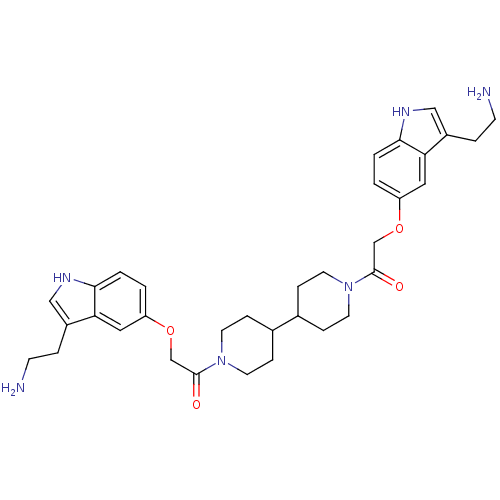
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(1'-{2-[3...)Show SMILES NCCc1c[nH]c2ccc(OCC(=O)N3CCC(CC3)C3CCN(CC3)C(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C34H44N6O4/c35-11-5-25-19-37-31-3-1-27(17-29(25)31)43-21-33(41)39-13-7-23(8-14-39)24-9-15-40(16-10-24)34(42)22-44-28-2-4-32-30(18-28)26(6-12-36)20-38-32/h1-4,17-20,23-24,37-38H,5-16,21-22,35-36H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410424
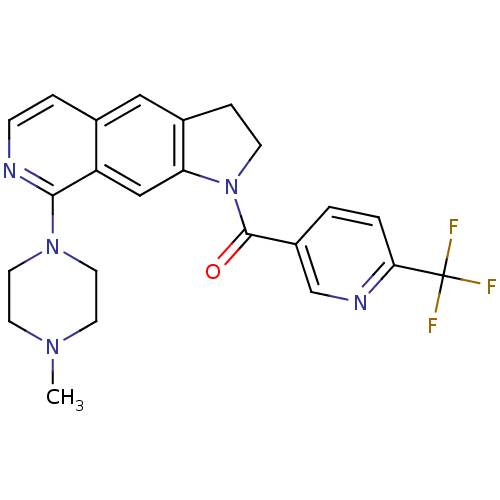
(CHEMBL199088)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(nc4)C(F)(F)F)c3cc12 Show InChI InChI=1S/C23H22F3N5O/c1-29-8-10-30(11-9-29)21-18-13-19-16(12-15(18)4-6-27-21)5-7-31(19)22(32)17-2-3-20(28-14-17)23(24,25)26/h2-4,6,12-14H,5,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM81499
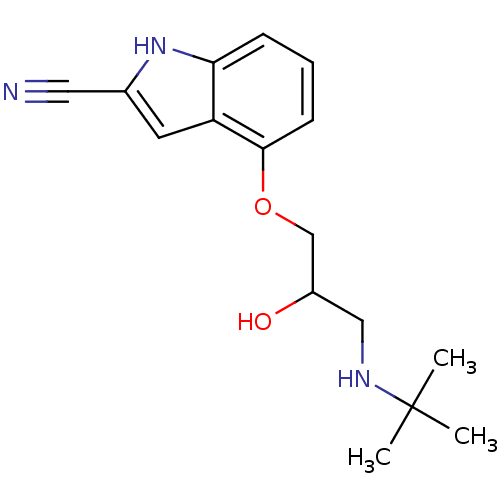
(CAS_155346 | CYANOPINDOLOL | CYANOPINDOLOL(+/-) | ...)Show InChI InChI=1S/C16H21N3O2/c1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14/h4-7,12,18-20H,9-10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM30712
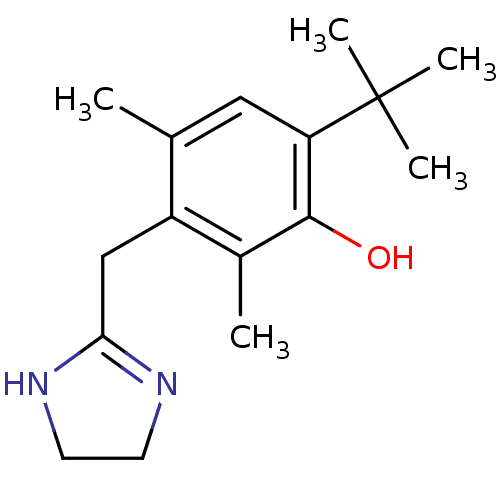
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by PDSP Ki Database
| |
Biochem Biophys Res Commun 184: 752-9 (1992)
Article DOI: 10.1016/0006-291x(92)90654-4
BindingDB Entry DOI: 10.7270/Q2ST7NB1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054979

(2-{5-[2-(4-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]...)Show SMILES NCCc1c[nH]c2ccc(OCCN3CCN(CCOc4ccc5[nH]cc(CCN)c5c4)CC3)cc12 Show InChI InChI=1S/C28H38N6O2/c29-7-5-21-19-31-27-3-1-23(17-25(21)27)35-15-13-33-9-11-34(12-10-33)14-16-36-24-2-4-28-26(18-24)22(6-8-30)20-32-28/h1-4,17-20,31-32H,5-16,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
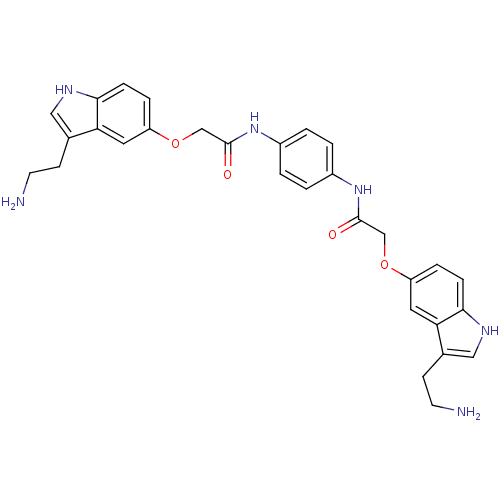
(Homo sapiens (Human)) | BDBM50054981
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[3-...)Show SMILES NCCc1c[nH]c2ccc(OCC(=O)Nc3ccc(NC(=O)COc4ccc5[nH]cc(CCN)c5c4)cc3)cc12 Show InChI InChI=1S/C30H32N6O4/c31-11-9-19-15-33-27-7-5-23(13-25(19)27)39-17-29(37)35-21-1-2-22(4-3-21)36-30(38)18-40-24-6-8-28-26(14-24)20(10-12-32)16-34-28/h1-8,13-16,33-34H,9-12,17-18,31-32H2,(H,35,37)(H,36,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Cricetulus griseus (Chinese hamster) (Cricetulus b...) | BDBM136322

(US8859534, 8)Show SMILES Clc1cccnc1N1CCC(CC1)NC(=O)c1cc2cccc(N3CCN(CCc4ccccn4)CC3)c2o1 Show InChI InChI=1S/C30H33ClN6O2/c31-25-7-4-13-33-29(25)37-15-10-24(11-16-37)34-30(38)27-21-22-5-3-8-26(28(22)39-27)36-19-17-35(18-20-36)14-9-23-6-1-2-12-32-23/h1-8,12-13,21,24H,9-11,14-20H2,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Acturum Life Science AB
US Patent
| Assay Description
Assays that were used to measure affinity of the compounds of the present invention for 5-HT1A and 5-HT1B receptors are described in J. Recept Signal... |
US Patent US8859534 (2014)
BindingDB Entry DOI: 10.7270/Q23R0RKV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
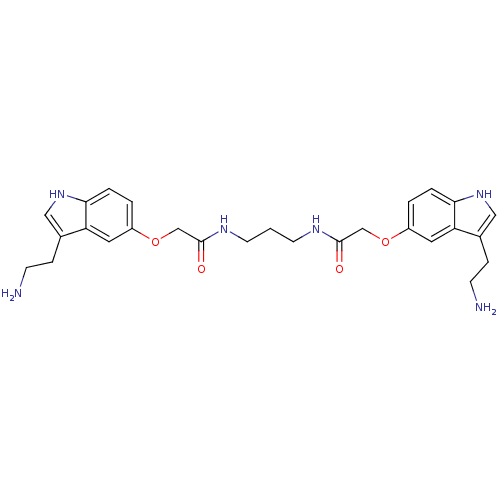
(Homo sapiens (Human)) | BDBM50054973
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(3-{2-[3-...)Show SMILES NCCc1c[nH]c2ccc(OCC(=O)NCCCNC(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C27H34N6O4/c28-8-6-18-14-32-24-4-2-20(12-22(18)24)36-16-26(34)30-10-1-11-31-27(35)17-37-21-3-5-25-23(13-21)19(7-9-29)15-33-25/h2-5,12-15,32-33H,1,6-11,16-17,28-29H2,(H,30,34)(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50061299

(2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...)Show SMILES CN1CCN(CC1)c1cccc2ccc(OCC(=O)N3CCN(CC3)c3ccccc3C)cc12 Show InChI InChI=1S/C28H34N4O2/c1-22-6-3-4-8-26(22)30-16-18-32(19-17-30)28(33)21-34-24-11-10-23-7-5-9-27(25(23)20-24)31-14-12-29(2)13-15-31/h3-11,20H,12-19,21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE
Curated by ChEMBL
| Assay Description
Receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor in Cos-7 cells |
J Med Chem 40: 3974-8 (1998)
Article DOI: 10.1021/jm9703552
BindingDB Entry DOI: 10.7270/Q2FT8K57 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-hydroxytryptamine 1B receptor using [3H]-5-HT trifluoroacetate as radioligand |
J Med Chem 41: 2243-51 (1998)
Article DOI: 10.1021/jm970513p
BindingDB Entry DOI: 10.7270/Q28K7872 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
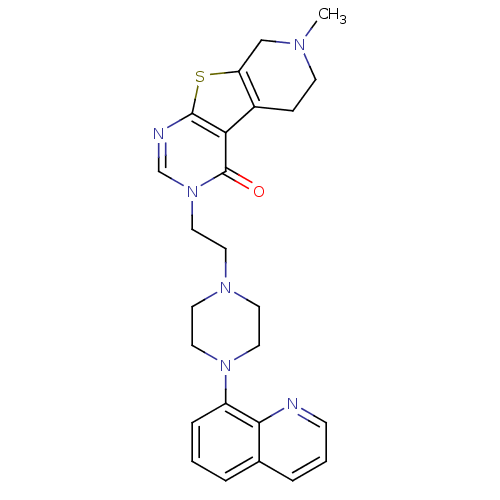
(Homo sapiens (Human)) | BDBM50175479
(7-methyl-3-[2-(4-quinolin-8-yl-piperazin-1-yl)-eth...)Show SMILES CN1CCc2c(C1)sc1ncn(CCN3CCN(CC3)c3cccc4cccnc34)c(=O)c21 Show InChI InChI=1S/C25H28N6OS/c1-28-9-7-19-21(16-28)33-24-22(19)25(32)31(17-27-24)15-12-29-10-13-30(14-11-29)20-6-2-4-18-5-3-8-26-23(18)20/h2-6,8,17H,7,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR |
Bioorg Med Chem Lett 15: 5567-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.077
BindingDB Entry DOI: 10.7270/Q2CR5SX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50413083
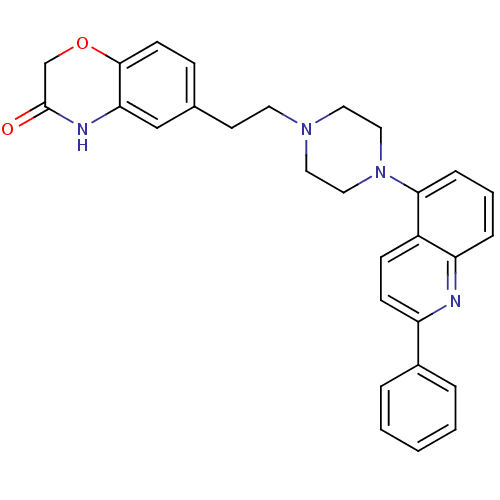
(CHEMBL484059)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4nc(ccc34)-c3ccccc3)cc2N1 Show InChI InChI=1S/C29H28N4O2/c34-29-20-35-28-12-9-21(19-26(28)31-29)13-14-32-15-17-33(18-16-32)27-8-4-7-25-23(27)10-11-24(30-25)22-5-2-1-3-6-22/h1-12,19H,13-18,20H2,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50249134
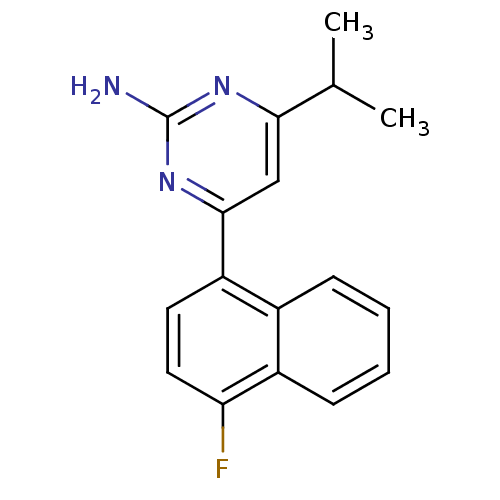
(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor (unknown origin) |
Bioorg Med Chem Lett 25: 3451-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.012
BindingDB Entry DOI: 10.7270/Q2MW2JXN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054978

(2-(5-{3-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...)Show SMILES NCCc1c[nH]c2ccc(OCc3cccc(COc4ccc5[nH]cc(CCN)c5c4)c3)cc12 Show InChI InChI=1S/C28H30N4O2/c29-10-8-21-15-31-27-6-4-23(13-25(21)27)33-17-19-2-1-3-20(12-19)18-34-24-5-7-28-26(14-24)22(9-11-30)16-32-28/h1-7,12-16,31-32H,8-11,17-18,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054986
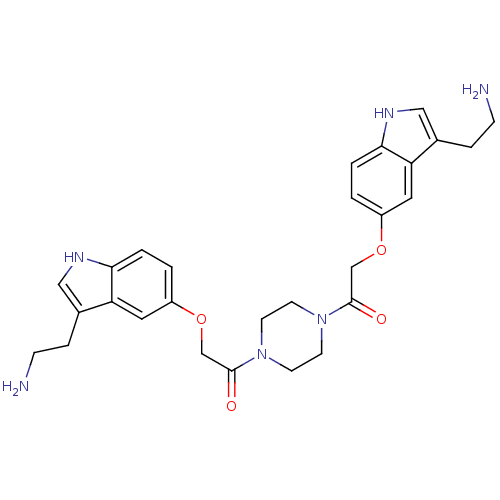
(2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...)Show SMILES NCCc1c[nH]c2ccc(OCC(=O)N3CCN(CC3)C(=O)COc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C28H34N6O4/c29-7-5-19-15-31-25-3-1-21(13-23(19)25)37-17-27(35)33-9-11-34(12-10-33)28(36)18-38-22-2-4-26-24(14-22)20(6-8-30)16-32-26/h1-4,13-16,31-32H,5-12,17-18,29-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM81498
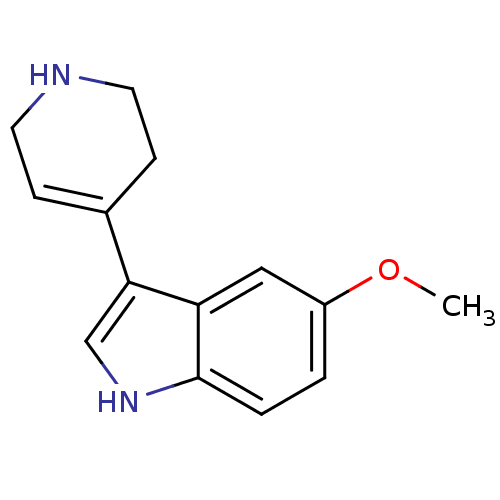
(5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...)Show InChI InChI=1S/C14H16N2O/c1-17-11-2-3-14-12(8-11)13(9-16-14)10-4-6-15-7-5-10/h2-4,8-9,15-16H,5-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054985
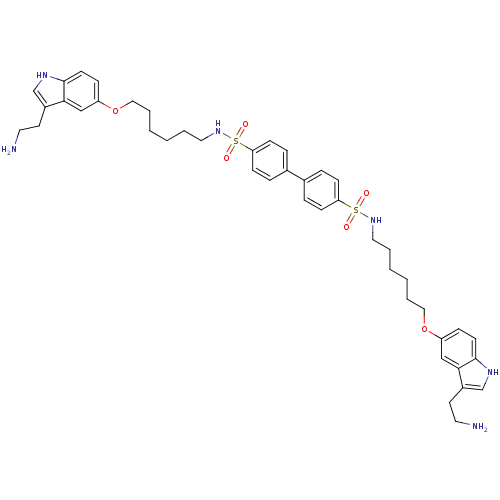
(Biphenyl-4,4'-disulfonic acid bis-({6-[3-(2-amino-...)Show SMILES NCCc1c[nH]c2ccc(OCCCCCCNS(=O)(=O)c3ccc(cc3)-c3ccc(cc3)S(=O)(=O)NCCCCCCOc3ccc4[nH]cc(CCN)c4c3)cc12 Show InChI InChI=1S/C44H56N6O6S2/c45-23-21-35-31-47-43-19-13-37(29-41(35)43)55-27-7-3-1-5-25-49-57(51,52)39-15-9-33(10-16-39)34-11-17-40(18-12-34)58(53,54)50-26-6-2-4-8-28-56-38-14-20-44-42(30-38)36(22-24-46)32-48-44/h9-20,29-32,47-50H,1-8,21-28,45-46H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre
Curated by ChEMBL
| Assay Description
Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1B receptor in CHO-K1 cells |
J Med Chem 39: 4920-7 (1997)
Article DOI: 10.1021/jm960552l
BindingDB Entry DOI: 10.7270/Q23B5Z7M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
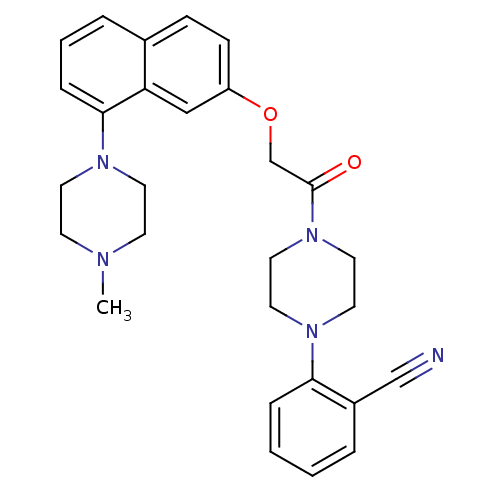
(Homo sapiens (Human)) | BDBM50454685
(CHEMBL2112660)Show SMILES CN1CCN(CC1)c1cccc2ccc(OCC(=O)N3CCN(CC3)c3ccccc3C#N)cc12 Show InChI InChI=1S/C28H31N5O2/c1-30-11-13-32(14-12-30)27-8-4-6-22-9-10-24(19-25(22)27)35-21-28(34)33-17-15-31(16-18-33)26-7-3-2-5-23(26)20-29/h2-10,19H,11-18,21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE
Curated by ChEMBL
| Assay Description
Receptor binding affinity for cloned human 5-hydroxytryptamine 1B receptor in Cos-7 cells |
J Med Chem 40: 3974-8 (1998)
Article DOI: 10.1021/jm9703552
BindingDB Entry DOI: 10.7270/Q2FT8K57 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
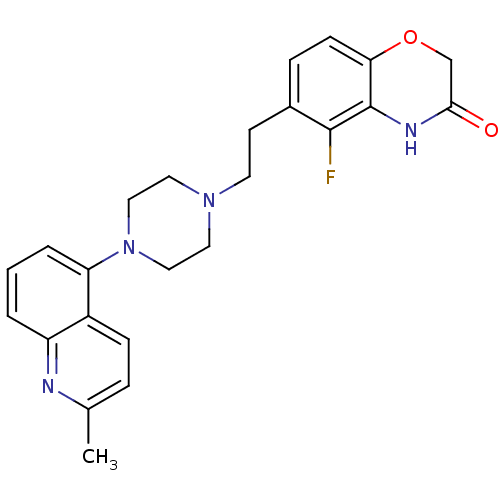
(Homo sapiens (Human)) | BDBM50413073
(CHEMBL496520)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2F)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-7-18-19(26-16)3-2-4-20(18)29-13-11-28(12-14-29)10-9-17-6-8-21-24(23(17)25)27-22(30)15-31-21/h2-8H,9-15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50413079
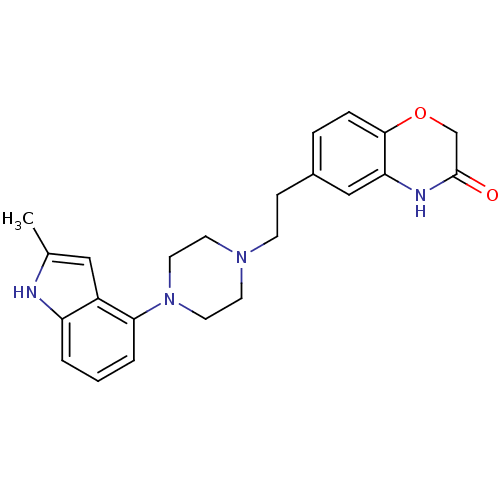
(CHEMBL444398)Show SMILES Cc1cc2c(cccc2[nH]1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C23H26N4O2/c1-16-13-18-19(24-16)3-2-4-21(18)27-11-9-26(10-12-27)8-7-17-5-6-22-20(14-17)25-23(28)15-29-22/h2-6,13-14,24H,7-12,15H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50175485
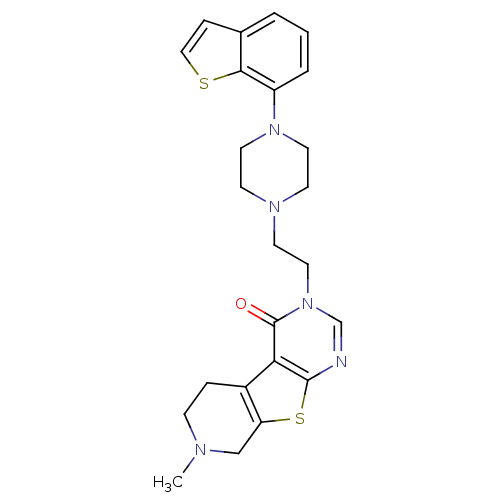
(3-[2-(4-benzo[b]thiophen-7-yl-piperazin-1-yl)-ethy...)Show SMILES CN1CCc2c(C1)sc1ncn(CCN3CCN(CC3)c3cccc4ccsc34)c(=O)c21 Show InChI InChI=1S/C24H27N5OS2/c1-26-7-5-18-20(15-26)32-23-21(18)24(30)29(16-25-23)13-10-27-8-11-28(12-9-27)19-4-2-3-17-6-14-31-22(17)19/h2-4,6,14,16H,5,7-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR |
Bioorg Med Chem Lett 15: 5567-73 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.077
BindingDB Entry DOI: 10.7270/Q2CR5SX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50306587
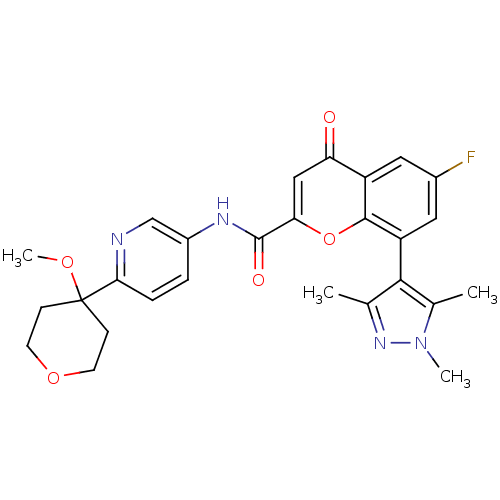
(6-Fluoro-N-(6-(4-methoxytetrahydro-2H-pyran-4-yl)p...)Show SMILES COC1(CCOCC1)c1ccc(NC(=O)c2cc(=O)c3cc(F)cc(-c4c(C)nn(C)c4C)c3o2)cn1 |(27.97,-16.65,;26.65,-17.44,;26.66,-18.99,;26.66,-20.53,;27.99,-21.29,;29.33,-20.52,;29.33,-18.98,;27.99,-18.21,;25.33,-18.23,;24,-19,;22.66,-18.23,;22.67,-16.69,;21.33,-15.93,;20,-16.7,;20,-18.24,;18.66,-15.93,;18.66,-14.39,;17.31,-13.61,;17.31,-12.07,;15.98,-14.4,;14.65,-13.63,;13.32,-14.4,;11.99,-13.63,;13.32,-15.95,;14.65,-16.72,;14.65,-18.27,;15.9,-19.17,;17.36,-18.7,;15.42,-20.64,;13.88,-20.64,;12.98,-21.89,;13.41,-19.17,;11.94,-18.7,;15.99,-15.94,;17.33,-16.71,;23.99,-15.92,;25.32,-16.68,)| Show InChI InChI=1S/C27H27FN4O5/c1-15-24(16(2)32(3)31-15)20-12-17(28)11-19-21(33)13-22(37-25(19)20)26(34)30-18-5-6-23(29-14-18)27(35-4)7-9-36-10-8-27/h5-6,11-14H,7-10H2,1-4H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor expressed in CHO cells |
J Med Chem 53: 1876-80 (2010)
Article DOI: 10.1021/jm901200t
BindingDB Entry DOI: 10.7270/Q25D8RZ0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data