

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candidapepsin-2 (Candida albicans) | BDBM50402348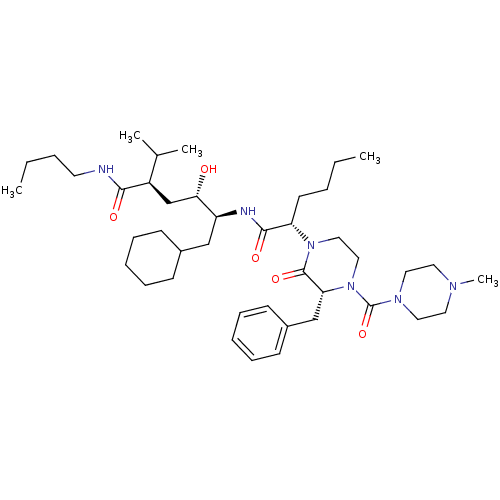 (CHEMBL2206678) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402357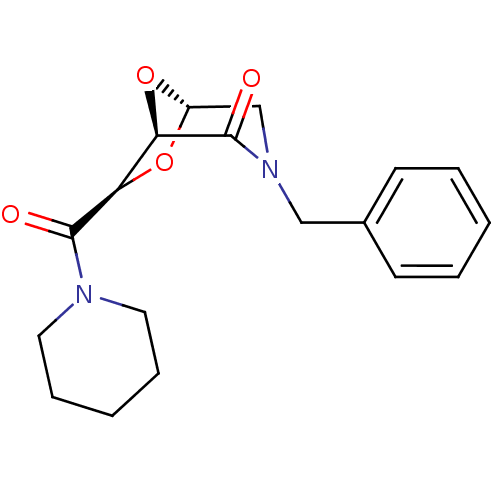 (CHEMBL589301) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402356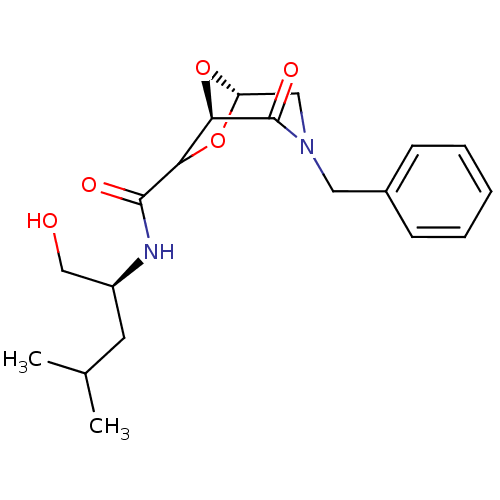 (CHEMBL2206670) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402349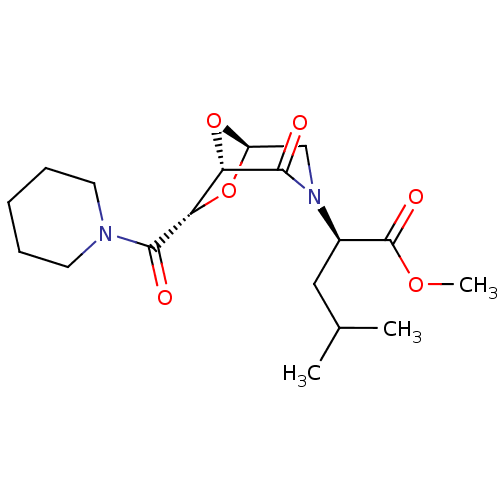 (CHEMBL2206677) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402350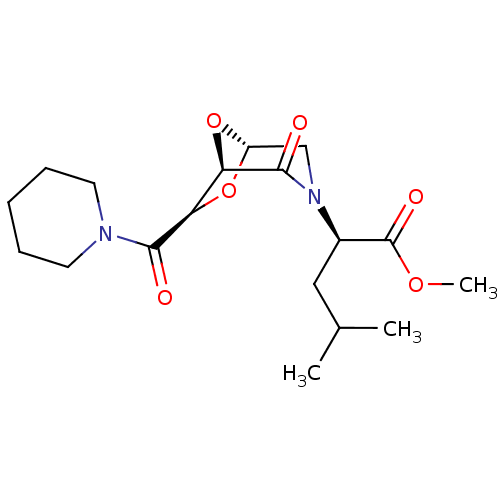 (CHEMBL2206676) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402352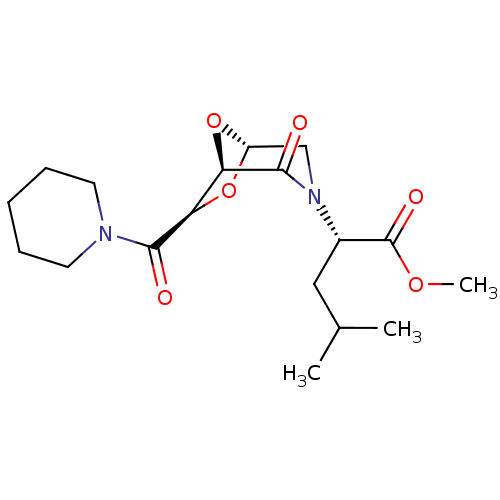 (CHEMBL2206674) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402358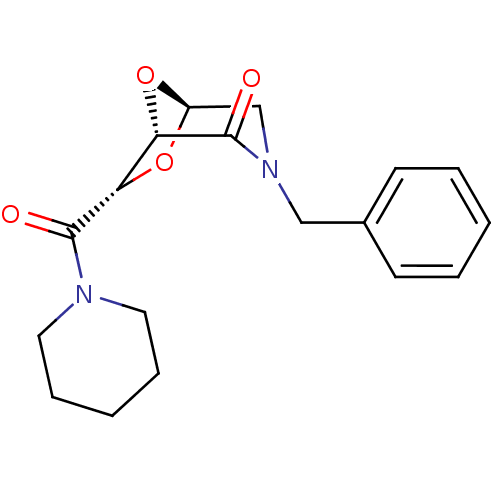 (CHEMBL2206669) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402355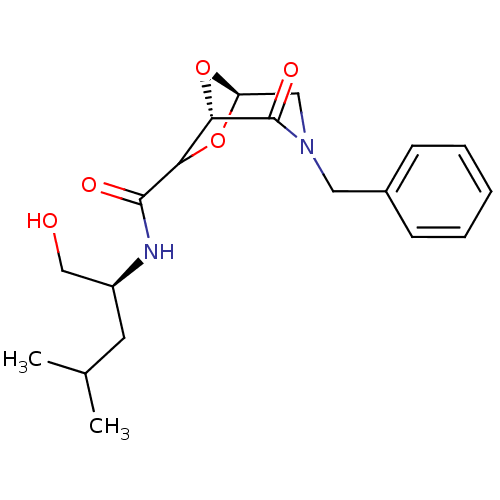 (CHEMBL2206671) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402354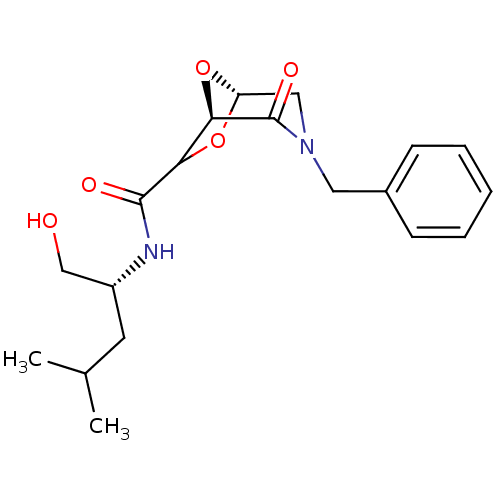 (CHEMBL2206672) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402351 (CHEMBL2206675) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Candidapepsin-2 (Candida albicans) | BDBM50402353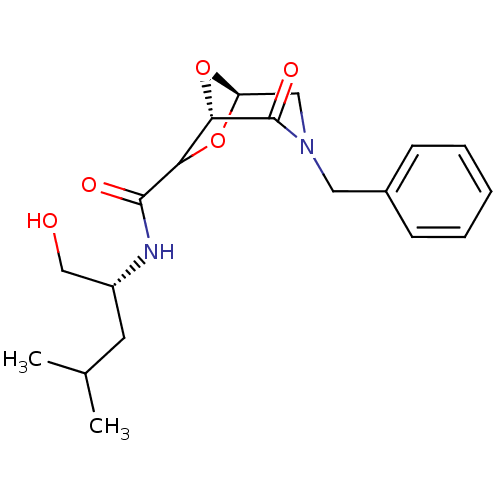 (CHEMBL2206673) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of candida albicans SAP2 using 0.05% BSA as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 7206-13 (2012) Article DOI: 10.1016/j.bmc.2012.09.031 BindingDB Entry DOI: 10.7270/Q2BP03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||