 Found 6696 hits Enz. Inhib. hit(s) with Target = 'Adenosine deaminase'
Found 6696 hits Enz. Inhib. hit(s) with Target = 'Adenosine deaminase' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
Bioorg Med Chem Lett 22: 7214-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.053
BindingDB Entry DOI: 10.7270/Q2MC916H |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
Bioorg Med Chem Lett 22: 7214-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.053
BindingDB Entry DOI: 10.7270/Q2MC916H |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of adenosine deaminase from calf intestine; Range of 0.01-0.001 nM |
J Med Chem 37: 201-5 (1994)
BindingDB Entry DOI: 10.7270/Q2KD1ZHQ |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity towards calf spleen adenosine deaminase was determined |
J Med Chem 47: 1044-50 (2004)
Article DOI: 10.1021/jm0304257
BindingDB Entry DOI: 10.7270/Q2GM882D |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50407749

(CHEMBL2112110)Show SMILES OC[C@@H]1O[C@@H](C[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |c:17| Show InChI InChI=1S/C12H17N3O4/c16-5-9-8(18)4-10(19-9)15-6-14-11-7(17)2-1-3-13-12(11)15/h3,6-10,16-18H,1-2,4-5H2/t7-,8-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Binding affinity (Ki) at calf intestinal adenosine deaminase. |
J Med Chem 35: 4180-4 (1992)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2SQ9118 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50378885
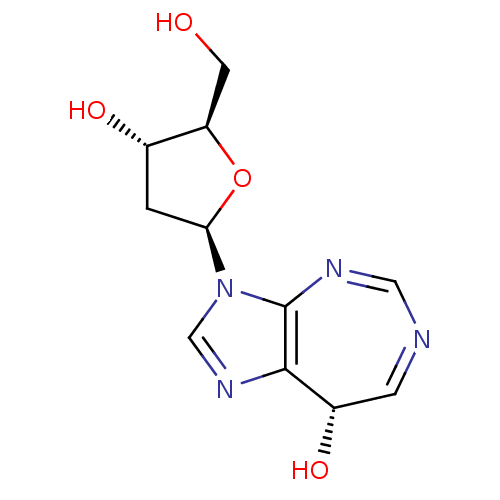
(CHEMBL1651378)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@@H](O)C=NC=Nc12 |r,c:15,17| Show InChI InChI=1S/C11H14N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h2,4-9,16-18H,1,3H2/t6-,7-,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50519495

(CHEMBL34023)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@@H](O)CN=CNc12 |c:17| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6+,8+,9+,11+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ADA assessed as reduction in formation of ammonia using adenosine as substrate incubated for 15 mins by spectroph... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity towards calf spleen adenosine deaminase was determined |
J Med Chem 47: 1044-50 (2004)
Article DOI: 10.1021/jm0304257
BindingDB Entry DOI: 10.7270/Q2GM882D |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50378886
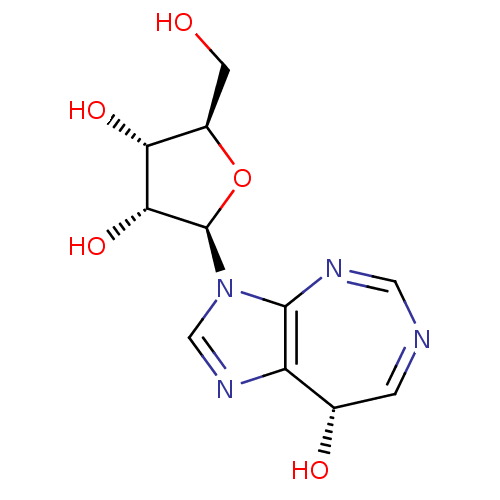
(CHEMBL1651377)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@@H](O)C=NC=Nc12 |r,c:16,18| Show InChI InChI=1S/C11H14N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h1,3-6,8-9,11,16-19H,2H2/t5-,6+,8+,9+,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50369126
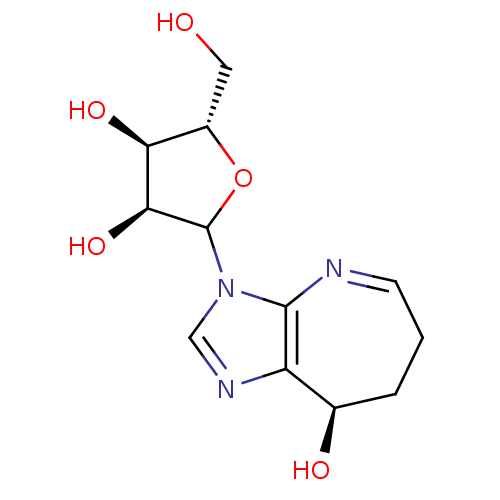
(CONFORMYCIN)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |r,c:18| Show InChI InChI=1S/C12H17N3O5/c16-4-7-9(18)10(19)12(20-7)15-5-14-8-6(17)2-1-3-13-11(8)15/h3,5-7,9-10,12,16-19H,1-2,4H2/t6-,7+,9+,10+,12?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity against Adenosine deaminase from calf intestinal mucosa was determined |
Bioorg Med Chem Lett 11: 2893-6 (2001)
BindingDB Entry DOI: 10.7270/Q28K79MD |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0330 | -59.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Fujisawa Pharmaceutical Co., Ltd.
| Assay Description
The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a... |
Bioorg Med Chem Lett 13: 1115-8 (2003)
Article DOI: 10.1016/S0960-894X(03)00026-X
BindingDB Entry DOI: 10.7270/Q29Z936D |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50370598
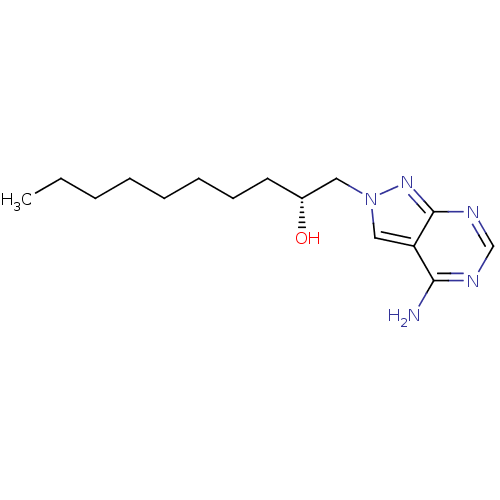
(CHEMBL1651379)Show InChI InChI=1S/C15H25N5O/c1-2-3-4-5-6-7-8-12(21)9-20-10-13-14(16)17-11-18-15(13)19-20/h10-12,21H,2-9H2,1H3,(H2,16,17,18,19)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University
Curated by ChEMBL
| Assay Description
Inhibition of adenosine deaminase |
J Med Chem 54: 107-21 (2011)
Article DOI: 10.1021/jm101286g
BindingDB Entry DOI: 10.7270/Q2K64K1Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50370598

(CHEMBL1651379)Show InChI InChI=1S/C15H25N5O/c1-2-3-4-5-6-7-8-12(21)9-20-10-13-14(16)17-11-18-15(13)19-20/h10-12,21H,2-9H2,1H3,(H2,16,17,18,19)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibitory constant against bovine spleen Adenosine deaminase |
J Med Chem 48: 5162-74 (2005)
Article DOI: 10.1021/jm050136d
BindingDB Entry DOI: 10.7270/Q2542PCH |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50146972
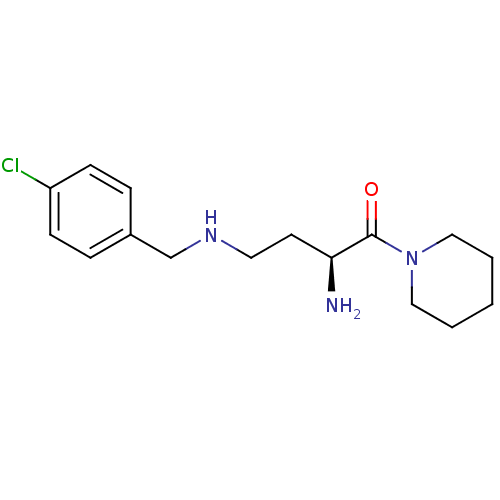
((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...)Show InChI InChI=1S/C16H24ClN3O/c17-14-6-4-13(5-7-14)12-19-9-8-15(18)16(21)20-10-2-1-3-11-20/h4-7,15,19H,1-3,8-12,18H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry University of Antwerp
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 16: 4777-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.082
BindingDB Entry DOI: 10.7270/Q2GM86XS |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of adenosine deaminase |
J Med Chem 24: 1383-5 (1982)
BindingDB Entry DOI: 10.7270/Q22N52T8 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50171394
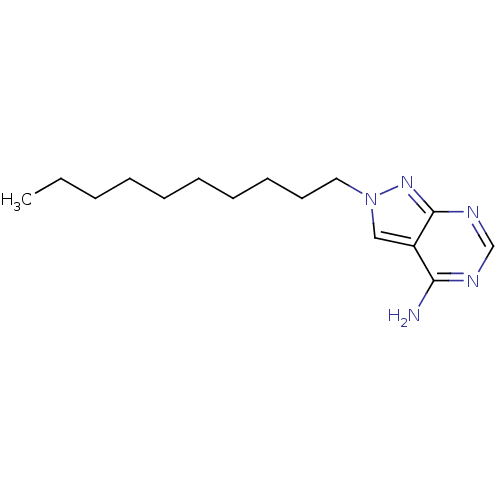
(2-Decyl-2H-pyrazolo[3,4-d]pyrimidin-4-ylamine | CH...)Show InChI InChI=1S/C15H25N5/c1-2-3-4-5-6-7-8-9-10-20-11-13-14(16)17-12-18-15(13)19-20/h11-12H,2-10H2,1H3,(H2,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibitory constant against bovine spleen Adenosine deaminase |
J Med Chem 48: 5162-74 (2005)
Article DOI: 10.1021/jm050136d
BindingDB Entry DOI: 10.7270/Q2542PCH |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 50: 2391-8 (2007)
Article DOI: 10.1021/jm061321+
BindingDB Entry DOI: 10.7270/Q2NC6407 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116748
BindingDB Entry DOI: 10.7270/Q2MG7TGW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324523
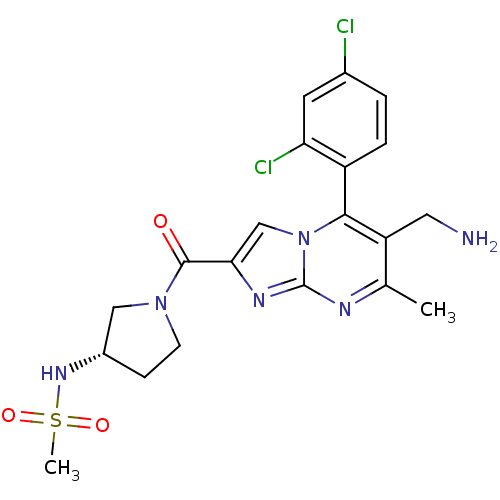
(CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CC[C@@H](C1)NS(C)(=O)=O |r,wD:25.30,(7.23,-5.85,;5.92,-6.66,;4.56,-5.95,;3.26,-6.75,;1.79,-6.32,;.92,-7.59,;1.87,-8.81,;3.31,-8.29,;4.66,-9.02,;5.97,-8.21,;7.33,-8.93,;8.64,-8.12,;4.63,-10.55,;3.28,-11.29,;3.25,-12.83,;4.57,-13.63,;4.54,-15.17,;5.92,-12.87,;5.95,-11.34,;7.29,-10.6,;-.62,-7.63,;-1.43,-6.33,;-1.34,-8.99,;-.88,-10.46,;-2.12,-11.36,;-3.37,-10.46,;-2.89,-8.99,;-4.91,-10.48,;-5.99,-9.37,;-7.4,-10.02,;-6.68,-7.99,;-4.84,-8.35,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-11-15(8-23)18(14-4-3-12(21)7-16(14)22)28-10-17(25-20(28)24-11)19(29)27-6-5-13(9-27)26-32(2,30)31/h3-4,7,10,13,26H,5-6,8-9,23H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ADA assessed as reduction in formation of ammonia using adenosine as substrate incubated for 15 mins by spectroph... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356582
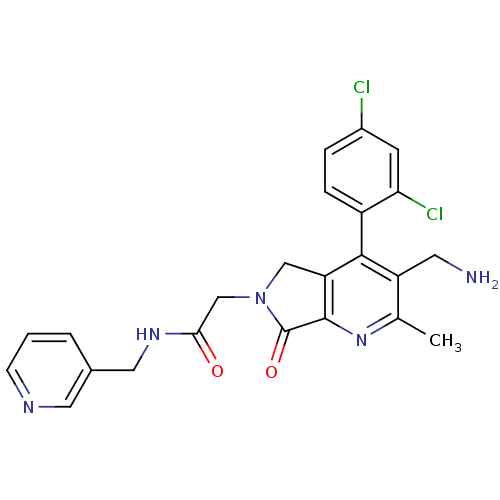
(CHEMBL1910126)Show SMILES Cc1nc2C(=O)N(CC(=O)NCc3cccnc3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(14.01,-46.7,;12.68,-47.48,;11.34,-46.71,;10.02,-47.49,;8.55,-47.01,;8.08,-45.55,;7.65,-48.25,;6.11,-48.26,;5.34,-49.59,;6.12,-50.92,;3.8,-49.59,;3.04,-50.93,;1.5,-50.93,;.74,-52.26,;-.8,-52.27,;-1.58,-50.93,;-.81,-49.6,;.73,-49.59,;8.55,-49.5,;10.01,-49.03,;11.35,-49.8,;12.68,-49.03,;14.02,-49.79,;15.35,-49.02,;11.35,-51.33,;10.01,-52.1,;10.01,-53.64,;11.35,-54.41,;11.35,-55.95,;12.69,-53.64,;12.68,-52.1,;14.01,-51.33,)| Show InChI InChI=1S/C23H21Cl2N5O2/c1-13-17(8-26)21(16-5-4-15(24)7-19(16)25)18-11-30(23(32)22(18)29-13)12-20(31)28-10-14-3-2-6-27-9-14/h2-7,9H,8,10-12,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356591

(CHEMBL1910117)Show SMILES CN(C)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(23.14,-19.79,;23.91,-21.12,;23.14,-22.45,;25.45,-21.12,;26.22,-22.45,;26.22,-19.78,;27.75,-19.78,;28.65,-21.03,;30.12,-20.55,;30.12,-19.01,;31.45,-18.24,;32.78,-19,;34.12,-18.23,;32.79,-20.55,;34.13,-21.32,;35.46,-20.55,;31.45,-21.32,;31.46,-22.86,;30.12,-23.63,;30.12,-25.17,;31.45,-25.94,;31.45,-27.48,;32.79,-25.16,;32.79,-23.62,;34.12,-22.85,;28.66,-18.54,;28.18,-17.07,)| Show InChI InChI=1S/C19H20Cl2N4O2/c1-10-13(7-22)17(12-5-4-11(20)6-15(12)21)14-8-25(9-16(26)24(2)3)19(27)18(14)23-10/h4-6H,7-9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50171396
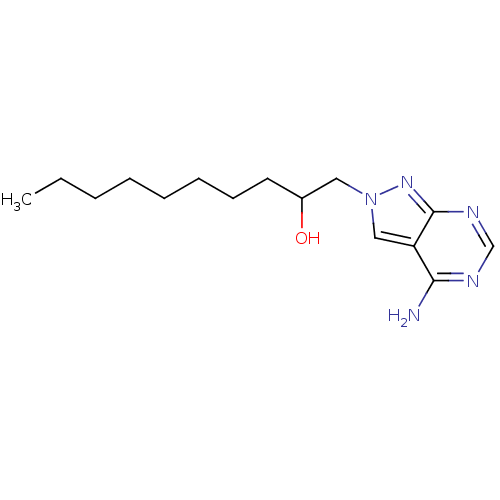
(1-((R)-4-Amino-pyrazolo[3,4-d]pyrimidin-2-yl)-deca...)Show InChI InChI=1S/C15H25N5O/c1-2-3-4-5-6-7-8-12(21)9-20-10-13-14(16)17-11-18-15(13)19-20/h10-12,21H,2-9H2,1H3,(H2,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibitory constant against bovine spleen Adenosine deaminase |
J Med Chem 48: 5162-74 (2005)
Article DOI: 10.1021/jm050136d
BindingDB Entry DOI: 10.7270/Q2542PCH |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
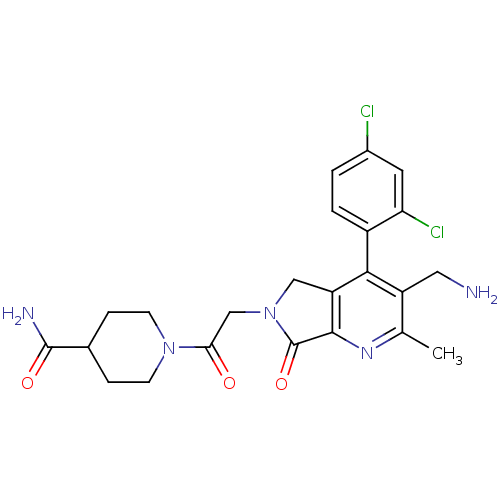
(Homo sapiens (Human)) | BDBM50356589
(CHEMBL1910119)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCC(CC3)C(N)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(6.41,-30.16,;5.08,-30.93,;3.75,-30.17,;2.42,-30.95,;.95,-30.47,;.48,-29,;.05,-31.71,;-1.49,-31.71,;-2.25,-33.05,;-1.48,-34.38,;-3.79,-33.05,;-4.56,-31.72,;-6.09,-31.72,;-6.87,-33.05,;-6.1,-34.38,;-4.56,-34.39,;-8.41,-33.04,;-9.18,-31.71,;-9.18,-34.38,;.95,-32.96,;2.41,-32.48,;3.75,-33.26,;5.09,-32.48,;6.42,-33.25,;7.75,-32.48,;3.75,-34.79,;2.41,-35.56,;2.41,-37.1,;3.75,-37.87,;3.75,-39.41,;5.09,-37.09,;5.08,-35.56,;6.41,-34.78,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-12-16(9-26)20(15-3-2-14(24)8-18(15)25)17-10-30(23(33)21(17)28-12)11-19(31)29-6-4-13(5-7-29)22(27)32/h2-3,8,13H,4-7,9-11,26H2,1H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356585

(CHEMBL1910123)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(22.17,-39.13,;20.84,-39.9,;19.5,-39.14,;18.17,-39.91,;16.71,-39.44,;16.24,-37.97,;15.8,-40.68,;14.27,-40.68,;13.5,-42.02,;14.28,-43.35,;11.96,-42.02,;11.05,-40.78,;9.59,-41.26,;9.59,-42.8,;11.06,-43.27,;16.71,-41.93,;18.17,-41.45,;19.51,-42.22,;20.84,-41.45,;22.18,-42.22,;23.51,-41.45,;19.51,-43.76,;18.17,-44.53,;18.17,-46.07,;19.51,-46.84,;19.51,-48.38,;20.84,-46.06,;20.84,-44.52,;22.17,-43.75,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-6-2-3-7-26/h4-5,8H,2-3,6-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324525
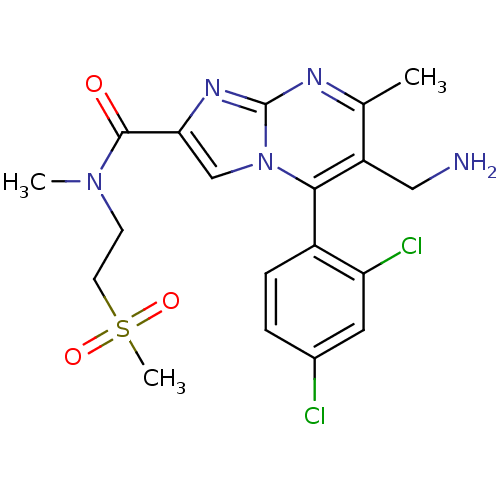
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCS(C)(=O)=O)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(2.96,-5.01,;2.15,-3.7,;.61,-3.75,;-.19,-2.43,;-1.73,-2.48,;-2.54,-1.17,;-3.23,-2.88,;-1.74,-4.02,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.91,;10.83,-3.64,;12.13,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.25,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,)| Show InChI InChI=1S/C19H21Cl2N5O3S/c1-11-14(9-22)17(13-5-4-12(20)8-15(13)21)26-10-16(24-19(26)23-11)18(27)25(2)6-7-30(3,28)29/h4-5,8,10H,6-7,9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356584
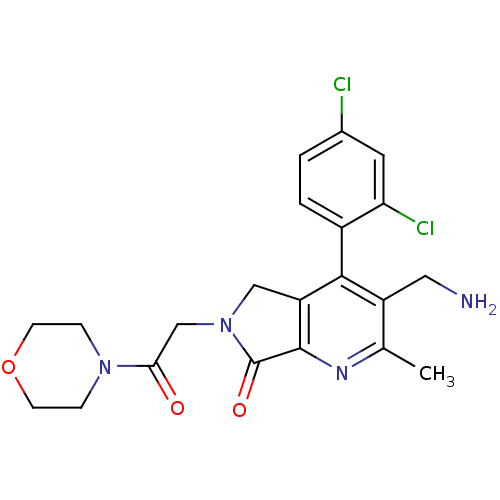
(CHEMBL1910124)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCOCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(23.9,-37.72,;22.57,-38.49,;21.24,-37.73,;19.91,-38.51,;18.45,-38.03,;17.97,-36.56,;17.54,-39.27,;16.01,-39.27,;15.24,-40.61,;16.01,-41.94,;13.7,-40.61,;12.93,-39.28,;11.4,-39.28,;10.62,-40.61,;11.39,-41.94,;12.94,-41.95,;18.44,-40.52,;19.91,-40.04,;21.24,-40.82,;22.58,-40.04,;23.91,-40.81,;25.25,-40.04,;21.24,-42.35,;19.91,-43.12,;19.91,-44.66,;21.24,-45.43,;21.24,-46.97,;22.58,-44.65,;22.57,-43.12,;23.9,-42.34,)| Show InChI InChI=1S/C21H22Cl2N4O3/c1-12-15(9-24)19(14-3-2-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-4-6-30-7-5-26/h2-3,8H,4-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356592

(CHEMBL1910116)Show SMILES CNC(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(7.18,-19.79,;7.95,-21.12,;9.49,-21.12,;10.26,-22.45,;10.26,-19.78,;11.79,-19.78,;12.69,-21.03,;14.16,-20.55,;14.16,-19.01,;15.49,-18.24,;16.82,-19,;18.16,-18.23,;16.83,-20.55,;18.17,-21.32,;19.5,-20.55,;15.49,-21.32,;15.5,-22.86,;14.16,-23.63,;14.16,-25.17,;15.49,-25.94,;15.49,-27.48,;16.83,-25.16,;16.83,-23.62,;18.16,-22.85,;12.7,-18.54,;12.22,-17.07,)| Show InChI InChI=1S/C18H18Cl2N4O2/c1-9-12(6-21)16(11-4-3-10(19)5-14(11)20)13-7-24(8-15(25)22-2)18(26)17(13)23-9/h3-5H,6-8,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356583

(CHEMBL1910125)Show SMILES Cc1nc2C(=O)N(CC(=O)Nc3ccnn3C)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(39.71,-37.55,;38.38,-38.32,;37.04,-37.56,;35.71,-38.33,;34.25,-37.86,;33.78,-36.39,;33.34,-39.1,;31.81,-39.1,;31.04,-40.44,;31.81,-41.77,;29.5,-40.44,;28.73,-41.77,;27.21,-41.94,;26.89,-43.44,;28.22,-44.21,;29.37,-43.18,;30.87,-43.5,;34.25,-40.35,;35.71,-39.87,;37.05,-40.64,;38.38,-39.87,;39.72,-40.64,;41.05,-39.87,;37.05,-42.18,;35.71,-42.95,;35.71,-44.49,;37.04,-45.26,;37.05,-46.8,;38.38,-44.48,;38.38,-42.94,;39.71,-42.17,)| Show InChI InChI=1S/C21H20Cl2N6O2/c1-11-14(8-24)19(13-4-3-12(22)7-16(13)23)15-9-29(21(31)20(15)26-11)10-18(30)27-17-5-6-25-28(17)2/h3-7H,8-10,24H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050511
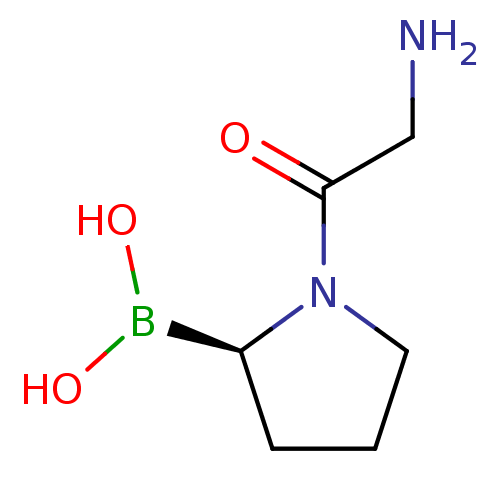
((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Inhibition of human placental DPP4 |
J Med Chem 51: 6005-13 (2008)
Article DOI: 10.1021/jm800390n
BindingDB Entry DOI: 10.7270/Q2GM874W |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11108
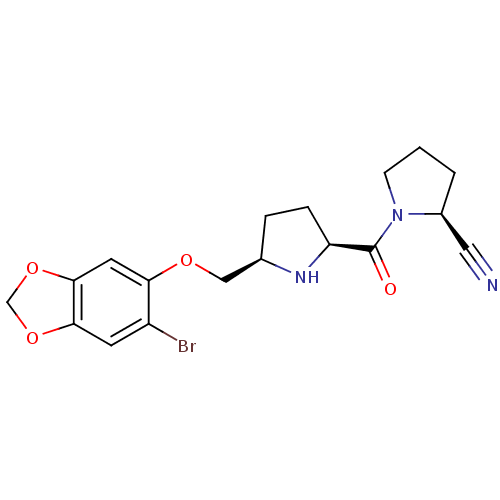
((2S)-1-{[(2S,5R)-5-{[(6-bromo-2H-1,3-benzodioxol-5...)Show SMILES Brc1cc2OCOc2cc1OC[C@H]1CC[C@H](N1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C18H20BrN3O4/c19-13-6-16-17(26-10-25-16)7-15(13)24-9-11-3-4-14(21-11)18(23)22-5-1-2-12(22)8-20/h6-7,11-12,14,21H,1-5,9-10H2/t11-,12+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50288303

((R)-3-((S)-2-Amino-3-methyl-pentanoyl)-thiazolidin...)Show InChI InChI=1S/C10H17N3OS/c1-3-7(2)9(12)10(14)13-6-15-5-8(13)4-11/h7-9H,3,5-6,12H2,1-2H3/t7?,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human Dipeptidyl peptidase IV |
Bioorg Med Chem Lett 6: 2745-2748 (1996)
Article DOI: 10.1016/S0960-894X(96)00491-X
BindingDB Entry DOI: 10.7270/Q2MP537C |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356590

(CHEMBL1910118)Show SMILES CCN(CC)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(39.88,-17.76,;39.11,-19.1,;39.89,-20.43,;39.12,-21.76,;39.89,-23.09,;41.43,-20.43,;42.2,-21.76,;42.19,-19.09,;43.73,-19.09,;44.63,-20.33,;46.09,-19.86,;46.1,-18.32,;47.43,-17.55,;48.76,-18.31,;50.09,-17.53,;48.77,-19.86,;50.1,-20.63,;51.43,-19.86,;47.43,-20.63,;47.43,-22.17,;46.09,-22.94,;46.09,-24.47,;47.43,-25.25,;47.43,-26.79,;48.77,-24.47,;48.76,-22.93,;50.09,-22.16,;44.63,-17.84,;44.16,-16.38,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-16-19(14-7-6-13(22)8-17(14)23)15(9-24)12(3)25-20(16)21(27)29/h6-8H,4-5,9-11,24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50519494

(CHEMBL1234234)Show SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:19| Show InChI InChI=1S/C12H18N4O4S/c1-21-3-7-9(18)10(19)12(20-7)16-5-15-8-6(17)2-13-4-14-11(8)16/h4-7,9-10,12,17-19H,2-3H2,1H3,(H,13,14)/t6-,7-,9-,10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11103
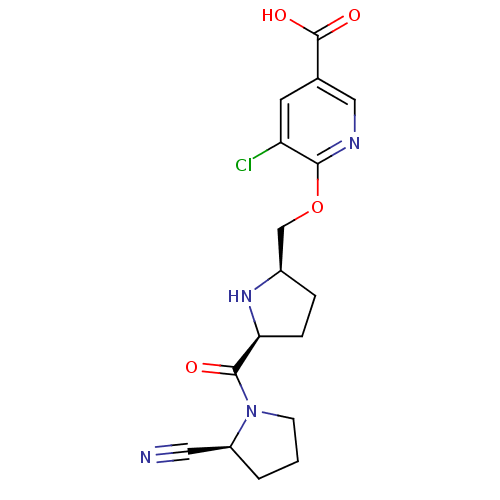
(2-cyanopyrrolidine 21aj | 5-chloro-6-{[(2R,5S)-5-{...)Show SMILES OC(=O)c1cnc(OC[C@H]2CC[C@H](N2)C(=O)N2CCC[C@H]2C#N)c(Cl)c1 |r| Show InChI InChI=1S/C17H19ClN4O4/c18-13-6-10(17(24)25)8-20-15(13)26-9-11-3-4-14(21-11)16(23)22-5-1-2-12(22)7-19/h6,8,11-12,14,21H,1-5,9H2,(H,24,25)/t11-,12+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... |
J Med Chem 49: 3520-35 (2006)
Article DOI: 10.1021/jm051283e
BindingDB Entry DOI: 10.7270/Q2WS8RG1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356587
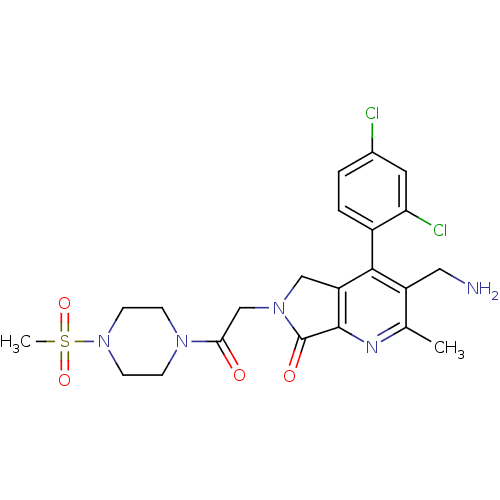
(CHEMBL1910121)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCN(CC3)S(C)(=O)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(48.61,-29.07,;47.28,-29.84,;45.95,-29.08,;44.62,-29.86,;43.16,-29.38,;42.68,-27.91,;42.25,-30.62,;40.72,-30.62,;39.95,-31.96,;40.72,-33.29,;38.41,-31.96,;37.64,-30.63,;36.11,-30.63,;35.33,-31.96,;36.1,-33.29,;37.65,-33.3,;33.79,-31.95,;33.03,-30.62,;32.45,-32.72,;33.78,-33.49,;43.15,-31.87,;44.62,-31.4,;45.95,-32.17,;47.29,-31.39,;48.62,-32.16,;49.96,-31.39,;45.95,-33.7,;44.62,-34.47,;44.62,-36.01,;45.95,-36.78,;45.95,-38.32,;47.29,-36,;47.28,-34.47,;48.61,-33.69,)| Show InChI InChI=1S/C22H25Cl2N5O4S/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-28(22(31)21(17)26-13)12-19(30)27-5-7-29(8-6-27)34(2,32)33/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data