 Found 6123 hits Enz. Inhib. hit(s) with Target = 'Beta-2 adrenergic receptor'
Found 6123 hits Enz. Inhib. hit(s) with Target = 'Beta-2 adrenergic receptor' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595424
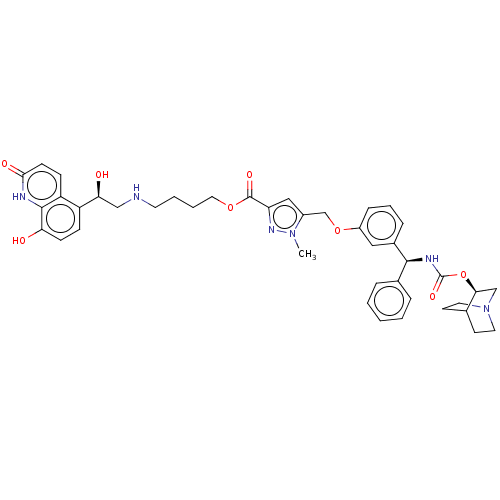
(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
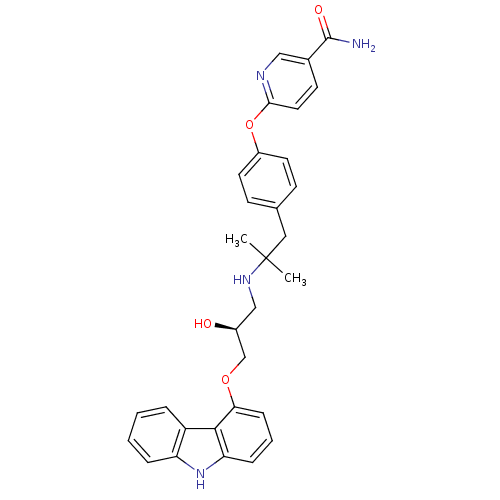
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569290
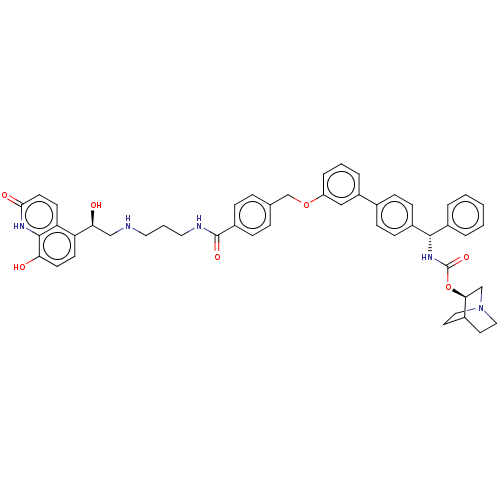
(CHEMBL4871517)Show SMILES O[C@@H](CNCCCNC(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:33.34,1.0,wD:28.30,(31.17,-6.07,;32.5,-6.84,;33.84,-6.06,;35.17,-6.83,;36.5,-6.05,;37.84,-6.82,;39.17,-6.05,;40.51,-6.81,;41.84,-6.04,;41.83,-4.5,;43.17,-6.8,;43.18,-8.34,;44.51,-9.11,;45.84,-8.33,;47.18,-9.1,;48.51,-8.32,;49.85,-9.08,;49.85,-10.62,;51.19,-11.38,;52.52,-10.61,;52.51,-9.06,;51.17,-8.3,;53.84,-8.28,;55.17,-9.04,;56.51,-8.27,;56.5,-6.72,;55.16,-5.96,;53.84,-6.74,;57.83,-5.94,;59.17,-6.7,;60.5,-5.92,;60.49,-4.38,;61.84,-6.69,;63.17,-5.91,;63.15,-4.38,;64.49,-3.61,;65.83,-4.37,;65.83,-5.91,;64.5,-6.68,;65.2,-5.33,;63.71,-4.93,;57.82,-4.4,;59.15,-3.63,;59.15,-2.09,;57.81,-1.33,;56.47,-2.11,;56.49,-3.65,;45.83,-6.78,;44.5,-6.02,;32.51,-8.38,;31.18,-9.15,;31.18,-10.69,;32.51,-11.46,;32.51,-13,;33.84,-10.69,;35.17,-11.46,;36.51,-10.69,;37.85,-11.46,;36.52,-9.14,;35.18,-8.36,;33.84,-9.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569294
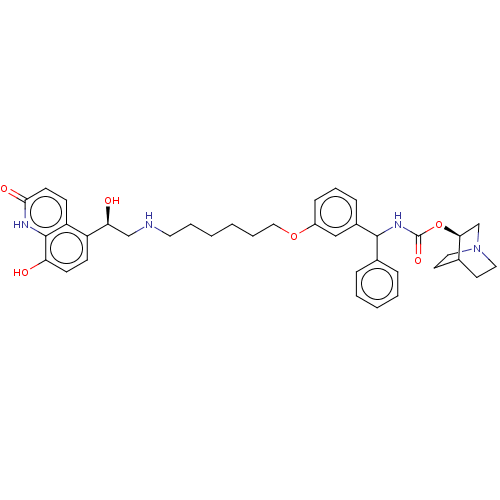
(CHEMBL4863525)Show SMILES O[C@@H](CNCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:22.22,1.0,(-.19,-37.45,;1.15,-38.22,;2.48,-37.45,;3.82,-38.21,;5.15,-37.44,;6.49,-38.2,;7.82,-37.43,;9.15,-38.2,;10.48,-37.42,;11.82,-38.19,;13.15,-37.41,;14.49,-38.19,;14.48,-39.72,;15.82,-40.49,;17.15,-39.72,;17.14,-38.17,;15.8,-37.41,;18.47,-37.38,;19.81,-38.15,;21.14,-37.37,;21.13,-35.83,;22.48,-38.13,;23.81,-37.35,;23.79,-35.82,;25.13,-35.05,;26.47,-35.82,;26.47,-37.36,;25.14,-38.13,;25.84,-36.77,;24.35,-36.38,;18.46,-35.85,;19.79,-35.08,;19.79,-33.54,;18.45,-32.77,;17.12,-33.56,;17.13,-35.09,;1.16,-39.76,;-.18,-40.53,;-.18,-42.07,;1.16,-42.85,;1.16,-44.39,;2.49,-42.07,;3.82,-42.84,;5.16,-42.07,;6.49,-42.85,;5.16,-40.53,;3.82,-39.75,;2.49,-40.52,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595404

(CHEMBL5184455)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8.01,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.55,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595423
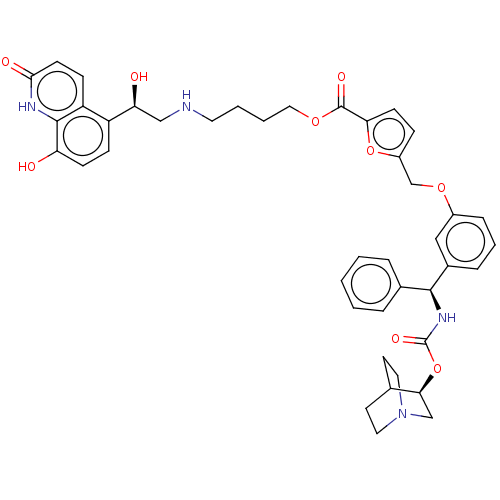
(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569293
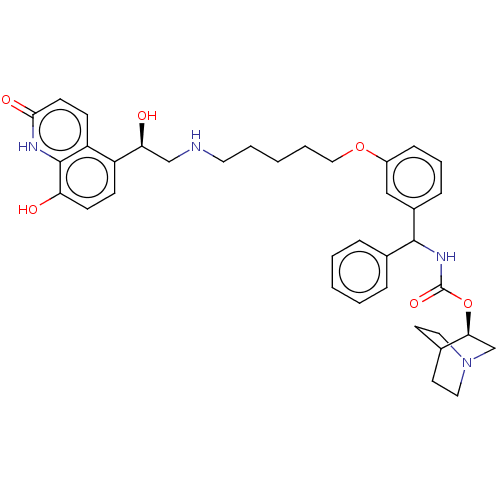
(CHEMBL4874819)Show SMILES O[C@@H](CNCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:21.21,(24.25,-39.74,;25.59,-40.51,;26.92,-39.73,;28.26,-40.5,;29.59,-39.72,;30.92,-40.49,;32.25,-39.72,;33.59,-40.48,;34.92,-39.71,;36.26,-40.47,;37.6,-39.7,;37.59,-38.17,;38.91,-37.39,;40.26,-38.15,;40.26,-39.7,;38.93,-40.47,;41.6,-40.46,;42.93,-39.69,;44.26,-40.45,;44.27,-42,;45.59,-39.67,;46.93,-40.44,;46.92,-41.99,;48.25,-42.75,;49.59,-41.98,;49.59,-40.44,;48.25,-39.67,;49.01,-41,;47.47,-41.41,;41.6,-42,;40.26,-42.77,;40.27,-44.31,;41.6,-45.07,;42.94,-44.29,;42.93,-42.76,;25.59,-42.05,;24.26,-42.82,;24.26,-44.36,;25.6,-45.13,;25.6,-46.67,;26.93,-44.36,;28.26,-45.13,;29.6,-44.36,;30.93,-45.13,;29.6,-42.81,;28.26,-42.03,;26.93,-42.81,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595427
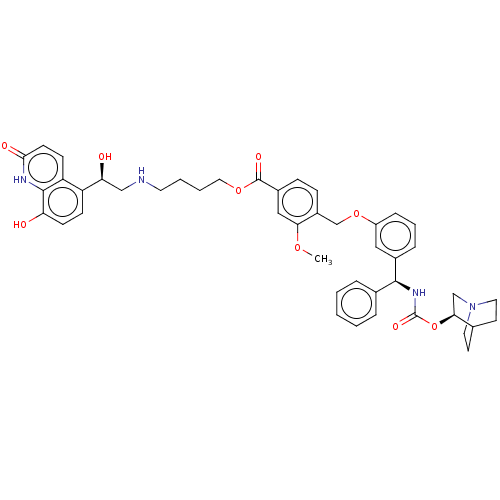
(CHEMBL5200887)Show SMILES COc1cc(ccc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:44.49,wD:16.18,21.22,(3.34,-1.15,;3.34,.39,;2,1.16,;.67,.39,;-.67,1.15,;-.67,2.69,;.67,3.47,;2,2.7,;3.33,3.47,;4.67,2.7,;6,3.47,;6,5.01,;7.33,5.78,;8.67,5.02,;8.67,3.48,;7.33,2.7,;10,2.71,;10,1.17,;8.67,.4,;7.34,1.16,;8.67,-1.14,;7.34,-1.92,;6.01,-1.15,;4.67,-1.92,;6.1,-1.92,;5.92,-3.46,;7.34,-3.46,;6.01,-4.23,;4.68,-3.46,;11.33,3.48,;11.33,5.02,;12.67,5.79,;14,5.02,;14,3.48,;12.67,2.71,;-2,.38,;-2,-1.16,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-7.33,.38,;-8.67,1.14,;-10,.37,;-11.33,1.14,;-12.67,.37,;-14,1.14,;-12.67,-1.17,;-14,-1.94,;-14,-3.48,;-12.66,-4.25,;-12.66,-5.79,;-11.33,-3.48,;-9.99,-4.25,;-8.66,-3.48,;-7.33,-4.24,;-8.66,-1.94,;-10,-1.17,;-11.33,-1.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595426
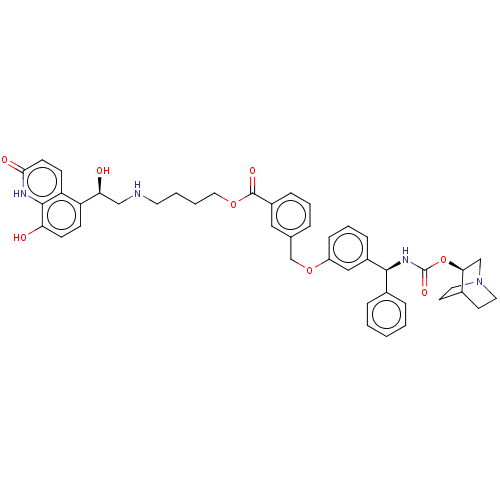
(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50167072
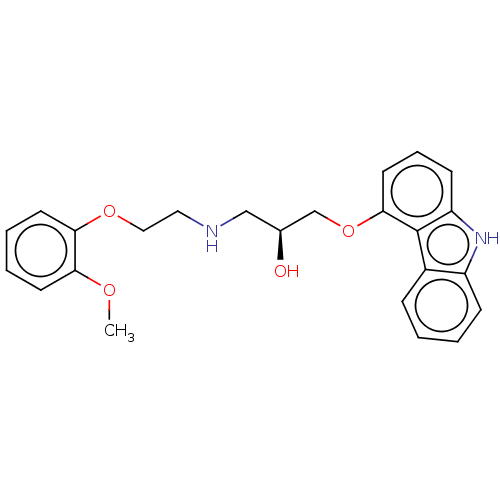
(CHEMBL3799125)Show SMILES COc1ccccc1OCCNC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg (FAU)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP-12177 from human beta2-adrenergic receptor expressed in CHO cells after 60 mins by radioligand competition binding assay |
J Med Chem 62: 5111-5131 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00349
BindingDB Entry DOI: 10.7270/Q2DZ0CRD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027663
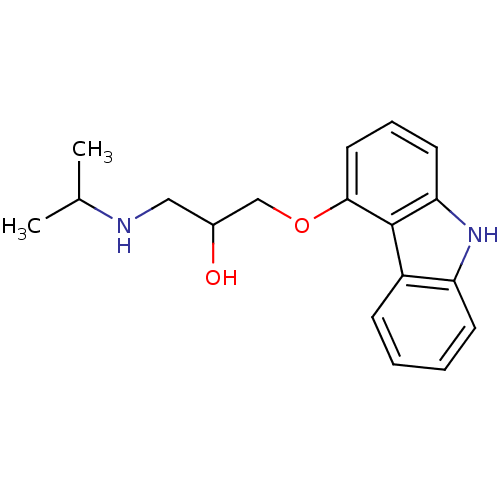
(1-(9H-Carbazol-4-yloxy)-3-isopropylamino-propan-2-...)Show InChI InChI=1S/C18H22N2O2/c1-12(2)19-10-13(21)11-22-17-9-5-8-16-18(17)14-6-3-4-7-15(14)20-16/h3-9,12-13,19-21H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg (FAU)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP-12177 from human beta2-adrenergic receptor expressed in CHO cells after 60 mins by radioligand competition binding assay |
J Med Chem 62: 5111-5131 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00349
BindingDB Entry DOI: 10.7270/Q2DZ0CRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50167072

(CHEMBL3799125)Show SMILES COc1ccccc1OCCNC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C24H26N2O4/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20/h2-12,17,25-27H,13-16H2,1H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 receptor expressed in CHO cells |
Bioorg Med Chem 24: 2641-53 (2016)
Article DOI: 10.1016/j.bmc.2016.04.028
BindingDB Entry DOI: 10.7270/Q2ZC84S3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595403
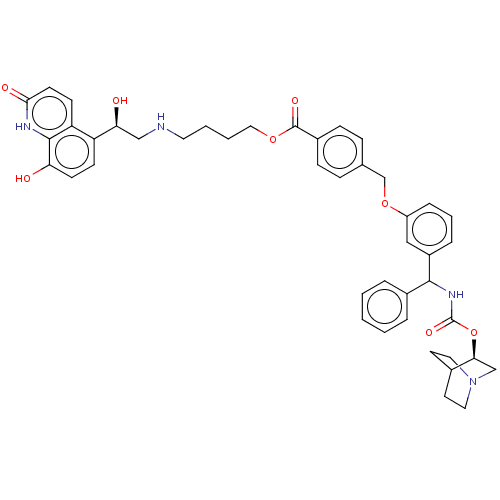
(CHEMBL5208201)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)C(NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.66,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-3.99,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;14.67,3.46,;14.67,5,;13.34,5.77,;14.05,4.54,;12.63,3.93,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.33,-6.54,;-13.33,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569292
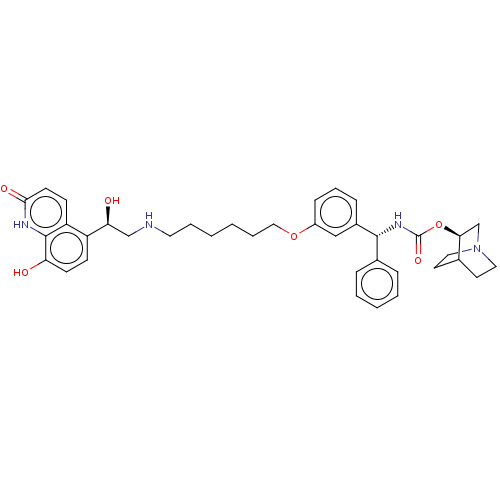
(CHEMBL4857743)Show SMILES O[C@@H](CNCCCCCCOc1cccc(c1)[C@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:17.18,22.22,1.0,(52.87,-39.55,;54.2,-40.32,;55.53,-39.55,;56.87,-40.31,;58.2,-39.54,;59.54,-40.3,;60.87,-39.53,;62.21,-40.3,;63.54,-39.52,;64.87,-40.29,;66.2,-39.51,;67.54,-40.29,;67.53,-41.82,;68.87,-42.59,;70.2,-41.82,;70.19,-40.27,;68.86,-39.51,;71.53,-39.48,;72.86,-40.25,;74.19,-39.47,;74.19,-37.93,;75.53,-40.23,;76.86,-39.45,;76.85,-37.93,;78.19,-37.15,;79.52,-37.92,;79.52,-39.46,;78.2,-40.23,;78.89,-38.87,;77.41,-38.48,;71.52,-37.95,;72.85,-37.18,;72.84,-35.64,;71.5,-34.87,;70.17,-35.66,;70.18,-37.19,;54.21,-41.86,;52.88,-42.63,;52.88,-44.17,;54.21,-44.95,;54.21,-46.49,;55.54,-44.17,;56.87,-44.94,;58.21,-44.17,;59.55,-44.95,;58.22,-42.63,;56.88,-41.85,;55.54,-42.62,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595425
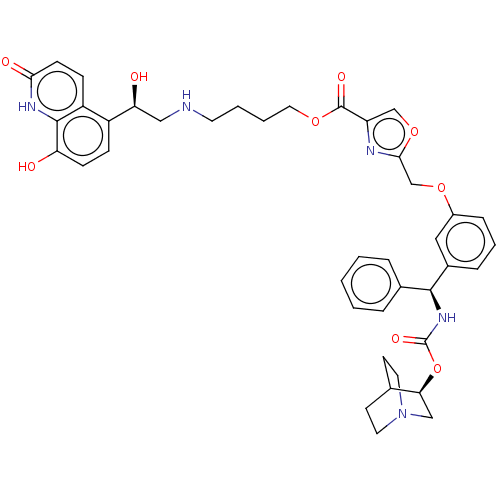
(CHEMBL5208957)Show SMILES O[C@@H](CNCCCCOC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569299
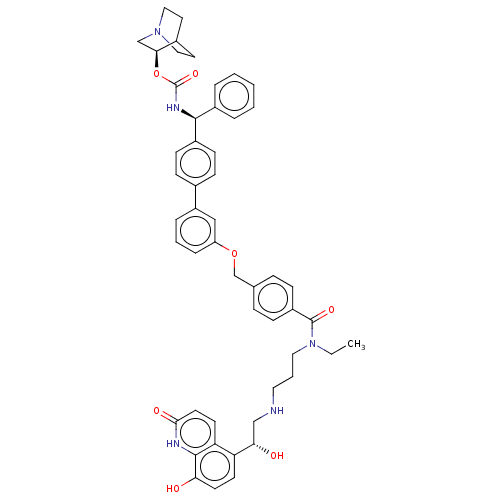
(CHEMBL4866806)Show SMILES CCN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:47.50,8.8,wD:42.46,(11.54,-25.97,;12.87,-25.19,;12.87,-23.65,;11.53,-22.89,;10.2,-23.66,;8.87,-22.89,;7.54,-23.67,;6.2,-22.9,;4.87,-23.68,;3.53,-22.91,;4.87,-25.22,;3.54,-25.99,;3.54,-27.53,;4.88,-28.3,;4.88,-29.84,;6.21,-27.53,;7.54,-28.3,;8.88,-27.53,;10.21,-28.3,;8.88,-25.98,;7.54,-25.2,;6.21,-25.98,;14.2,-22.88,;14.2,-21.34,;15.54,-23.64,;15.54,-25.18,;16.87,-25.94,;18.21,-25.17,;19.54,-25.94,;20.87,-25.16,;22.21,-25.92,;22.22,-27.46,;23.55,-28.22,;24.88,-27.45,;24.87,-25.9,;23.53,-25.14,;26.2,-25.12,;27.54,-25.88,;28.87,-25.11,;28.86,-23.56,;27.53,-22.8,;26.21,-23.58,;30.19,-22.78,;31.53,-23.54,;32.86,-22.76,;32.85,-21.22,;34.2,-23.52,;35.53,-22.75,;35.52,-21.22,;36.85,-20.44,;38.19,-21.21,;38.19,-22.75,;36.87,-23.52,;37.56,-22.17,;36.07,-21.77,;30.19,-21.24,;31.52,-20.47,;31.51,-18.93,;30.17,-18.17,;28.84,-18.95,;28.85,-20.49,;18.2,-23.62,;16.86,-22.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595405
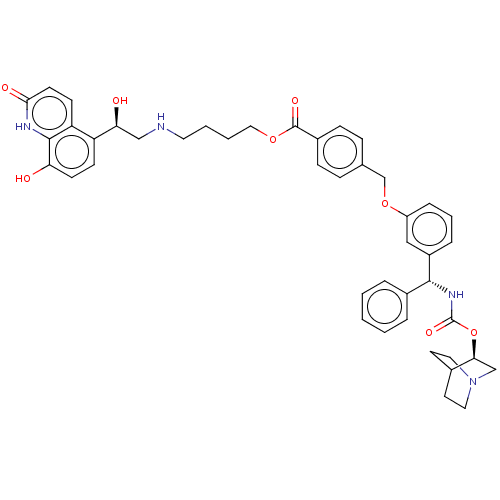
(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50526679
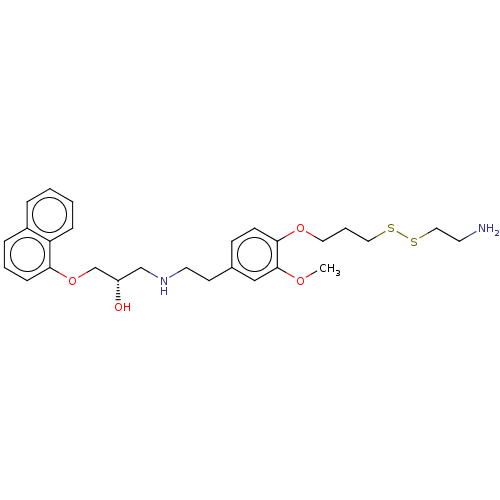
(CHEMBL4456301)Show SMILES COc1cc(CCNC[C@H](O)COc2cccc3ccccc23)ccc1OCCCSSCCN |r| Show InChI InChI=1S/C27H36N2O4S2/c1-31-27-18-21(10-11-26(27)32-15-5-16-34-35-17-13-28)12-14-29-19-23(30)20-33-25-9-4-7-22-6-2-3-8-24(22)25/h2-4,6-11,18,23,29-30H,5,12-17,19-20,28H2,1H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor expressed in CHO cell membranes |
Bioorg Med Chem 27: 2959-2971 (2019)
Article DOI: 10.1016/j.bmc.2019.05.034
BindingDB Entry DOI: 10.7270/Q2FB56CD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818
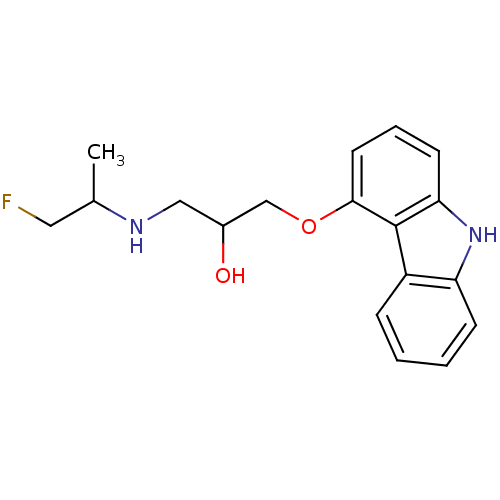
(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569300
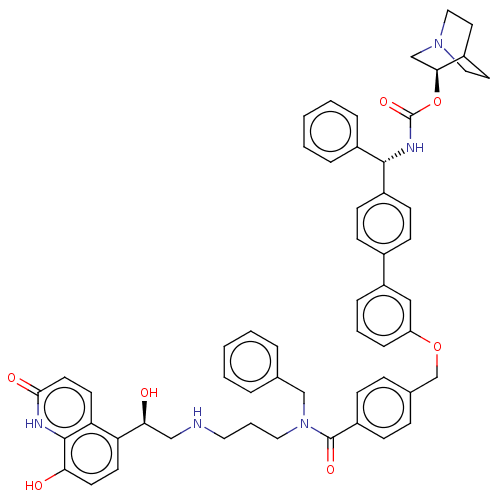
(CHEMBL4871702)Show SMILES O[C@@H](CNCCCN(Cc1ccccc1)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:40.42,1.0,wD:35.38,(46.1,-20.06,;47.43,-20.83,;48.76,-20.05,;50.1,-20.82,;51.43,-20.05,;52.77,-20.81,;54.1,-20.04,;55.44,-20.81,;55.44,-22.35,;56.53,-23.43,;56.13,-24.91,;57.21,-25.99,;58.7,-25.59,;59.09,-24.1,;58,-23.03,;56.77,-20.03,;56.76,-18.49,;58.11,-20.8,;58.11,-22.33,;59.44,-23.1,;60.78,-22.33,;62.11,-23.09,;63.44,-22.31,;64.78,-23.08,;64.79,-24.61,;66.12,-25.38,;67.45,-24.6,;67.44,-23.05,;66.11,-22.3,;68.78,-22.27,;70.11,-23.04,;71.44,-22.26,;71.43,-20.71,;70.1,-19.96,;68.78,-20.73,;72.77,-19.93,;74.11,-20.69,;75.44,-19.91,;75.43,-18.37,;76.77,-20.68,;78.1,-19.9,;78.09,-18.37,;79.43,-17.6,;80.77,-18.36,;80.77,-19.9,;79.44,-20.68,;80.14,-19.32,;78.65,-18.92,;72.76,-18.39,;74.09,-17.62,;74.08,-16.08,;72.75,-15.32,;71.41,-16.1,;71.42,-17.64,;60.77,-20.78,;59.43,-20.02,;47.44,-22.37,;46.11,-23.14,;46.1,-24.69,;47.44,-25.46,;47.44,-27,;48.77,-24.68,;50.1,-25.45,;51.44,-24.68,;52.78,-25.46,;51.45,-23.14,;50.11,-22.36,;48.77,-23.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569295
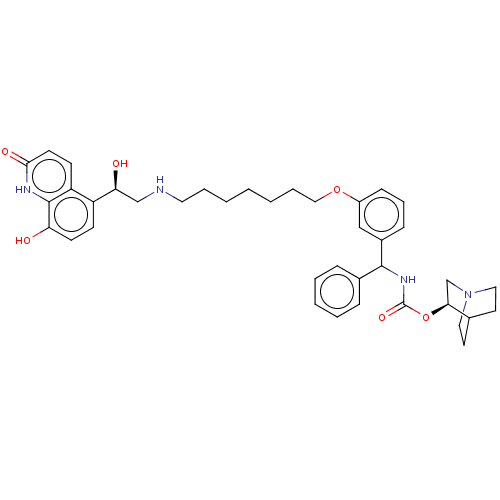
(CHEMBL4854418)Show SMILES O[C@@H](CNCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.23,(39.22,-23.81,;40.55,-24.58,;41.88,-23.8,;43.22,-24.57,;44.55,-23.79,;45.89,-24.56,;47.22,-23.79,;48.55,-24.55,;49.89,-23.78,;51.22,-24.54,;52.55,-23.77,;53.89,-24.54,;55.22,-23.76,;55.21,-22.23,;56.54,-21.45,;57.88,-22.22,;57.88,-23.76,;56.55,-24.53,;59.22,-24.53,;60.55,-23.75,;61.89,-24.51,;61.89,-26.06,;63.22,-23.74,;64.55,-24.5,;64.55,-26.05,;65.88,-26.81,;67.21,-26.05,;67.21,-24.5,;65.87,-23.73,;66.63,-25.06,;65.1,-25.47,;59.22,-26.07,;57.89,-26.83,;57.89,-28.37,;59.23,-29.14,;60.56,-28.36,;60.55,-26.82,;40.56,-26.12,;39.23,-26.89,;39.23,-28.43,;40.56,-29.2,;40.56,-30.74,;41.89,-28.43,;43.22,-29.2,;44.56,-28.43,;45.89,-29.2,;44.56,-26.88,;43.23,-26.1,;41.89,-26.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318987
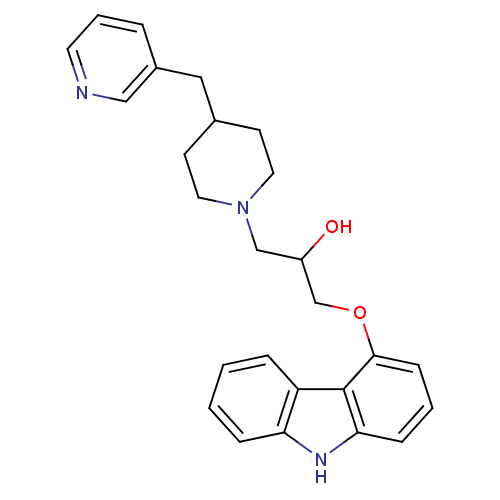
(1-(9H-carbazol-4-yloxy)-3-(4-(pyridin-3-ylmethyl)p...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(Cc2cccnc2)CC1 Show InChI InChI=1S/C26H29N3O2/c30-21(17-29-13-10-19(11-14-29)15-20-5-4-12-27-16-20)18-31-25-9-3-8-24-26(25)22-6-1-2-7-23(22)28-24/h1-9,12,16,19,21,28,30H,10-11,13-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50098668
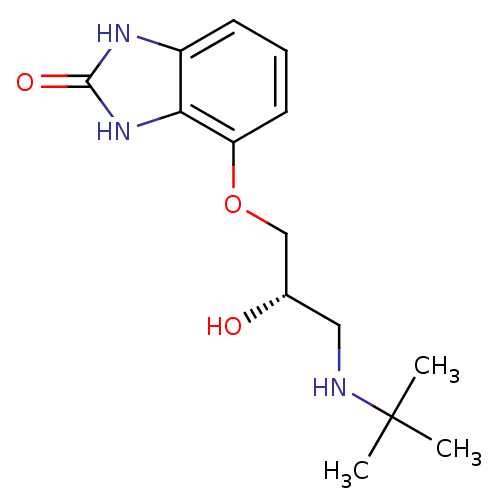
(4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...)Show SMILES CC(C)(C)NC[C@H](O)COc1cccc2[nH]c(=O)[nH]c12 Show InChI InChI=1S/C14H21N3O3/c1-14(2,3)15-7-9(18)8-20-11-6-4-5-10-12(11)17-13(19)16-10/h4-6,9,15,18H,7-8H2,1-3H3,(H2,16,17,19)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors |
J Med Chem 44: 1456-66 (2001)
BindingDB Entry DOI: 10.7270/Q2T72J53 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027663

(1-(9H-Carbazol-4-yloxy)-3-isopropylamino-propan-2-...)Show InChI InChI=1S/C18H22N2O2/c1-12(2)19-10-13(21)11-22-17-9-5-8-16-18(17)14-6-3-4-7-15(14)20-16/h3-9,12-13,19-21H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter |
Bioorg Med Chem Lett 18: 5391-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.046
BindingDB Entry DOI: 10.7270/Q2FB52RP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM85818

(Fluorocarazolol,(R) | Fluorocarazolol,(S))Show InChI InChI=1S/C18H21FN2O2/c1-12(9-19)20-10-13(22)11-23-17-8-4-7-16-18(17)14-5-2-3-6-15(14)21-16/h2-8,12-13,20-22H,9-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 157: 111-4 (2001)
Article DOI: 10.1007/s002130100844
BindingDB Entry DOI: 10.7270/Q22V2DPJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50274012
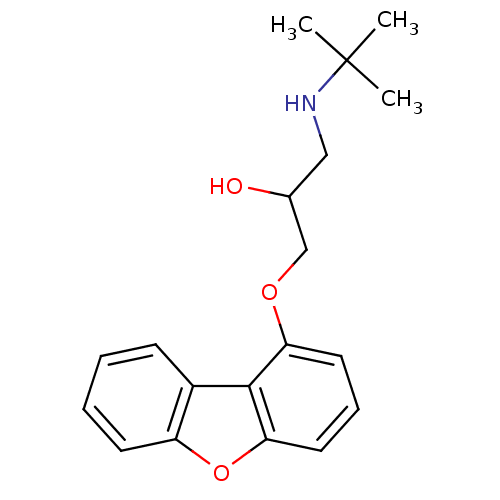
(1-tert-Butylamino-3-(dibenzofuran-1-yloxy)-propan-...)Show InChI InChI=1S/C19H23NO3/c1-19(2,3)20-11-13(21)12-22-16-9-6-10-17-18(16)14-7-4-5-8-15(14)23-17/h4-10,13,20-21H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter |
Bioorg Med Chem Lett 18: 5391-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.046
BindingDB Entry DOI: 10.7270/Q2FB52RP |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569302
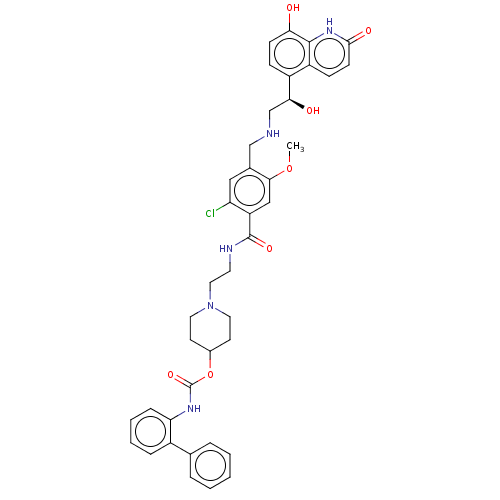
(CHEMBL4878138)Show SMILES COc1cc(C(=O)NCCN2CCC(CC2)OC(=O)Nc2ccccc2-c2ccccc2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50526681
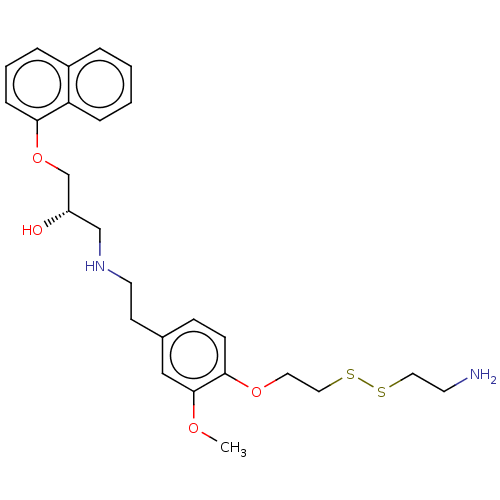
(CHEMBL4537221)Show SMILES COc1cc(CCNC[C@H](O)COc2cccc3ccccc23)ccc1OCCSSCCN |r| Show InChI InChI=1S/C26H34N2O4S2/c1-30-26-17-20(9-10-25(26)31-14-16-34-33-15-12-27)11-13-28-18-22(29)19-32-24-8-4-6-21-5-2-3-7-23(21)24/h2-10,17,22,28-29H,11-16,18-19,27H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor expressed in CHO cell membranes |
Bioorg Med Chem 27: 2959-2971 (2019)
Article DOI: 10.1016/j.bmc.2019.05.034
BindingDB Entry DOI: 10.7270/Q2FB56CD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
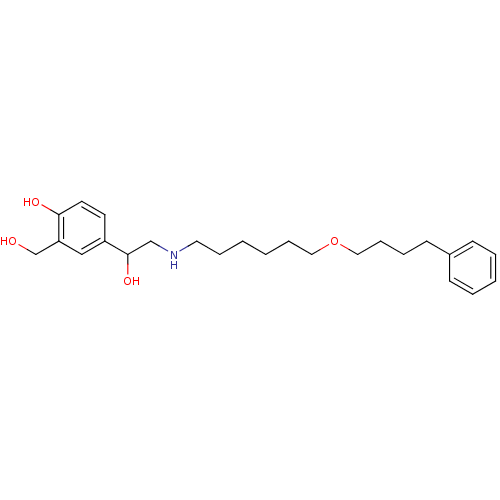
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 receptor expressed in CHO cells |
Bioorg Med Chem 24: 2641-53 (2016)
Article DOI: 10.1016/j.bmc.2016.04.028
BindingDB Entry DOI: 10.7270/Q2ZC84S3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569297
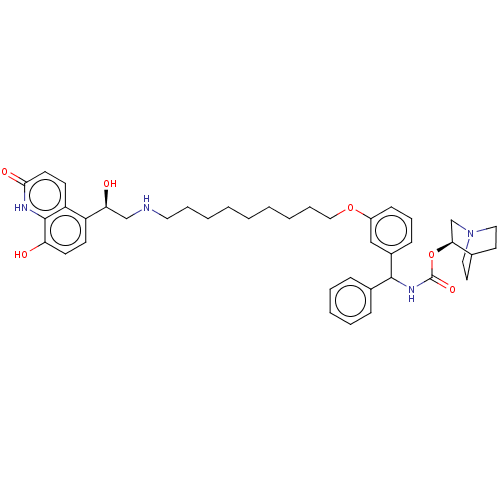
(CHEMBL4846536)Show SMILES O[C@@H](CNCCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:25.25,(48.77,-6.75,;50.1,-7.51,;51.43,-6.74,;52.77,-7.5,;54.1,-6.73,;55.44,-7.5,;56.77,-6.72,;58.11,-7.49,;59.44,-6.71,;60.77,-7.48,;62.1,-6.71,;63.44,-7.47,;64.77,-6.7,;66.11,-7.46,;67.44,-6.69,;67.43,-5.16,;68.75,-4.38,;70.1,-5.15,;70.1,-6.69,;68.77,-7.46,;71.44,-7.45,;72.77,-6.68,;74.11,-7.44,;74.11,-8.99,;75.44,-6.67,;76.77,-7.43,;76.77,-8.98,;78.1,-9.74,;79.43,-8.97,;79.43,-7.43,;78.09,-6.66,;78.85,-7.99,;77.32,-8.4,;71.44,-8.99,;70.11,-9.76,;70.11,-11.3,;71.45,-12.07,;72.78,-11.28,;72.77,-9.75,;50.11,-9.05,;48.78,-9.82,;48.78,-11.37,;50.11,-12.14,;50.11,-13.68,;51.44,-11.36,;52.77,-12.13,;54.11,-11.37,;55.44,-12.14,;54.12,-9.82,;52.78,-9.04,;51.44,-9.82,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569296
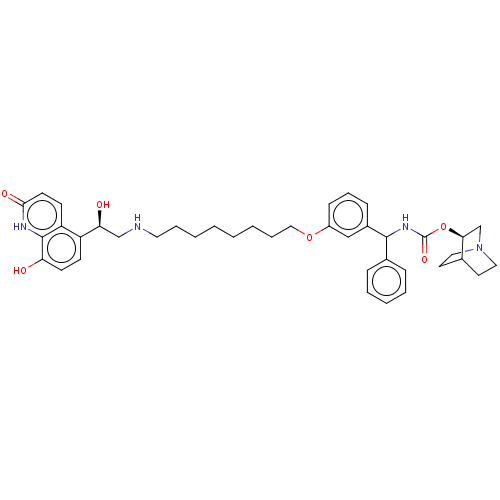
(CHEMBL4852629)Show SMILES O[C@@H](CNCCCCCCCCOc1cccc(c1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,24.24,(3.65,-24.81,;4.98,-25.58,;6.32,-24.81,;7.65,-25.57,;8.98,-24.8,;10.32,-25.56,;11.65,-24.79,;12.99,-25.56,;14.32,-24.78,;15.65,-25.55,;16.99,-24.77,;18.32,-25.54,;19.65,-24.77,;20.99,-25.42,;20.98,-26.95,;22.32,-27.72,;23.65,-26.94,;23.64,-25.4,;22.31,-24.64,;24.98,-24.61,;26.31,-25.37,;27.64,-24.6,;27.63,-23.06,;28.98,-25.36,;30.31,-24.58,;30.3,-23.05,;31.63,-22.28,;32.97,-23.05,;32.97,-24.59,;31.65,-25.36,;32.34,-24,;30.85,-23.61,;24.97,-23.08,;26.3,-22.31,;26.29,-20.77,;24.95,-20,;23.62,-20.79,;23.63,-22.32,;4.99,-27.12,;3.66,-27.89,;3.66,-29.44,;4.99,-30.21,;5,-31.75,;6.32,-29.43,;7.65,-30.2,;9,-29.43,;10.33,-30.21,;9,-27.89,;7.66,-27.11,;6.32,-27.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318989
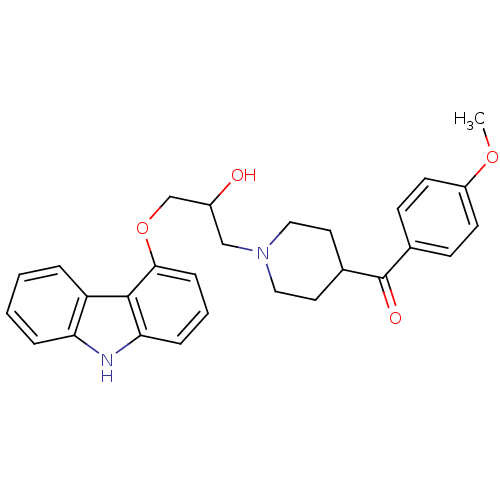
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES COc1ccc(cc1)C(=O)C1CCN(CC(O)COc2cccc3[nH]c4ccccc4c23)CC1 Show InChI InChI=1S/C28H30N2O4/c1-33-22-11-9-19(10-12-22)28(32)20-13-15-30(16-14-20)17-21(31)18-34-26-8-4-7-25-27(26)23-5-2-3-6-24(23)29-25/h2-12,20-21,29,31H,13-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318986
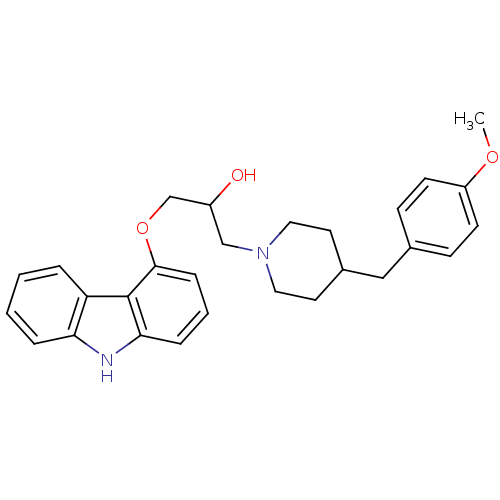
(1-(9H-carbazol-4-yloxy)-3-(4-(4-methoxybenzyl)pipe...)Show SMILES COc1ccc(CC2CCN(CC(O)COc3cccc4[nH]c5ccccc5c34)CC2)cc1 Show InChI InChI=1S/C28H32N2O3/c1-32-23-11-9-20(10-12-23)17-21-13-15-30(16-14-21)18-22(31)19-33-27-8-4-7-26-28(27)24-5-2-3-6-25(24)29-26/h2-12,21-22,29,31H,13-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318988
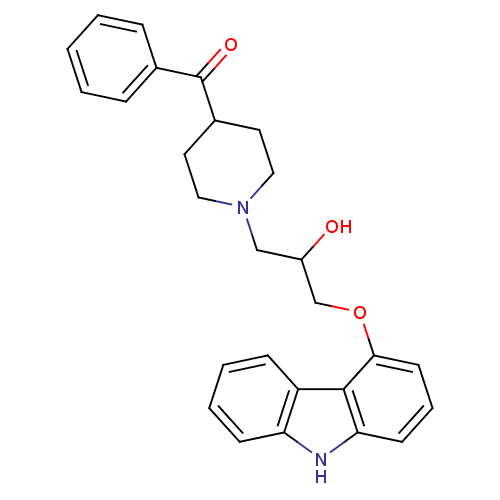
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C27H28N2O3/c30-21(17-29-15-13-20(14-16-29)27(31)19-7-2-1-3-8-19)18-32-25-12-6-11-24-26(25)22-9-4-5-10-23(22)28-24/h1-12,20-21,28,30H,13-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50292219
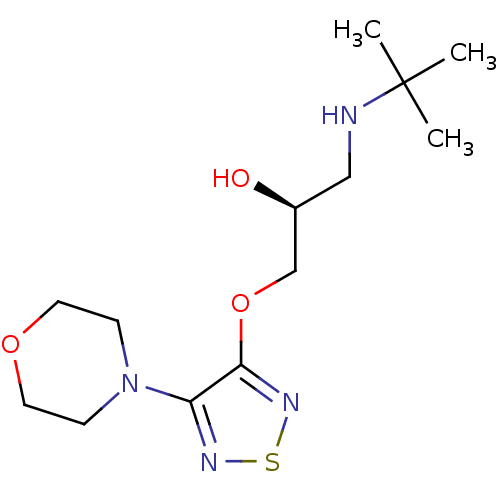
((-)-3-morpholino-4-(3-tert-butylamino-2-hydroxypro...)Show InChI InChI=1S/C13H24N4O3S/c1-13(2,3)14-8-10(18)9-20-12-11(15-21-16-12)17-4-6-19-7-5-17/h10,14,18H,4-9H2,1-3H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP12177 from human beta2 ADR expressed in HEK293T cell membrane after 90 mins by scintillation counting |
J Med Chem 61: 5380-5394 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00625
BindingDB Entry DOI: 10.7270/Q2XS5XX3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg (FAU)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP-12177 from human beta2-adrenergic receptor expressed in CHO cells after 60 mins by radioligand competition binding assay |
J Med Chem 62: 5111-5131 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00349
BindingDB Entry DOI: 10.7270/Q2DZ0CRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50166979
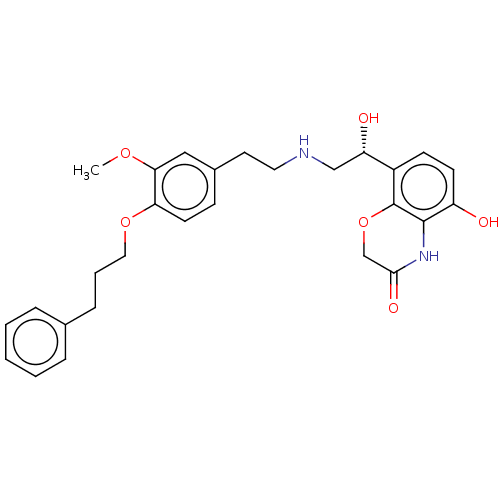
(CHEMBL3798748)Show SMILES COc1cc(CCNC[C@H](O)c2ccc(O)c3NC(=O)COc23)ccc1OCCCc1ccccc1 |r| Show InChI InChI=1S/C28H32N2O6/c1-34-25-16-20(9-12-24(25)35-15-5-8-19-6-3-2-4-7-19)13-14-29-17-23(32)21-10-11-22(31)27-28(21)36-18-26(33)30-27/h2-4,6-7,9-12,16,23,29,31-32H,5,8,13-15,17-18H2,1H3,(H,30,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 receptor expressed in CHO cells |
Bioorg Med Chem 24: 2641-53 (2016)
Article DOI: 10.1016/j.bmc.2016.04.028
BindingDB Entry DOI: 10.7270/Q2ZC84S3 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569298
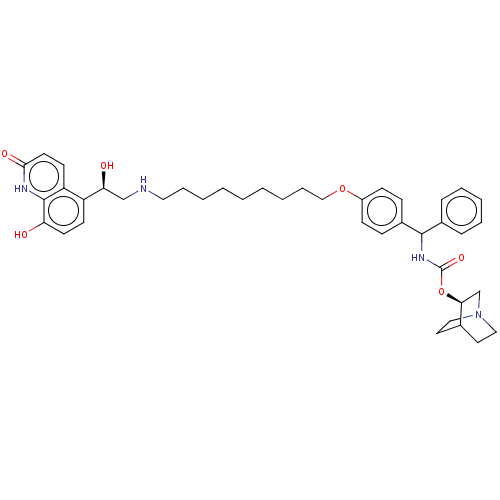
(CHEMBL4878395)Show SMILES O[C@@H](CNCCCCCCCCCOc1ccc(cc1)C(NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,25.25,(3.18,-8.47,;4.52,-9.24,;5.85,-8.47,;7.19,-9.23,;8.52,-8.46,;9.85,-9.22,;11.18,-8.45,;12.52,-9.22,;13.85,-8.44,;15.19,-9.21,;16.52,-8.43,;17.85,-9.2,;19.19,-8.43,;20.52,-9.19,;21.85,-8.42,;23.19,-9.19,;24.52,-8.41,;24.51,-6.87,;23.17,-6.11,;21.84,-6.88,;25.84,-6.1,;27.18,-6.86,;28.51,-6.08,;28.5,-4.54,;29.85,-6.84,;31.18,-6.06,;31.17,-4.53,;32.5,-3.76,;33.84,-4.53,;33.84,-6.07,;32.52,-6.84,;33.21,-5.48,;31.73,-5.09,;25.84,-4.56,;27.17,-3.79,;27.16,-2.25,;25.82,-1.48,;24.49,-2.27,;24.5,-3.8,;4.52,-10.78,;3.19,-11.55,;3.19,-13.09,;4.53,-13.86,;4.53,-15.4,;5.86,-13.09,;7.19,-13.86,;8.53,-13.09,;9.86,-13.87,;8.53,-11.55,;7.19,-10.77,;5.86,-11.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569301
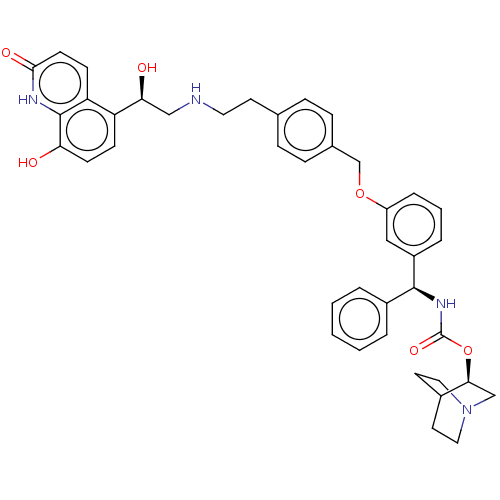
(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 125I-cyanopindolol from human adrenergic beta2 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127975
BindingDB Entry DOI: 10.7270/Q2F193HC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569301

(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318990
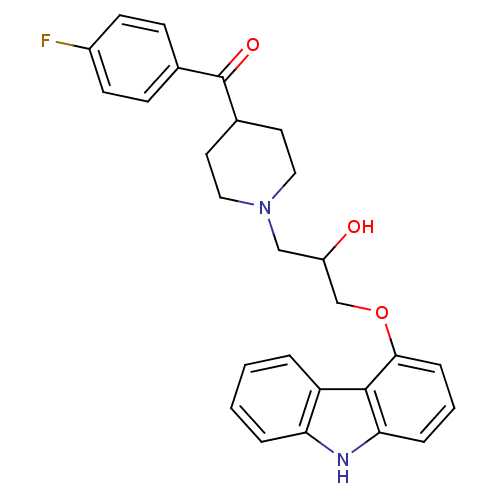
((1-(3-(9H-carbazol-4-yloxy)-2-hydroxypropyl)piperi...)Show SMILES OC(COc1cccc2[nH]c3ccccc3c12)CN1CCC(CC1)C(=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H27FN2O3/c28-20-10-8-18(9-11-20)27(32)19-12-14-30(15-13-19)16-21(31)17-33-25-7-3-6-24-26(25)22-4-1-2-5-23(22)29-24/h1-11,19,21,29,31H,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human adrenergic beta2 receptor expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 3399-404 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.009
BindingDB Entry DOI: 10.7270/Q2D21XSW |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324835

((R)-4-hydroxy-7-(1-hydroxy-2-(3-(methyl(phenyl)ami...)Show SMILES CN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)c1ccccc1 |r| Show InChI InChI=1S/C19H23N3O3S/c1-22(13-6-3-2-4-7-13)11-5-10-20-12-16(24)14-8-9-15(23)17-18(14)26-19(25)21-17/h2-4,6-9,16,20,23-24H,5,10-12H2,1H3,(H,21,25)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50274013
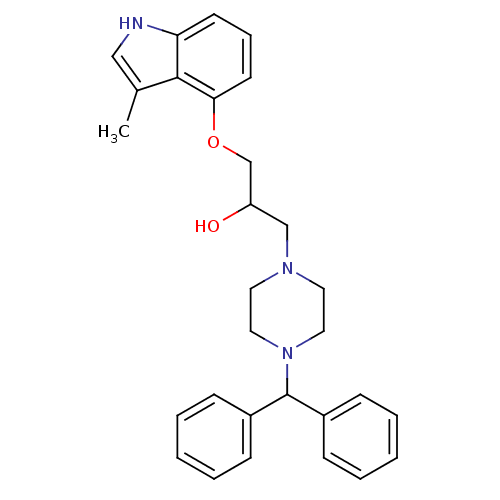
(1-(4-benzhydrylpiperazin-1-yl)-3-(3-methyl-1H-indo...)Show SMILES Cc1c[nH]c2cccc(OCC(O)CN3CCN(CC3)C(c3ccccc3)c3ccccc3)c12 Show InChI InChI=1S/C29H33N3O2/c1-22-19-30-26-13-8-14-27(28(22)26)34-21-25(33)20-31-15-17-32(18-16-31)29(23-9-4-2-5-10-23)24-11-6-3-7-12-24/h2-14,19,25,29-30,33H,15-18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited
Curated by ChEMBL
| Assay Description
Displacement of radioligand from human adrenergic beta2 receptor |
J Med Chem 54: 4283-311 (2011)
Article DOI: 10.1021/jm200371q
BindingDB Entry DOI: 10.7270/Q23N23RZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50470923
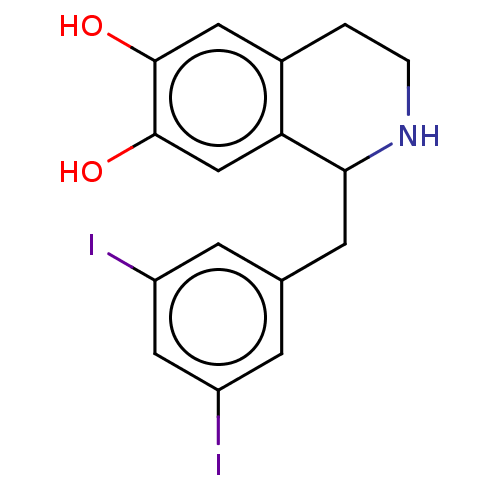
(CHEMBL122757)Show InChI InChI=1S/C16H15I2NO2/c17-11-3-9(4-12(18)7-11)5-14-13-8-16(21)15(20)6-10(13)1-2-19-14/h3-4,6-8,14,19-21H,1-2,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis
Curated by ChEMBL
| Assay Description
Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... |
J Med Chem 39: 3701-11 (1996)
Article DOI: 10.1021/jm960208o
BindingDB Entry DOI: 10.7270/Q2Q52SBM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50274013

(1-(4-benzhydrylpiperazin-1-yl)-3-(3-methyl-1H-indo...)Show SMILES Cc1c[nH]c2cccc(OCC(O)CN3CCN(CC3)C(c3ccccc3)c3ccccc3)c12 Show InChI InChI=1S/C29H33N3O2/c1-22-19-30-26-13-8-14-27(28(22)26)34-21-25(33)20-31-15-17-32(18-16-31)29(23-9-4-2-5-10-23)24-11-6-3-7-12-24/h2-14,19,25,29-30,33H,15-18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from human beta2 adrenoceptor by liquid scintillation counter |
Bioorg Med Chem Lett 18: 5391-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.046
BindingDB Entry DOI: 10.7270/Q2FB52RP |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Alexander Universit£t Erlangen-N£rnberg
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor expressed in CHO cell membranes |
Bioorg Med Chem 27: 2959-2971 (2019)
Article DOI: 10.1016/j.bmc.2019.05.034
BindingDB Entry DOI: 10.7270/Q2FB56CD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50019443
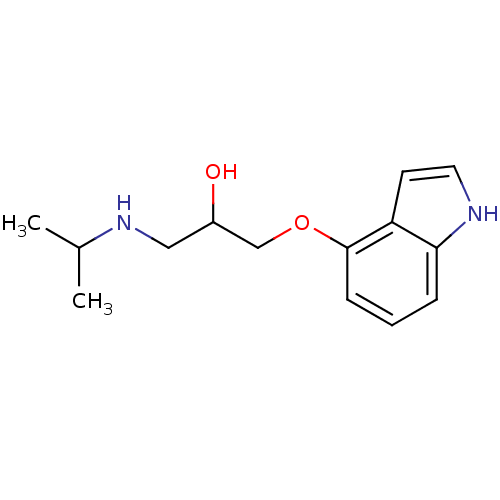
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Niigata College of Pharmacy
Curated by PDSP Ki Database
| |
Jpn J Pharmacol 52: 195-200 (1990)
Article DOI: 10.1254/jjp.52.195
BindingDB Entry DOI: 10.7270/Q2H41PXD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50518984
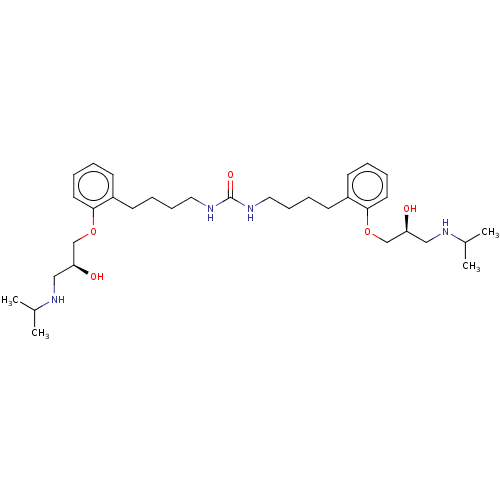
(CHEMBL4564189)Show SMILES CC(C)NC[C@H](O)COc1ccccc1CCCCNC(=O)NCCCCc1ccccc1OC[C@@H](O)CNC(C)C |r| Show InChI InChI=1S/C33H54N4O5/c1-25(2)36-21-29(38)23-41-31-17-7-5-13-27(31)15-9-11-19-34-33(40)35-20-12-10-16-28-14-6-8-18-32(28)42-24-30(39)22-37-26(3)4/h5-8,13-14,17-18,25-26,29-30,36-39H,9-12,15-16,19-24H2,1-4H3,(H2,34,35,40)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHA from beta2 adrenergic receptor (unknown origin) stably expressed in HEK293 cell membranes measured after 90 mins by scintilla... |
J Med Chem 62: 7806-7839 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00595
BindingDB Entry DOI: 10.7270/Q2PG1W4K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data