 Found 3562 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 4'
Found 3562 hits Enz. Inhib. hit(s) with Target = 'Carbonic anhydrase 4' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515869
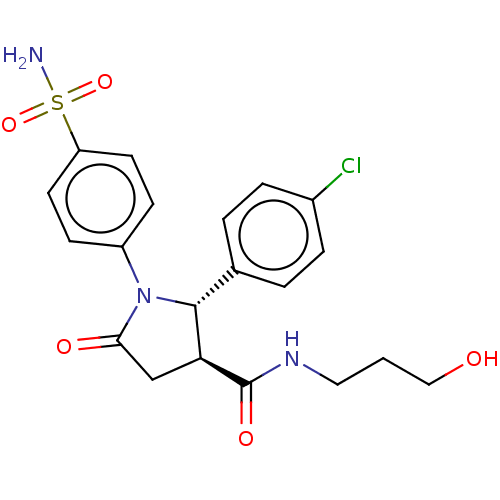
(CHEMBL4577408)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCCO)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22ClN3O5S/c21-14-4-2-13(3-5-14)19-17(20(27)23-10-1-11-25)12-18(26)24(19)15-6-8-16(9-7-15)30(22,28)29/h2-9,17,19,25H,1,10-12H2,(H,23,27)(H2,22,28,29)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515853
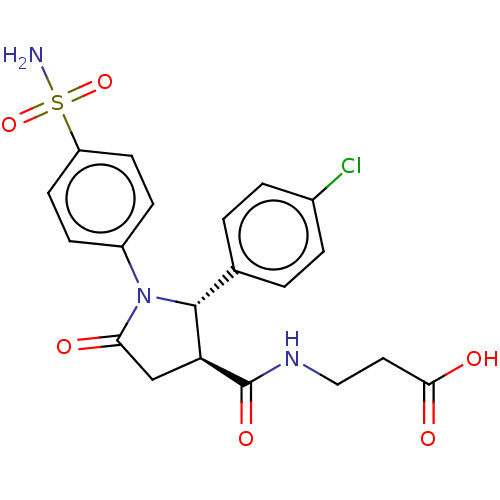
(CHEMBL4573177)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H20ClN3O6S/c21-13-3-1-12(2-4-13)19-16(20(28)23-10-9-18(26)27)11-17(25)24(19)14-5-7-15(8-6-14)31(22,29)30/h1-8,16,19H,9-11H2,(H,23,28)(H,26,27)(H2,22,29,30)/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50547697
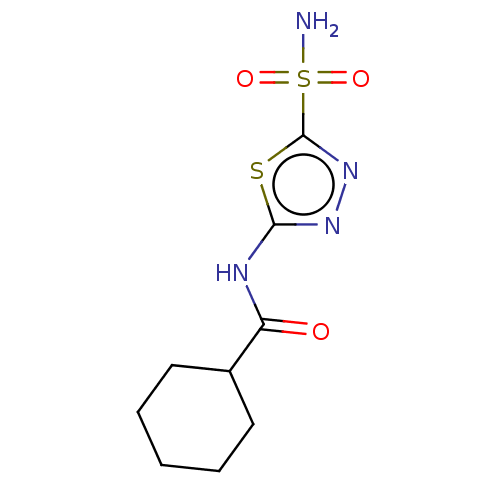
(CHEMBL4739913) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01390
BindingDB Entry DOI: 10.7270/Q2BC435H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50331834
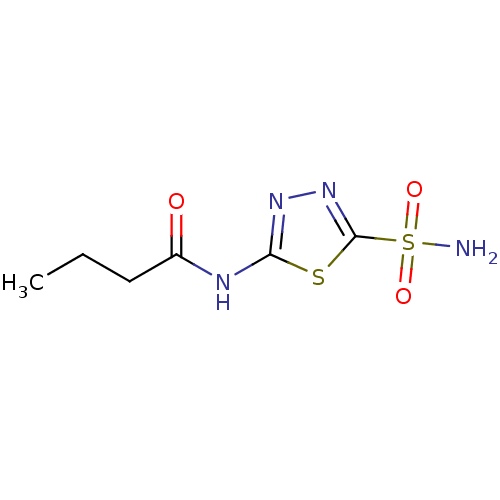
(5-butyramido-2-sulfamoyl-1,3,4-thiadiazole | CHEMB...)Show InChI InChI=1S/C6H10N4O3S2/c1-2-3-4(11)8-5-9-10-6(14-5)15(7,12)13/h2-3H2,1H3,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01390
BindingDB Entry DOI: 10.7270/Q2BC435H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515868
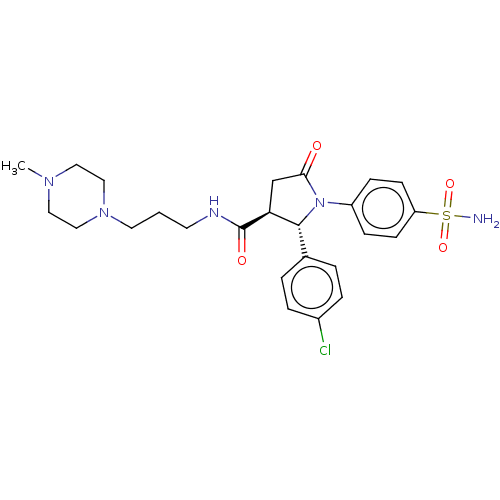
(CHEMBL4521611)Show SMILES CN1CCN(CCCNC(=O)[C@H]2CC(=O)N([C@@H]2c2ccc(Cl)cc2)c2ccc(cc2)S(N)(=O)=O)CC1 |r| Show InChI InChI=1S/C25H32ClN5O4S/c1-29-13-15-30(16-14-29)12-2-11-28-25(33)22-17-23(32)31(24(22)18-3-5-19(26)6-4-18)20-7-9-21(10-8-20)36(27,34)35/h3-10,22,24H,2,11-17H2,1H3,(H,28,33)(H2,27,34,35)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM16668
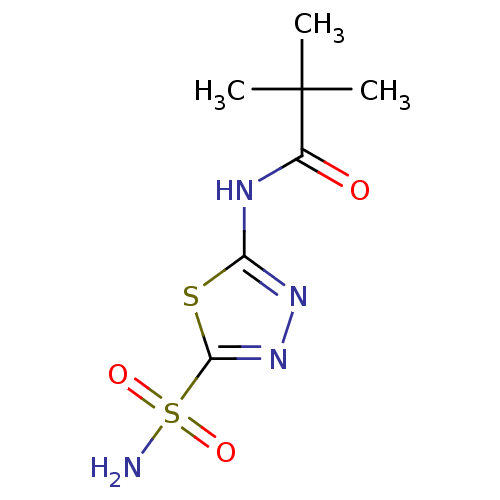
(2,2-dimethyl-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)...)Show InChI InChI=1S/C7H12N4O3S2/c1-7(2,3)4(12)9-5-10-11-6(15-5)16(8,13)14/h1-3H3,(H2,8,13,14)(H,9,10,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01390
BindingDB Entry DOI: 10.7270/Q2BC435H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50230195
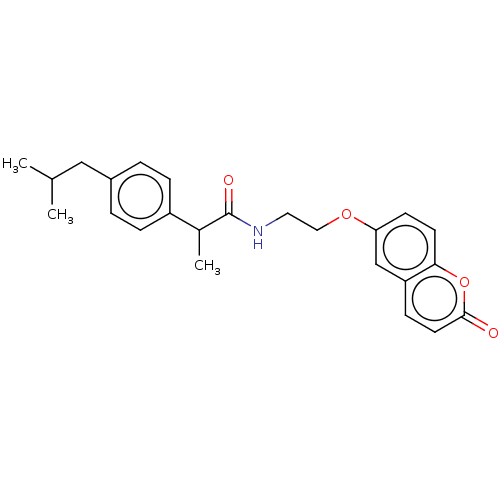
(CHEMBL4100903)Show SMILES CC(C)Cc1ccc(cc1)C(C)C(=O)NCCOc1ccc2oc(=O)ccc2c1 Show InChI InChI=1S/C24H27NO4/c1-16(2)14-18-4-6-19(7-5-18)17(3)24(27)25-12-13-28-21-9-10-22-20(15-21)8-11-23(26)29-22/h4-11,15-17H,12-14H2,1-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA4 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 60: 1159-1170 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01607
BindingDB Entry DOI: 10.7270/Q2PC34NX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50331835
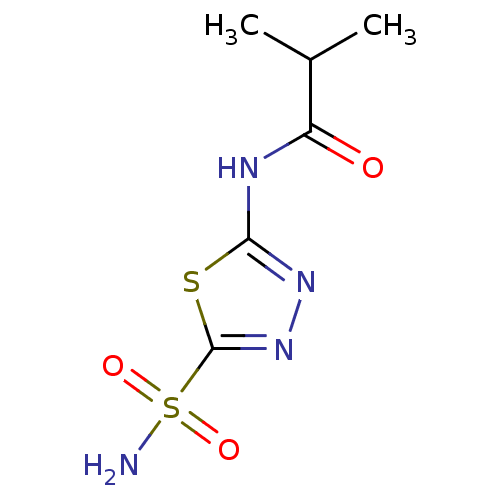
(5-(2-methyl-propylamido)-2-sulfamoyl-1,3,4-thiadia...)Show InChI InChI=1S/C6H10N4O3S2/c1-3(2)4(11)8-5-9-10-6(14-5)15(7,12)13/h3H,1-2H3,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01390
BindingDB Entry DOI: 10.7270/Q2BC435H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515873
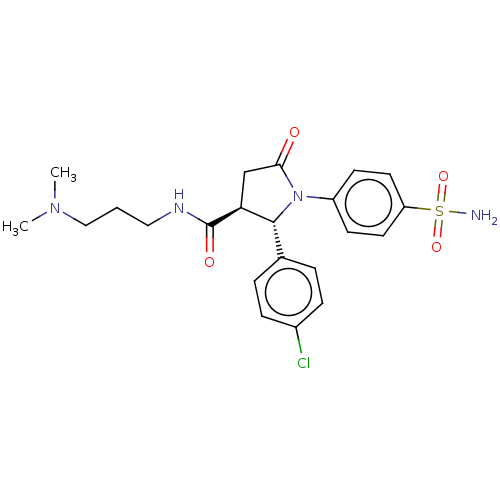
(CHEMBL4586855)Show SMILES CN(C)CCCNC(=O)[C@H]1CC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C22H27ClN4O4S/c1-26(2)13-3-12-25-22(29)19-14-20(28)27(21(19)15-4-6-16(23)7-5-15)17-8-10-18(11-9-17)32(24,30)31/h4-11,19,21H,3,12-14H2,1-2H3,(H,25,29)(H2,24,30,31)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50209283
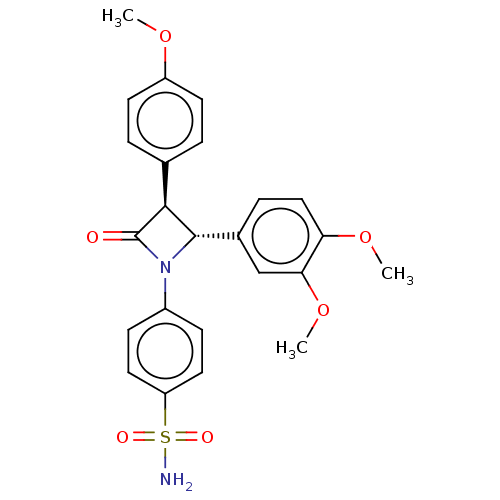
(CHEMBL3885230)Show SMILES COc1ccc(cc1)[C@H]1[C@@H](N(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C24H24N2O6S/c1-30-18-9-4-15(5-10-18)22-23(16-6-13-20(31-2)21(14-16)32-3)26(24(22)27)17-7-11-19(12-8-17)33(25,28)29/h4-14,22-23H,1-3H3,(H2,25,28,29)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay |
Bioorg Med Chem 25: 539-544 (2017)
Article DOI: 10.1016/j.bmc.2016.11.027
BindingDB Entry DOI: 10.7270/Q2G73GQQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515874

(CHEMBL4550339)Show SMILES CC(=O)OCCNC(=O)[C@H]1CC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C21H22ClN3O6S/c1-13(26)31-11-10-24-21(28)18-12-19(27)25(20(18)14-2-4-15(22)5-3-14)16-6-8-17(9-7-16)32(23,29)30/h2-9,18,20H,10-12H2,1H3,(H,24,28)(H2,23,29,30)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50087247
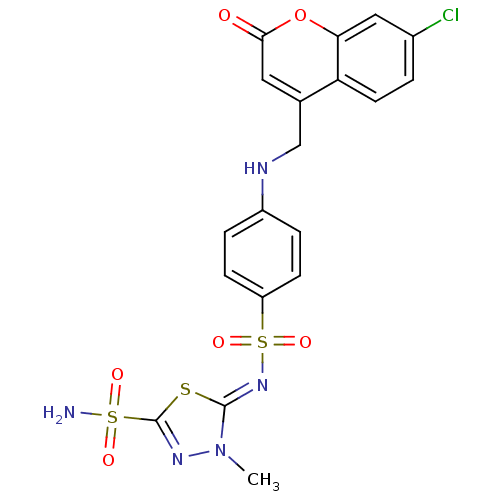
(5-{4-[(7-Chloro-2-oxo-2H-chromen-4-ylmethyl)-amino...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(NCc2cc(=O)oc3cc(Cl)ccc23)cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16ClN5O6S3/c1-25-18(32-19(23-25)33(21,27)28)24-34(29,30)14-5-3-13(4-6-14)22-10-11-8-17(26)31-16-9-12(20)2-7-15(11)16/h2-9,22H,10H2,1H3,(H2,21,27,28)/b24-18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of bovine Carbonic anhydrase IV determined by esterase method |
Bioorg Med Chem Lett 10: 673-6 (2000)
BindingDB Entry DOI: 10.7270/Q2RV0P61 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515843
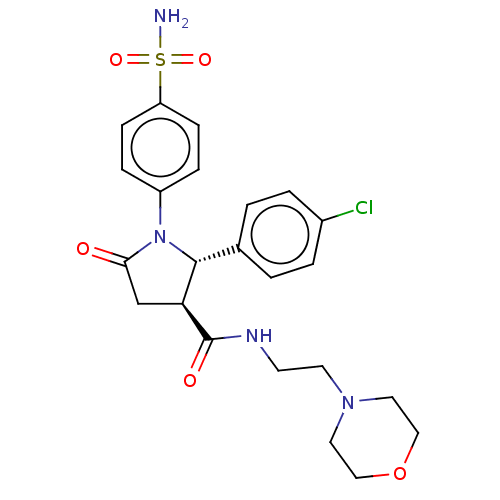
(CHEMBL4446872)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCN1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H27ClN4O5S/c24-17-3-1-16(2-4-17)22-20(23(30)26-9-10-27-11-13-33-14-12-27)15-21(29)28(22)18-5-7-19(8-6-18)34(25,31)32/h1-8,20,22H,9-15H2,(H,26,30)(H2,25,31,32)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50209288
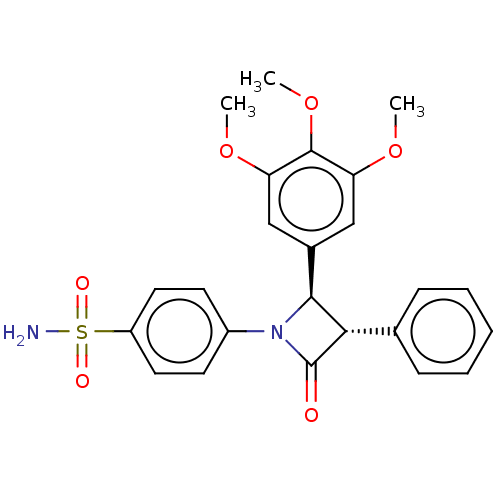
(CHEMBL3885451)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1[C@@H](C(=O)N1c1ccc(cc1)S(N)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C24H24N2O6S/c1-30-19-13-16(14-20(31-2)23(19)32-3)22-21(15-7-5-4-6-8-15)24(27)26(22)17-9-11-18(12-10-17)33(25,28)29/h4-14,21-22H,1-3H3,(H2,25,28,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay |
Bioorg Med Chem 25: 539-544 (2017)
Article DOI: 10.1016/j.bmc.2016.11.027
BindingDB Entry DOI: 10.7270/Q2G73GQQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50209225
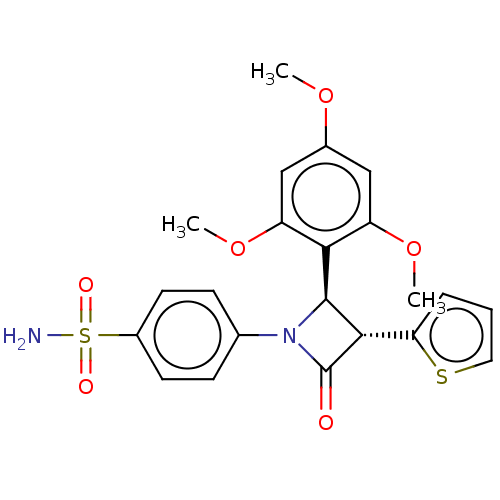
(CHEMBL3883807)Show SMILES COc1cc(OC)c([C@H]2[C@@H](C(=O)N2c2ccc(cc2)S(N)(=O)=O)c2cccs2)c(OC)c1 |r| Show InChI InChI=1S/C22H22N2O6S2/c1-28-14-11-16(29-2)19(17(12-14)30-3)21-20(18-5-4-10-31-18)22(25)24(21)13-6-8-15(9-7-13)32(23,26)27/h4-12,20-21H,1-3H3,(H2,23,26,27)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay |
Bioorg Med Chem 25: 539-544 (2017)
Article DOI: 10.1016/j.bmc.2016.11.027
BindingDB Entry DOI: 10.7270/Q2G73GQQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515878

(CHEMBL4569748)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H18ClN3O6S/c20-12-3-1-11(2-4-12)18-15(19(27)22-10-17(25)26)9-16(24)23(18)13-5-7-14(8-6-13)30(21,28)29/h1-8,15,18H,9-10H2,(H,22,27)(H,25,26)(H2,21,28,29)/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515877

(CHEMBL4462700)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCCn1ccnc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O4S/c24-17-4-2-16(3-5-17)22-20(23(31)27-10-1-12-28-13-11-26-15-28)14-21(30)29(22)18-6-8-19(9-7-18)34(25,32)33/h2-9,11,13,15,20,22H,1,10,12,14H2,(H,27,31)(H2,25,32,33)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50230205
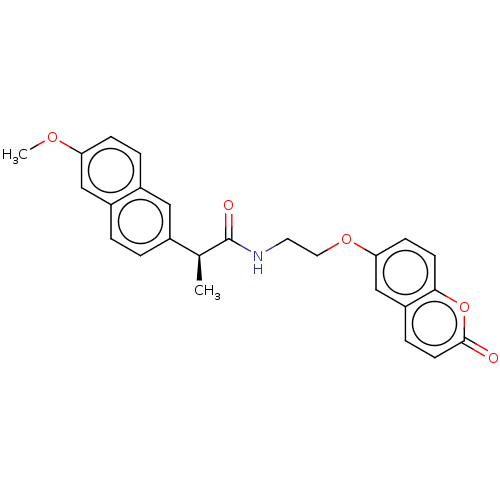
(CHEMBL4088636)Show SMILES COc1ccc2cc(ccc2c1)[C@H](C)C(=O)NCCOc1ccc2oc(=O)ccc2c1 |r| Show InChI InChI=1S/C25H23NO5/c1-16(17-3-4-19-14-21(29-2)7-5-18(19)13-17)25(28)26-11-12-30-22-8-9-23-20(15-22)6-10-24(27)31-23/h3-10,13-16H,11-12H2,1-2H3,(H,26,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA4 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 60: 1159-1170 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01607
BindingDB Entry DOI: 10.7270/Q2PC34NX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50331833
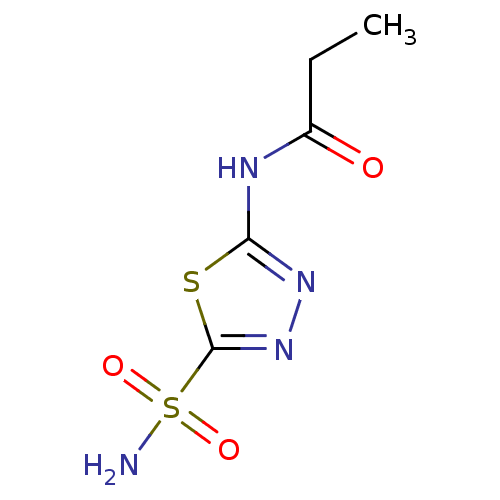
(5-propylamido-2-sulfamoyl-1,3,4-thiadiazole | CHEM...)Show InChI InChI=1S/C5H8N4O3S2/c1-2-3(10)7-4-8-9-5(13-4)14(6,11)12/h2H2,1H3,(H2,6,11,12)(H,7,8,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 expressed in Escherichia coli BL21 preincubated for 15 mins by phenol red dye-based stopped-flow CO2 hydration as... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01390
BindingDB Entry DOI: 10.7270/Q2BC435H |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50230197
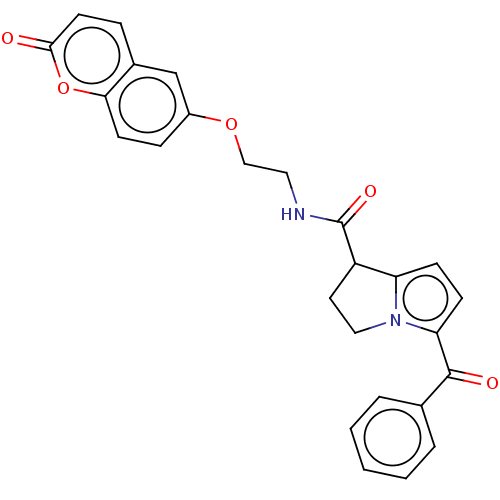
(CHEMBL4060402)Show SMILES O=C(NCCOc1ccc2oc(=O)ccc2c1)C1CCn2c1ccc2C(=O)c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c29-24-11-6-18-16-19(7-10-23(18)33-24)32-15-13-27-26(31)20-12-14-28-21(20)8-9-22(28)25(30)17-4-2-1-3-5-17/h1-11,16,20H,12-15H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA4 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 60: 1159-1170 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01607
BindingDB Entry DOI: 10.7270/Q2PC34NX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080684
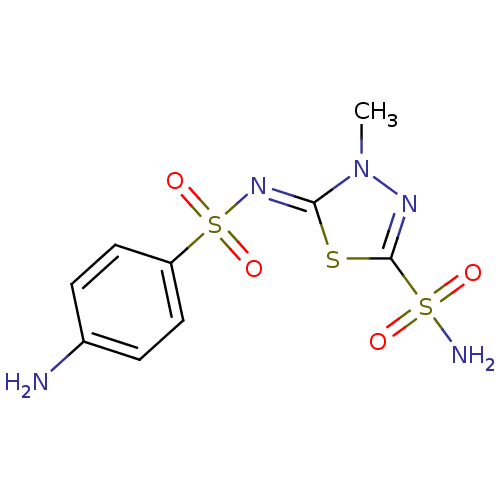
(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50114157

((R)-4-((8S,10S,12S,14R,15R,17S)-10,13-Dimethyl-3,7...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C[C@]4([H])CC(=O)CC[C@]4(C)[C@@]3([H])CC(=O)[C@]12C)[C@H](C)CCC(=O)OCCOc1ccc2nc(sc2c1)S(N)(=O)=O Show InChI InChI=1S/C33H42N2O8S2/c1-18(4-9-29(39)43-13-12-42-21-5-8-25-27(16-21)44-31(35-25)45(34,40)41)22-6-7-23-30-24(17-28(38)33(22,23)3)32(2)11-10-20(36)14-19(32)15-26(30)37/h5,8,16,18-19,22-24,30H,4,6-7,9-15,17H2,1-3H3,(H2,34,40,41)/t18-,19+,22-,23+,24+,30+,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes |
Bioorg Med Chem Lett 12: 1551-7 (2002)
BindingDB Entry DOI: 10.7270/Q2B56K7W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084563
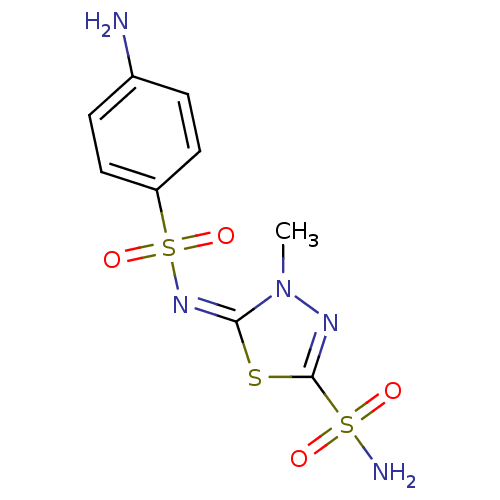
(5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 43: 292-300 (2000)
BindingDB Entry DOI: 10.7270/Q22806T7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50084563

(5-(4-Amino-benzenesulfonylimino)-4-methyl-4,5-dihy...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of bovine carbonic anhydrase IV from bovine lung microsomes |
Bioorg Med Chem Lett 10: 1117-20 (2000)
BindingDB Entry DOI: 10.7270/Q2WW7J6M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50230196
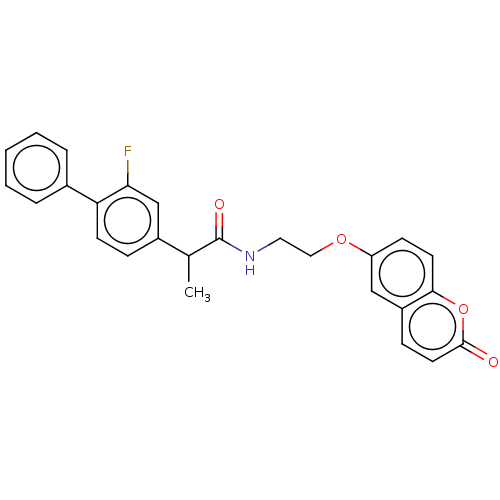
(CHEMBL4096350)Show SMILES CC(C(=O)NCCOc1ccc2oc(=O)ccc2c1)c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C26H22FNO4/c1-17(19-7-10-22(23(27)16-19)18-5-3-2-4-6-18)26(30)28-13-14-31-21-9-11-24-20(15-21)8-12-25(29)32-24/h2-12,15-17H,13-14H2,1H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CA4 preincubated for 15 mins by stopped-flow CO2 hydration assay |
J Med Chem 60: 1159-1170 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01607
BindingDB Entry DOI: 10.7270/Q2PC34NX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515846

(CHEMBL4586708)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCCN1CCCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H29ClN4O4S/c25-18-6-4-17(5-7-18)23-21(24(31)27-12-3-15-28-13-1-2-14-28)16-22(30)29(23)19-8-10-20(11-9-19)34(26,32)33/h4-11,21,23H,1-3,12-16H2,(H,27,31)(H2,26,32,33)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589822
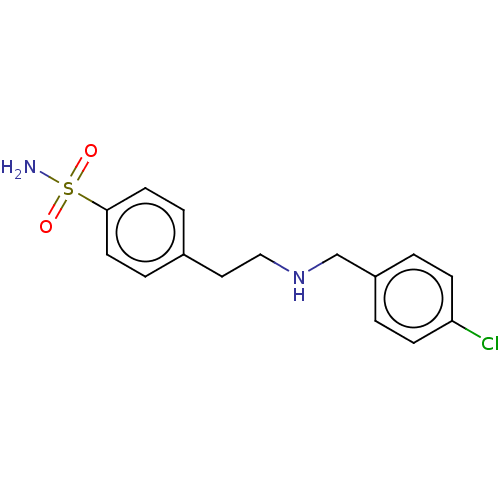
(CHEMBL5207894) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589823

(CHEMBL5199926) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589828
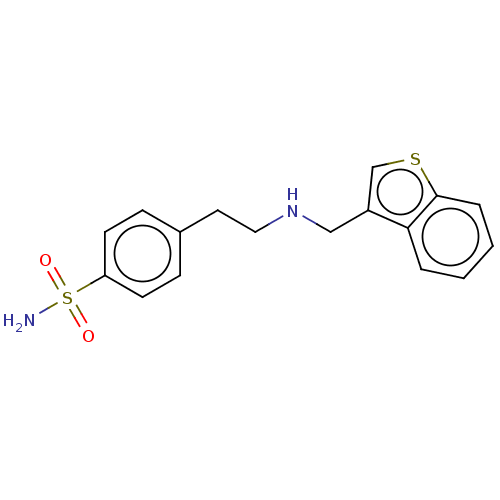
(CHEMBL5197745) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515848
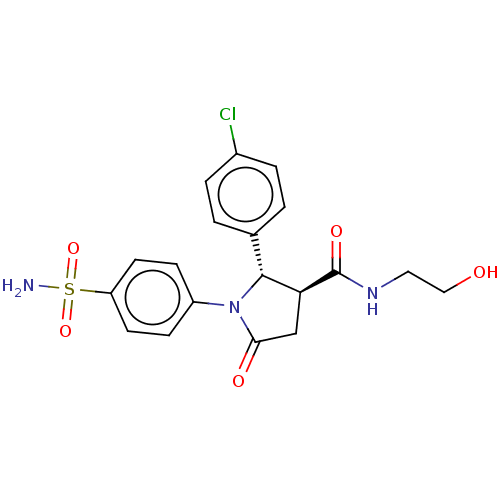
(CHEMBL4476050)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCO)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H20ClN3O5S/c20-13-3-1-12(2-4-13)18-16(19(26)22-9-10-24)11-17(25)23(18)14-5-7-15(8-6-14)29(21,27)28/h1-8,16,18,24H,9-11H2,(H,22,26)(H2,21,27,28)/t16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589824
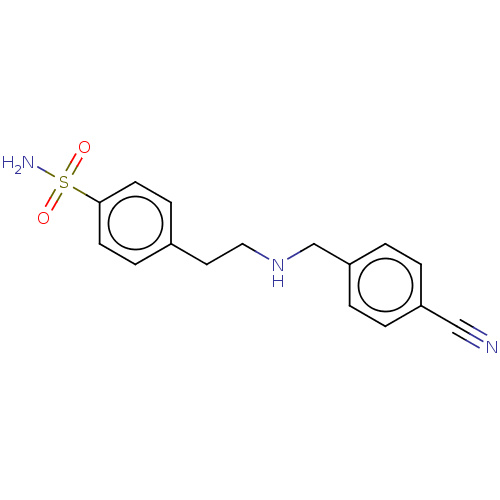
(CHEMBL5177103) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50082759
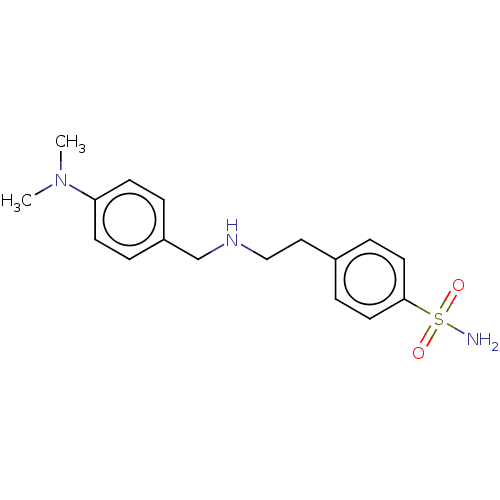
(CHEMBL3423268)Show InChI InChI=1S/C17H23N3O2S/c1-20(2)16-7-3-15(4-8-16)13-19-12-11-14-5-9-17(10-6-14)23(18,21)22/h3-10,19H,11-13H2,1-2H3,(H2,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589825

(CHEMBL5193273)Show SMILES COc1cc(ccc1CNCCc1ccc(cc1)S(N)(=O)=O)[N+]([O-])=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50082763
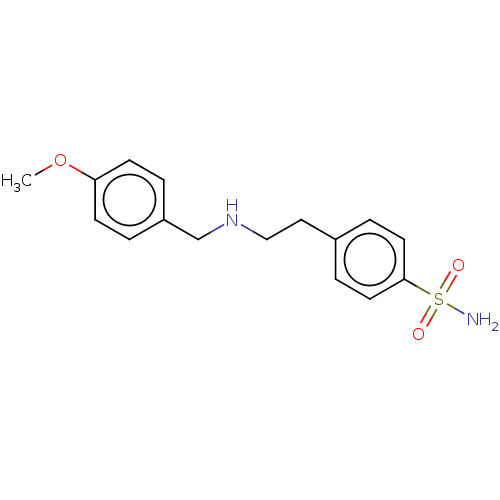
(CHEMBL3423262)Show InChI InChI=1S/C16H20N2O3S/c1-21-15-6-2-14(3-7-15)12-18-11-10-13-4-8-16(9-5-13)22(17,19)20/h2-9,18H,10-12H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589826
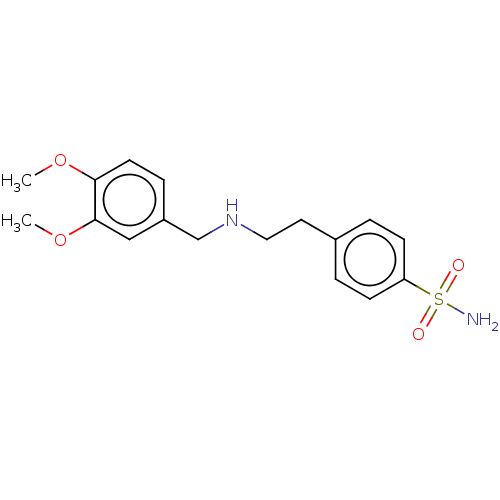
(CHEMBL5183301) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50589827
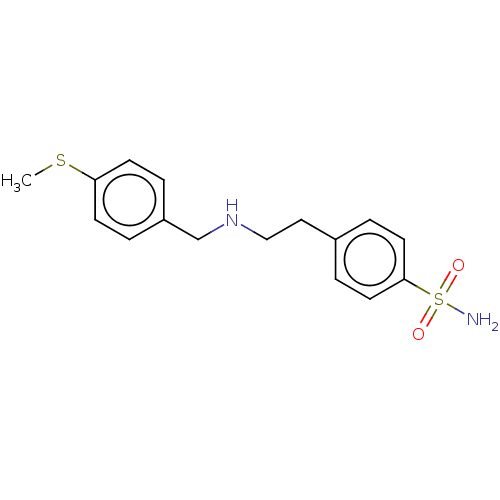
(CHEMBL5192003) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50004997

(CHEMBL2392427)Show InChI InChI=1S/C19H20N2O2S/c20-24(22,23)18-10-8-15(9-11-18)12-13-21-14-17-6-3-5-16-4-1-2-7-19(16)17/h1-11,21H,12-14H2,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00982
BindingDB Entry DOI: 10.7270/Q2DN491K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50209282
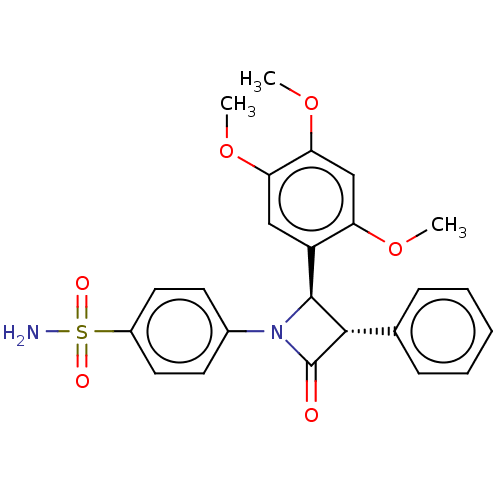
(CHEMBL3883491)Show SMILES COc1cc(OC)c(cc1OC)[C@H]1[C@@H](C(=O)N1c1ccc(cc1)S(N)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C24H24N2O6S/c1-30-19-14-21(32-3)20(31-2)13-18(19)23-22(15-7-5-4-6-8-15)24(27)26(23)16-9-11-17(12-10-16)33(25,28)29/h4-14,22-23H,1-3H3,(H2,25,28,29)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay |
Bioorg Med Chem 25: 539-544 (2017)
Article DOI: 10.1016/j.bmc.2016.11.027
BindingDB Entry DOI: 10.7270/Q2G73GQQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515866

(CHEMBL4593200)Show SMILES COC(=O)CCNC(=O)[C@H]1CC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C21H22ClN3O6S/c1-31-19(27)10-11-24-21(28)17-12-18(26)25(20(17)13-2-4-14(22)5-3-13)15-6-8-16(9-7-15)32(23,29)30/h2-9,17,20H,10-12H2,1H3,(H,24,28)(H2,23,29,30)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50114120
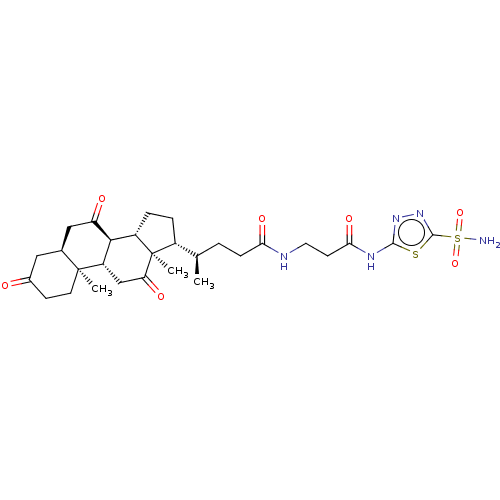
((R)-4-((2R,6S,7S,8S,10R,12S)-10,13-Dimethyl-3,7,12...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(=O)C[C@]4([H])CC(=O)CC[C@]4(C)[C@@]3([H])CC(=O)[C@]12C)[C@H](C)CCC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C29H41N5O7S2/c1-15(4-7-23(38)31-11-9-24(39)32-26-33-34-27(42-26)43(30,40)41)18-5-6-19-25-20(14-22(37)29(18,19)3)28(2)10-8-17(35)12-16(28)13-21(25)36/h15-16,18-20,25H,4-14H2,1-3H3,(H,31,38)(H2,30,40,41)(H,32,33,39)/t15-,16+,18-,19+,20+,25+,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes |
Bioorg Med Chem Lett 12: 1551-7 (2002)
BindingDB Entry DOI: 10.7270/Q2B56K7W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50114129
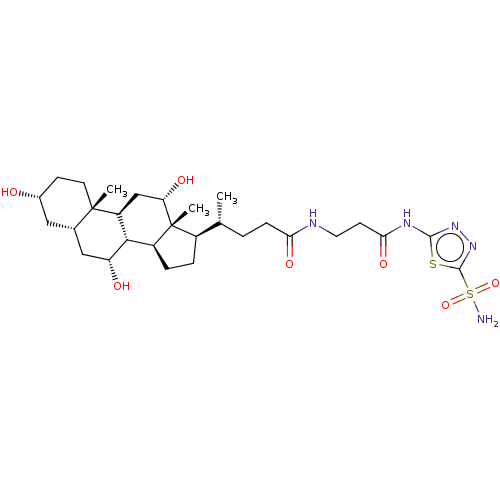
(4-((6R,7S,8S,10R,11S,12S,14S,15R)-3,12-Di(R)-hydro...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C29H47N5O7S2/c1-15(4-7-23(38)31-11-9-24(39)32-26-33-34-27(42-26)43(30,40)41)18-5-6-19-25-20(14-22(37)29(18,19)3)28(2)10-8-17(35)12-16(28)13-21(25)36/h15-22,25,35-37H,4-14H2,1-3H3,(H,31,38)(H2,30,40,41)(H,32,33,39)/t15-,16+,17-,18-,19+,20+,21-,22+,25+,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes |
Bioorg Med Chem Lett 12: 1551-7 (2002)
BindingDB Entry DOI: 10.7270/Q2B56K7W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515865
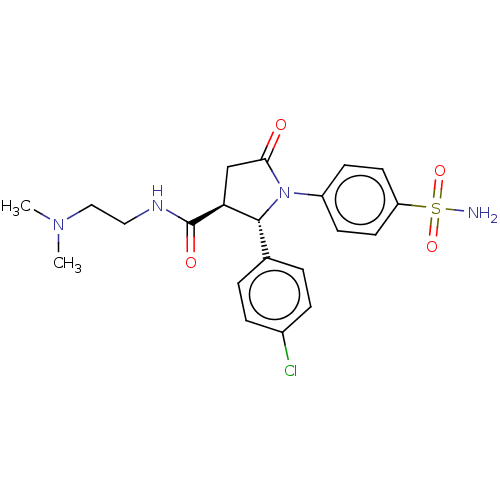
(CHEMBL4440330)Show SMILES CN(C)CCNC(=O)[C@H]1CC(=O)N([C@@H]1c1ccc(Cl)cc1)c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C21H25ClN4O4S/c1-25(2)12-11-24-21(28)18-13-19(27)26(20(18)14-3-5-15(22)6-4-14)16-7-9-17(10-8-16)31(23,29)30/h3-10,18,20H,11-13H2,1-2H3,(H,24,28)(H2,23,29,30)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50114147
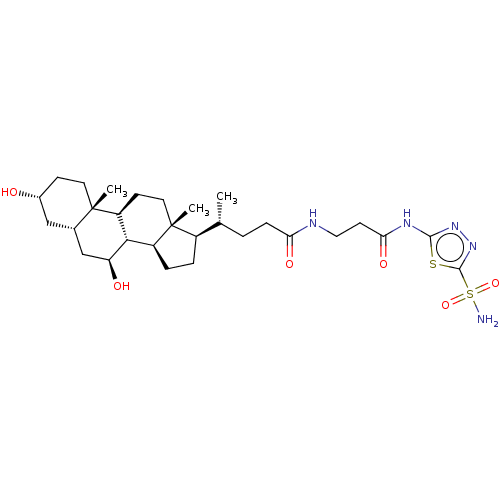
((R)-4-((5S,6R,8S,9S,12S,14R,17S)-7-Hydroxy-3-(R)-h...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C29H47N5O6S2/c1-16(4-7-23(37)31-13-10-24(38)32-26-33-34-27(41-26)42(30,39)40)19-5-6-20-25-21(9-12-29(19,20)3)28(2)11-8-18(35)14-17(28)15-22(25)36/h16-22,25,35-36H,4-15H2,1-3H3,(H,31,37)(H2,30,39,40)(H,32,33,38)/t16-,17+,18-,19-,20+,21+,22+,25+,28+,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), obtained from bovine lung microsomes |
Bioorg Med Chem Lett 12: 1551-7 (2002)
BindingDB Entry DOI: 10.7270/Q2B56K7W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50088778
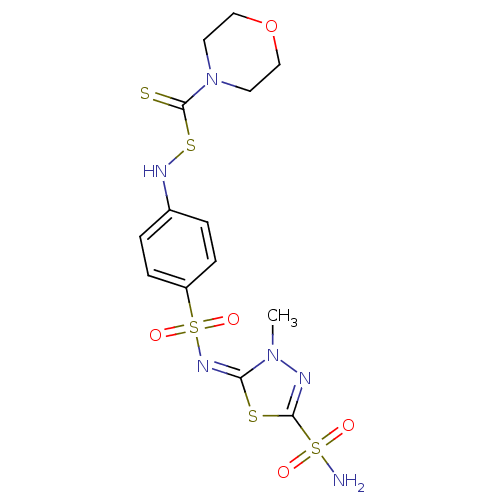
(CHEMBL275565 | N,N-dialkylthio-carbonylsulfenylami...)Show SMILES Cn1nc(s\c1=N\S(=O)(=O)c1ccc(NSC(=S)N2CCOCC2)cc1)S(N)(=O)=O Show InChI InChI=1S/C14H18N6O5S5/c1-19-12(27-13(16-19)29(15,21)22)18-30(23,24)11-4-2-10(3-5-11)17-28-14(26)20-6-8-25-9-7-20/h2-5,17H,6-9H2,1H3,(H2,15,21,22)/b18-12+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibition of bovine carbonic anhydrase IV from bovine lung microsomes |
Bioorg Med Chem Lett 10: 1117-20 (2000)
BindingDB Entry DOI: 10.7270/Q2WW7J6M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515867
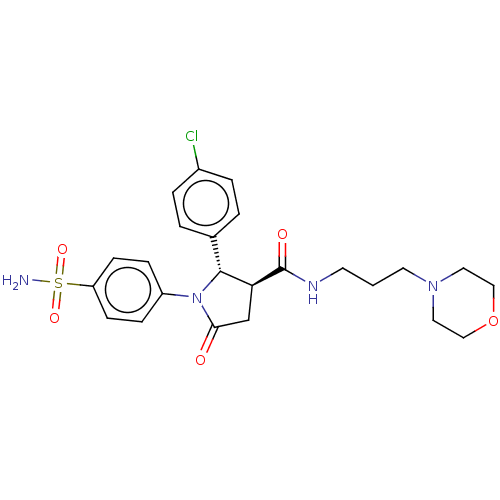
(CHEMBL4475435)Show SMILES NS(=O)(=O)c1ccc(cc1)N1[C@@H]([C@H](CC1=O)C(=O)NCCCN1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C24H29ClN4O5S/c25-18-4-2-17(3-5-18)23-21(24(31)27-10-1-11-28-12-14-34-15-13-28)16-22(30)29(23)19-6-8-20(9-7-19)35(26,32)33/h2-9,21,23H,1,10-16H2,(H,27,31)(H2,26,32,33)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Petersburg State University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111642
BindingDB Entry DOI: 10.7270/Q2C250SX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515559
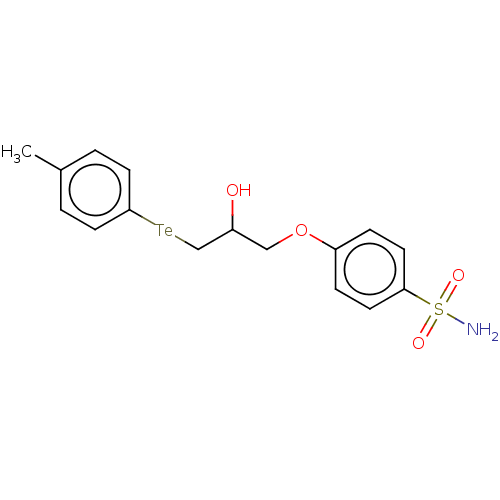
(CHEMBL4435078)Show SMILES [#6]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO4STe/c1-12-2-8-16(9-3-12)23-11-13(18)10-21-14-4-6-15(7-5-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50369931
(CHEMBL4167555)Show SMILES CN1CCN(C[C@H]1Cc1ccccc1)C(=O)c1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C19H23N3O3S/c1-21-11-12-22(14-17(21)13-15-5-3-2-4-6-15)19(23)16-7-9-18(10-8-16)26(20,24)25/h2-10,17H,11-14H2,1H3,(H2,20,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human membrane-anchored carbonic anhydrase 4 assessed as inhibition of CO2 hydration preincubated for 15 mins prior to testing by pheno... |
Eur J Med Chem 151: 363-375 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.002
BindingDB Entry DOI: 10.7270/Q25H7JTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM11631
(2-N-(4-amino-3,5-dibromobenzene)-1,3,4-thiadiazole...)Show SMILES Nc1c(Br)cc(cc1Br)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C8H7Br2N5O4S3/c9-4-1-3(2-5(10)6(4)11)22(18,19)15-7-13-14-8(20-7)21(12,16)17/h1-2H,11H2,(H,13,15)(H2,12,16,17) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Agricultural Sciences and Veterinary Medicine
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 46: 2187-96 (2003)
Article DOI: 10.1021/jm021123s
BindingDB Entry DOI: 10.7270/Q2XK8CR9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50209290
(CHEMBL3883755)Show SMILES COc1cc(OC)c([C@H]2[C@@H](C(=O)N2c2ccc(cc2)S(N)(=O)=O)c2ccccc2)c(OC)c1 |r| Show InChI InChI=1S/C24H24N2O6S/c1-30-17-13-19(31-2)22(20(14-17)32-3)23-21(15-7-5-4-6-8-15)24(27)26(23)16-9-11-18(12-10-16)33(25,28)29/h4-14,21,23H,1-3H3,(H2,25,28,29)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carbonic anhydrase-4 using CO2 as substrate preincubated for 10 mins by stopped-flow CO2 hydrase assay |
Bioorg Med Chem 25: 539-544 (2017)
Article DOI: 10.1016/j.bmc.2016.11.027
BindingDB Entry DOI: 10.7270/Q2G73GQQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50511590
(CHEMBL4587627)Show SMILES [#6]-c1ccccc1[Te;v2][#6]-c1ccc(cc1)S([#7])(=O)=O Show InChI InChI=1S/C14H15NO2STe/c1-11-4-2-3-5-14(11)19-10-12-6-8-13(9-7-12)18(15,16)17/h2-9H,10H2,1H3,(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA4 preincubated for 15 mins by measured for 10 to 100 secs by phenol red dye based stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128147
BindingDB Entry DOI: 10.7270/Q27S7SJ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data