

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 2 (GUINEA PIG) | BDBM50292408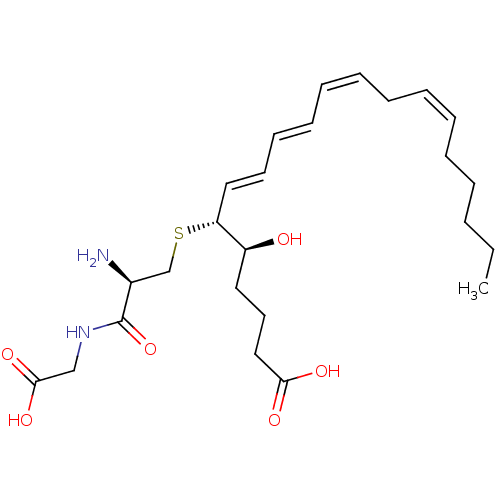 ((R-(R*,S*-(E,E,Z,Z)))-N-(S-(1-(4-Carboxy-1-hydroxy...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 258: 531-6 (1991) BindingDB Entry DOI: 10.7270/Q2BK19TF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359757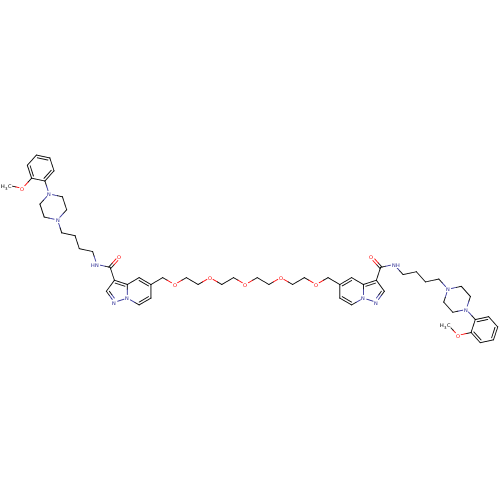 (CHEMBL1928139) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359763 (CHEMBL1928122) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359755 (CHEMBL1928137) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359742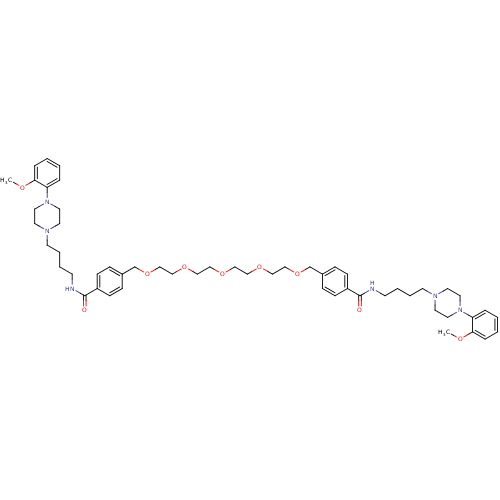 (CHEMBL1928126) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359751 (CHEMBL1928134) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359753 (CHEMBL1928136) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359762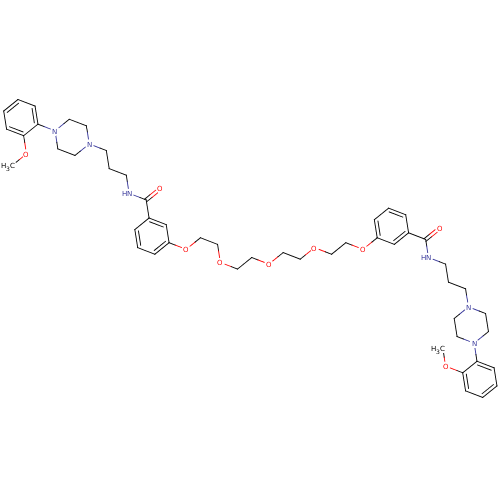 (CHEMBL1928121) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359743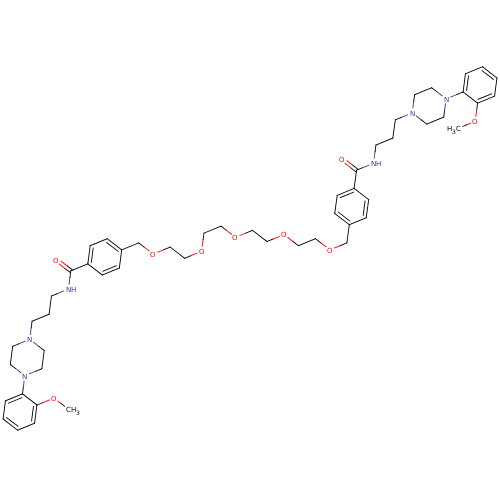 (CHEMBL1928125) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359745 (CHEMBL1928128) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258678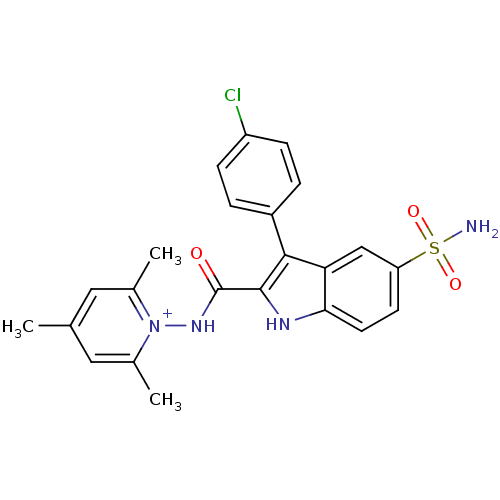 (1-(3-(4-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359756 (CHEMBL1928138) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359749 (CHEMBL1928132) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081194 (CHEMBL3422009 | US10544150, Compound 156) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359754 (CHEMBL1926700) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359744 (CHEMBL1928127) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359747 (CHEMBL1928130) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM54376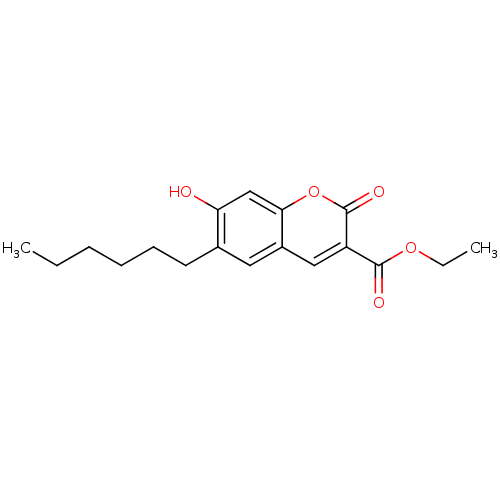 (6-Hexyl-7-hydroxy-2-oxo-2H-chromene-3-carboxylic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50146756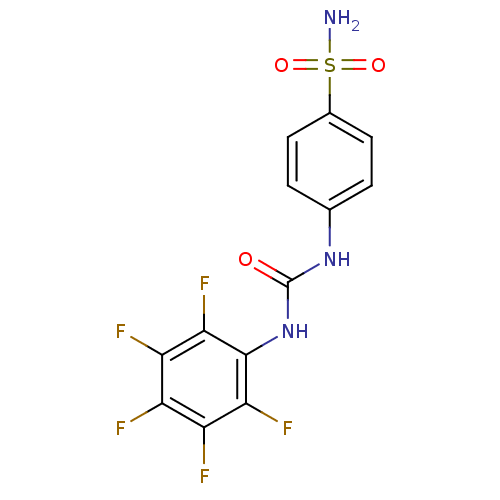 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258716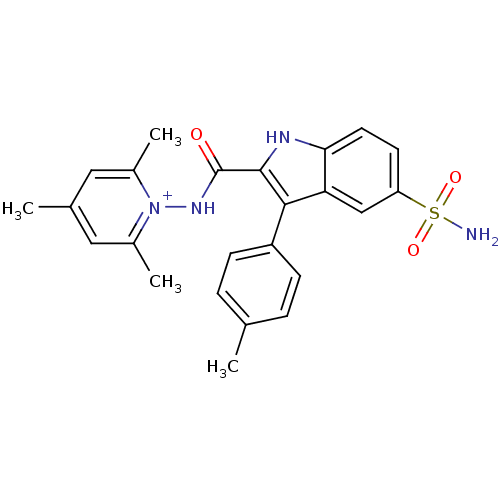 (1-({[5-(Aminosulfonyl)-3-(4-bromophenyl)-1H-indol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081196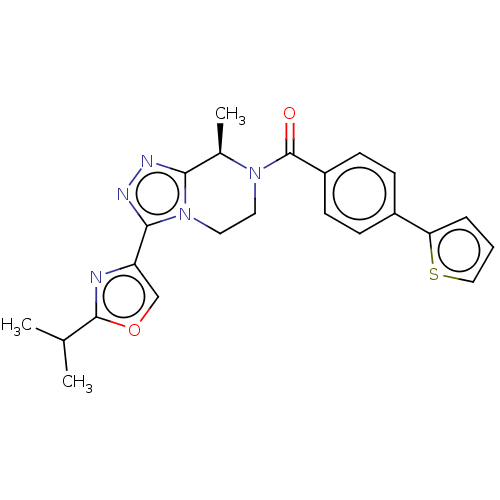 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081198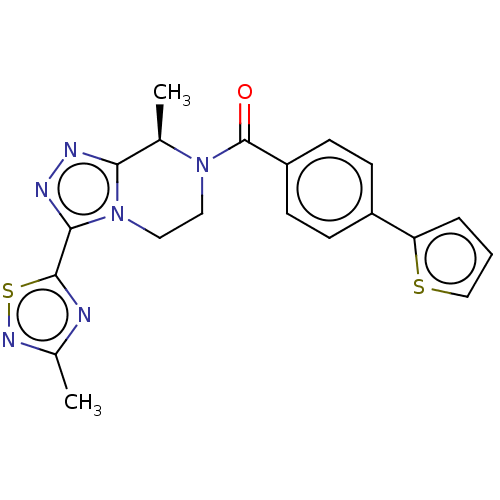 (CHEMBL3422018 | US10065961, Compound 24 | US947581...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50334354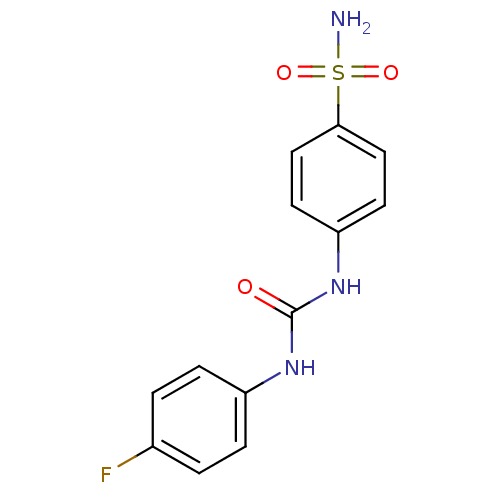 (4-(3-(4-fluorophenyl)ureido)benzenesulfonamide | 4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359746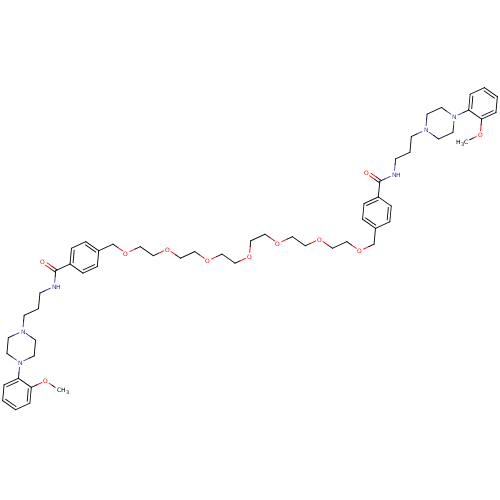 (CHEMBL1928129) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50334356 (4-(3-(4-iodophenyl)ureido)benzenesulfonamide | 4-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 2 (GUINEA PIG) | BDBM50009075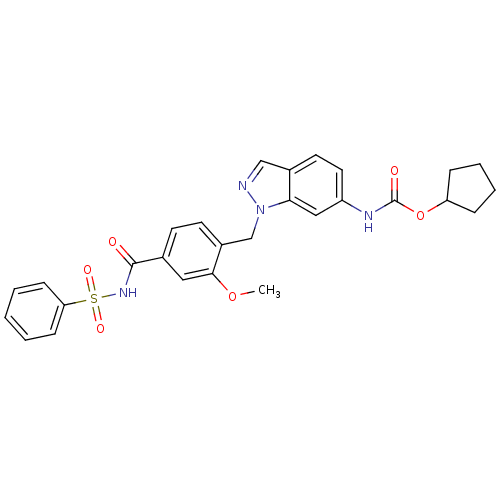 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 6.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 258: 531-6 (1991) BindingDB Entry DOI: 10.7270/Q2BK19TF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359761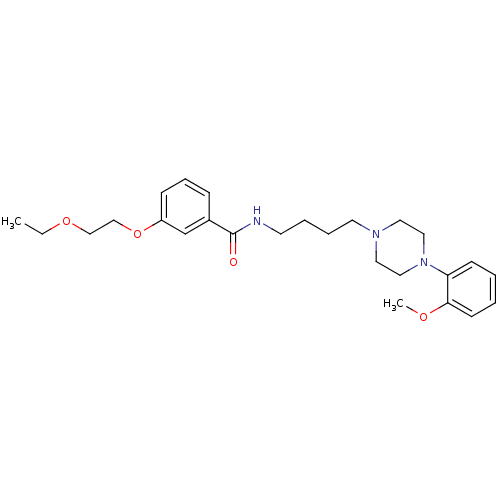 (CHEMBL1928120) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258792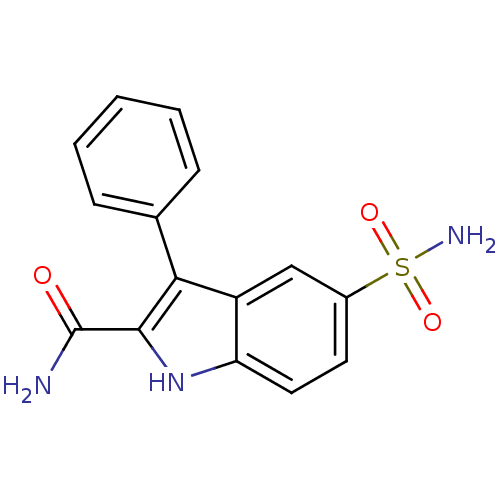 (3-phenyl-5-sulfamoyl-1H-indole-2-carboxamide | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50334355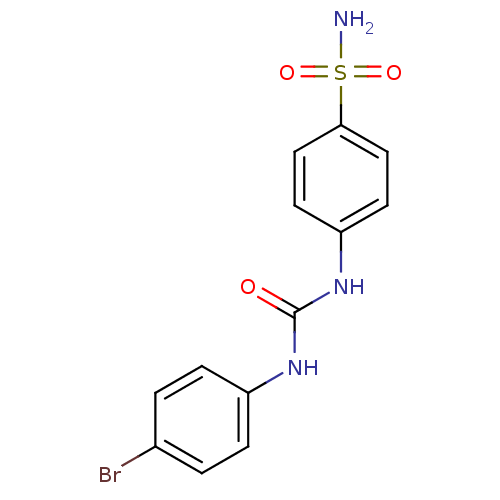 (4-(3-(4-bromophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258635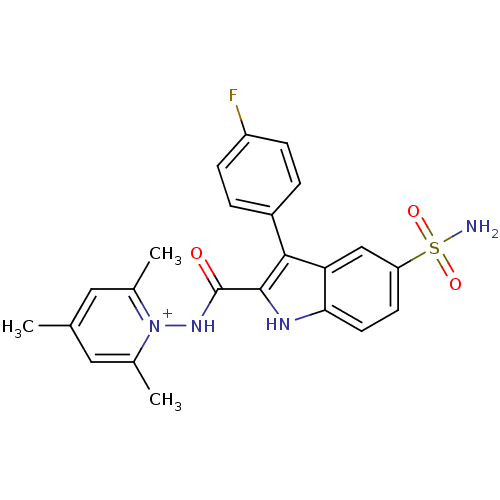 (1-(3-(4-fluorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258588 (1-(3-(2-fluorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258298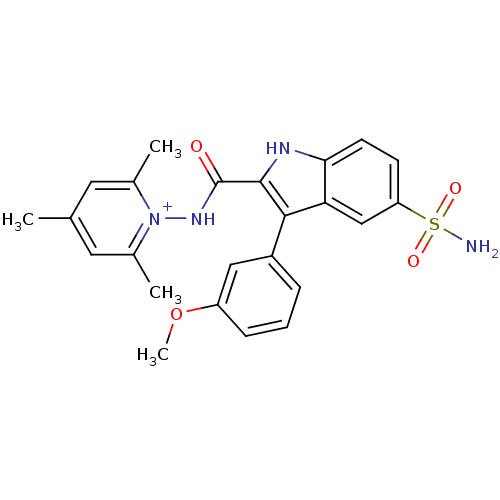 (1-(3-(3-methoxyphenyl)-5-sulfamoyl-1H-indole-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359748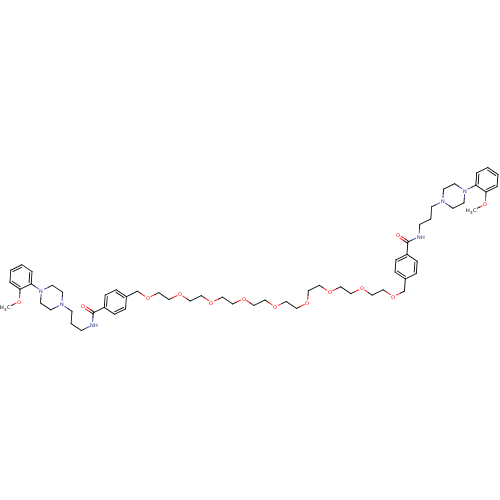 (CHEMBL1928131) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258587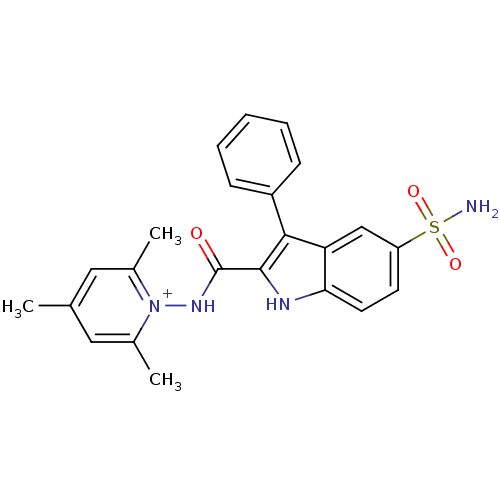 (1-(([5-(Aminosulfonyl)-3-phenyl-1H-indol-2-yl]carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359765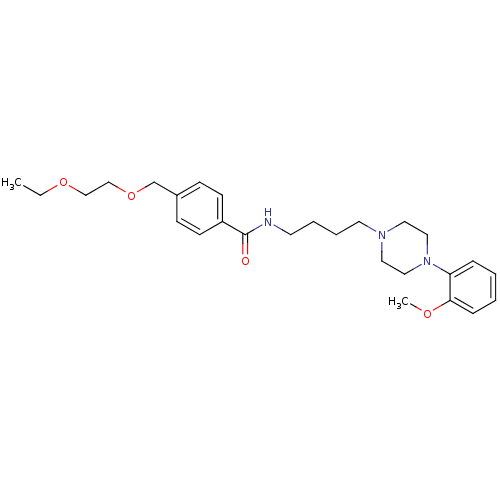 (CHEMBL1928124) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359750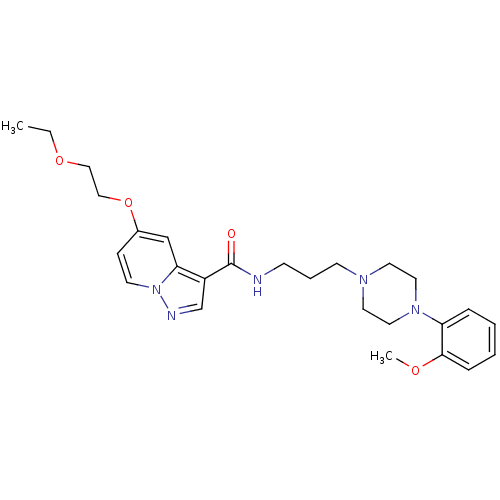 (CHEMBL1928133) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258299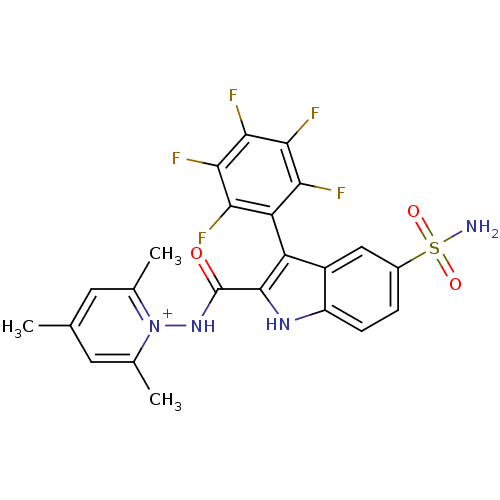 (1-({[5-(Aminosulfonyl)-3-(2,3,4,5,6-pentafluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258714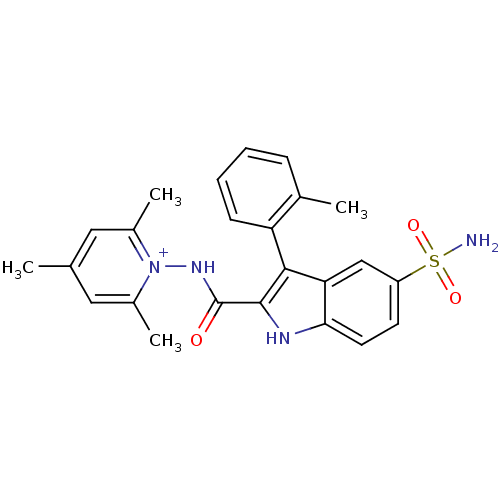 (1-({[5-(Aminosulfonyl)-3-(2-bromophenyl)-1H-indol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2 (Bos taurus) | BDBM50438367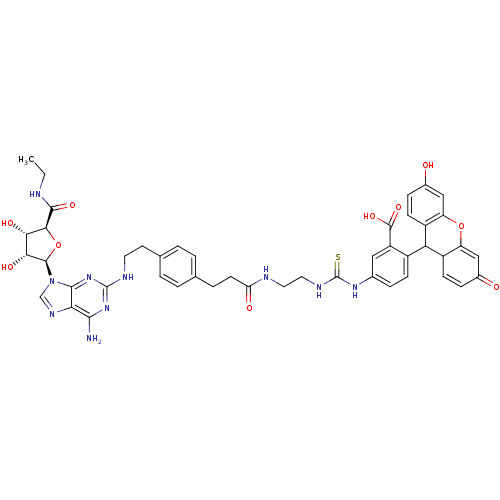 (CHEMBL2413102) | PDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from adenosine A2A receptor in bovine striatal membranes after 90 mins by liquid scintillation counting analysis | Bioorg Med Chem Lett 23: 26-36 (2012) Article DOI: 10.1016/j.bmcl.2012.10.112 BindingDB Entry DOI: 10.7270/Q2XK8H03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258634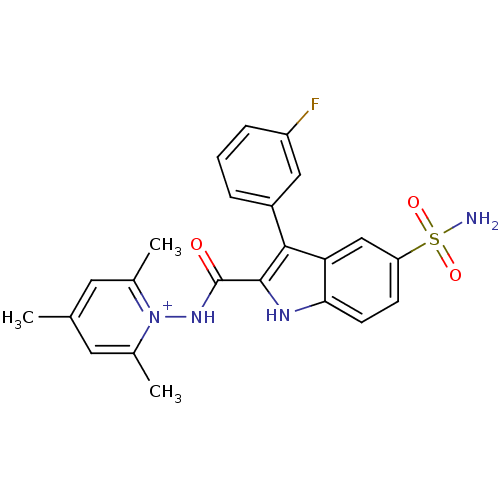 (1-(3-(3-fluorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258681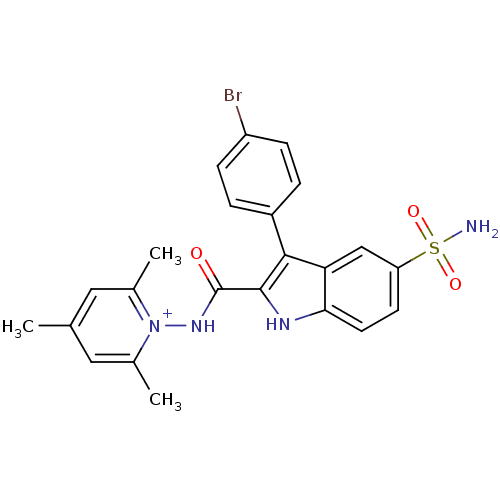 (1-(3-(4-bromophenyl)-5-sulfamoyl-1H-indole-2-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359760 (CHEMBL1928119) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359764 (CHEMBL1928123) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Sus scrofa) | BDBM50359752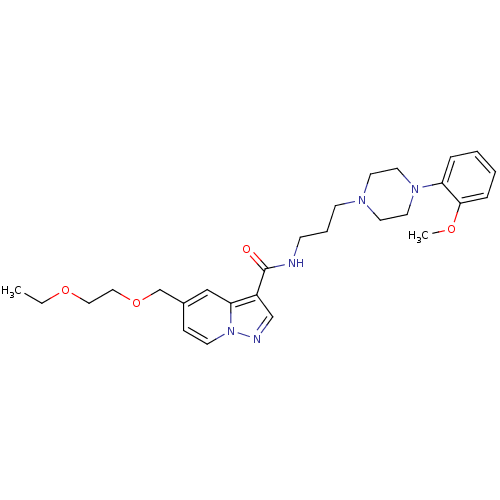 (CHEMBL1928135) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from pig adrenergic alpha1 receptor in cerebral cortex homogenate | Bioorg Med Chem 20: 455-66 (2011) Article DOI: 10.1016/j.bmc.2011.10.063 BindingDB Entry DOI: 10.7270/Q2R211TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081195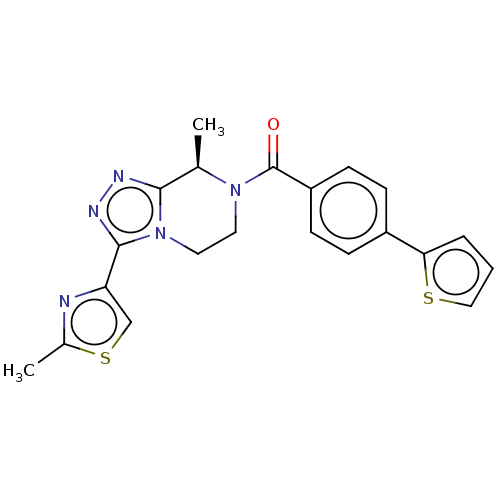 (CHEMBL3422010 | US10065961, Compound 1 | US1068329...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081197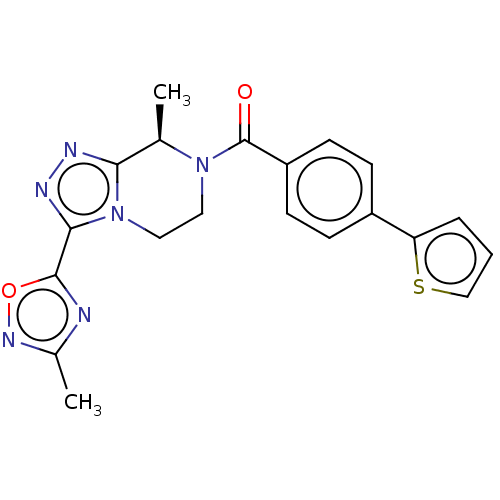 (CHEMBL3422017 | US10065961, Compound 23 | US106832...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258636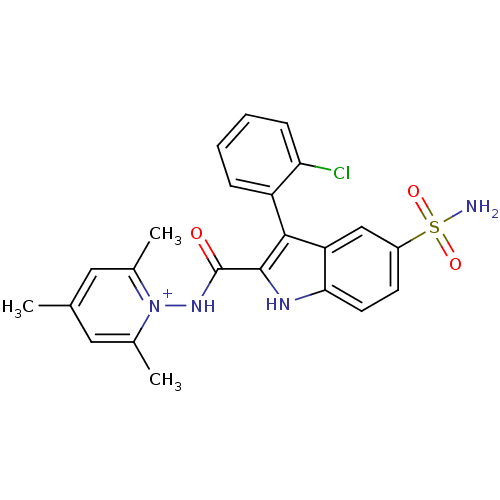 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50258680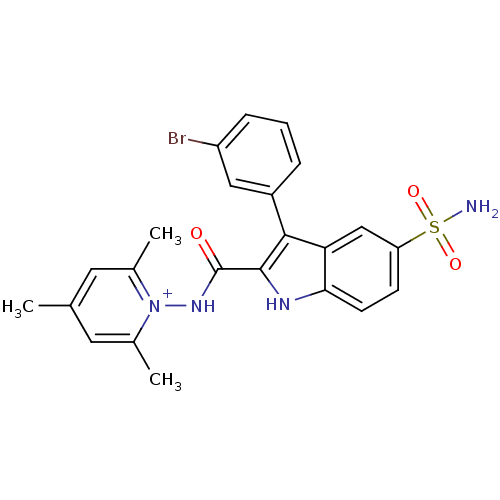 (1-(3-(3-bromophenyl)-5-sulfamoyl-1H-indole-2-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-carbonic anhydrase 1 (Mycobacterium tuberculosis) | BDBM50334364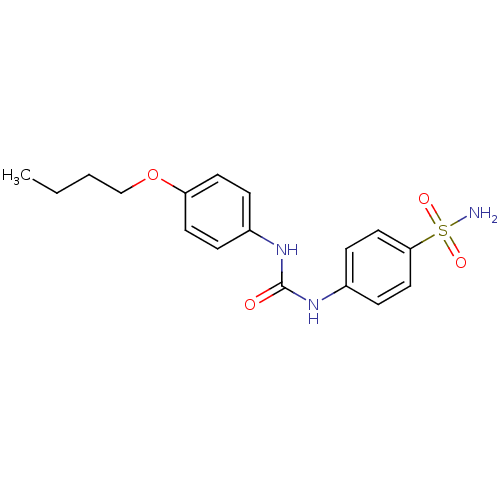 (4-(3-(4-butoxyphenyl)ureido)benzenesulfonamide | 4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant beta-carbonic anhydrase Rv1284 preincubated for 15 mins by stopped-flow CO2 hydrase assay | Bioorg Med Chem Lett 21: 102-5 (2010) Article DOI: 10.1016/j.bmcl.2010.11.064 BindingDB Entry DOI: 10.7270/Q2WS8TH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 353 total ) | Next | Last >> |