

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108110 (US8598206, Table 6, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human Complement C1r subcomponent using Val-Ser-Arg-pNA as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human Complement C1r subcomponent using Val-Ser-Arg-pNA as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50369774 (SEPIMOSTAT MESYLATE | Sepimostat) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Binding affinity against factor C1r. | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108106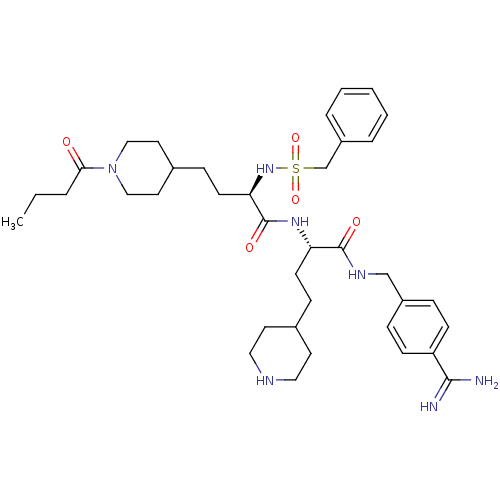 (US8598206, Table 6, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108095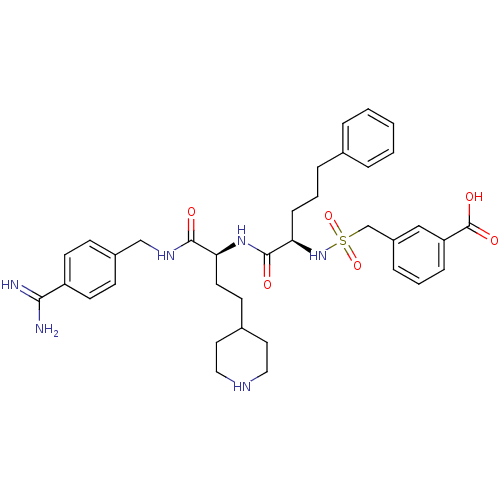 (US8598206, Table 6, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108105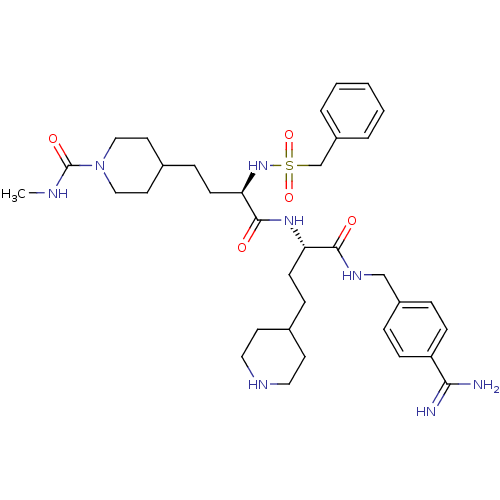 (US8598206, Table 6, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108107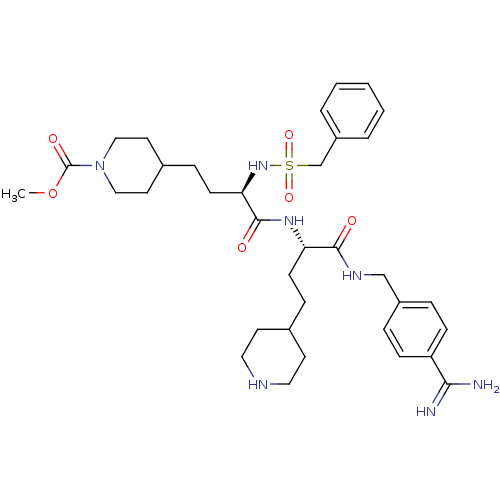 (US8598206, Table 6, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM47178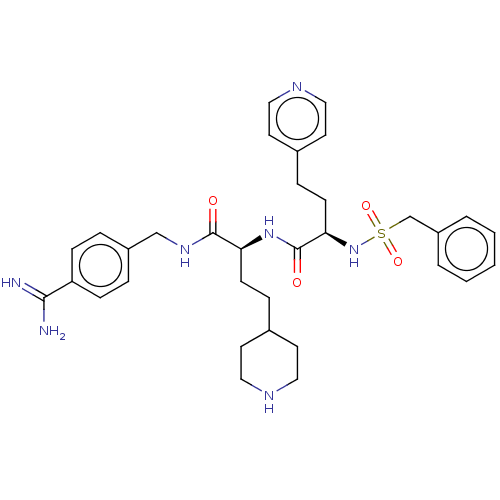 (BDBM108103 | US8598206, Table 6, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108116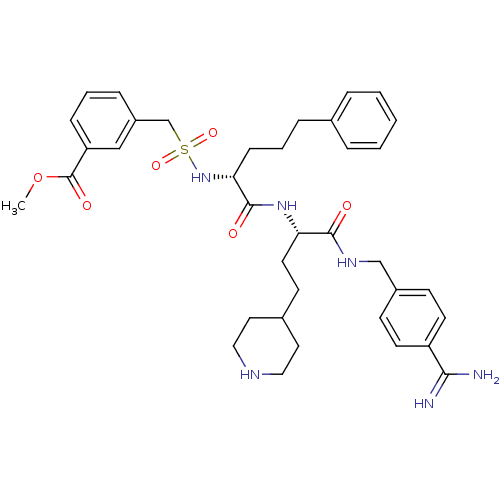 (US8598206, Table 6, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108108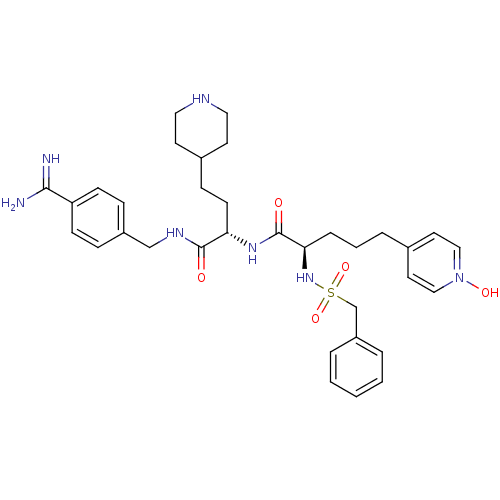 (US8598206, 117 | US8598206, 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108109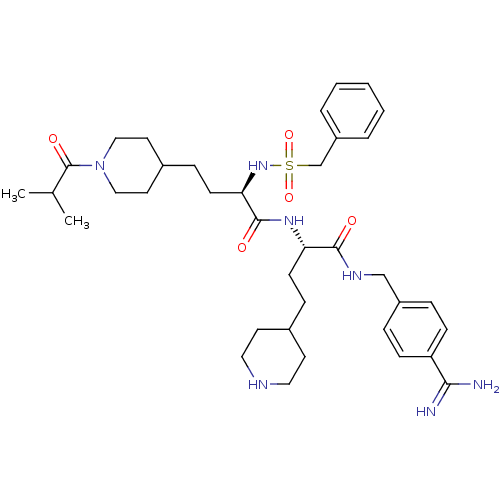 (US8598206, 118 | US8598206, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108109 (US8598206, 118 | US8598206, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108108 (US8598206, 117 | US8598206, 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108099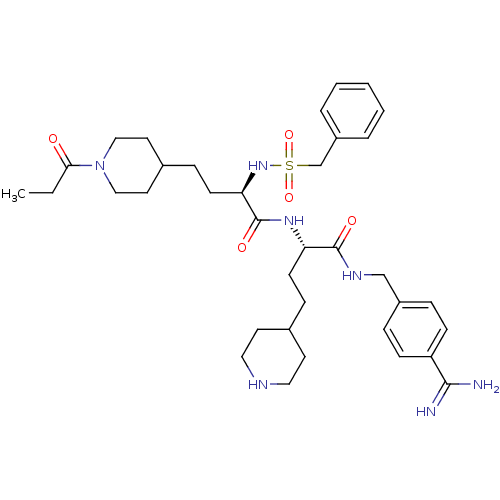 (US8598206, Table 6, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50425648 (CHEMBL2315243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human C1r | J Med Chem 56: 820-31 (2013) Article DOI: 10.1021/jm3012917 BindingDB Entry DOI: 10.7270/Q2RB75W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108096 (US8598206, Table 6, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108117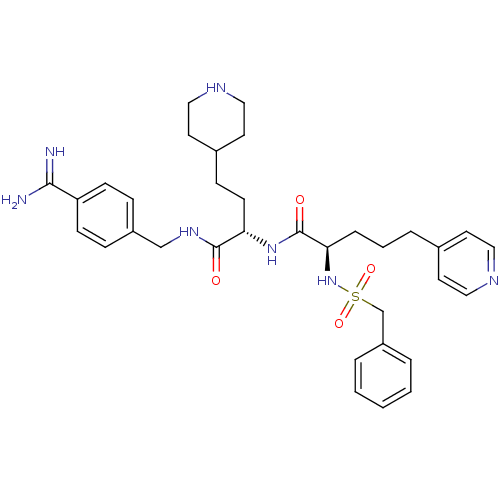 (US8598206, Table 6, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108104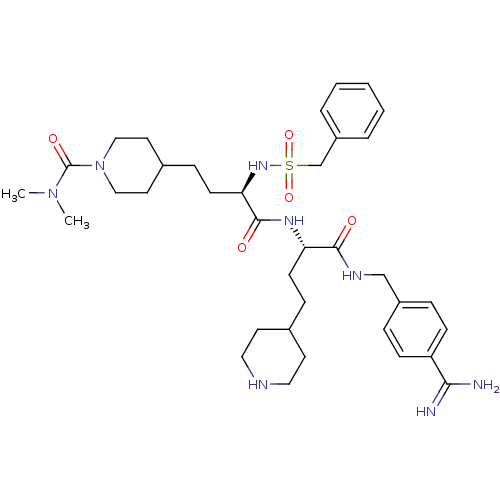 (US8598206, Table 6, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108112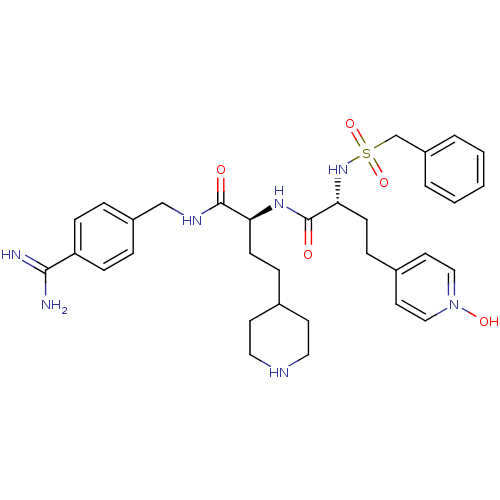 (US8598206, Table 6, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50425649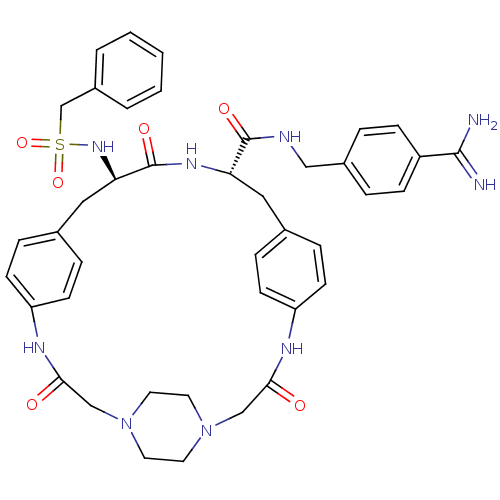 (CHEMBL2315239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human C1r | J Med Chem 56: 820-31 (2013) Article DOI: 10.1021/jm3012917 BindingDB Entry DOI: 10.7270/Q2RB75W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108102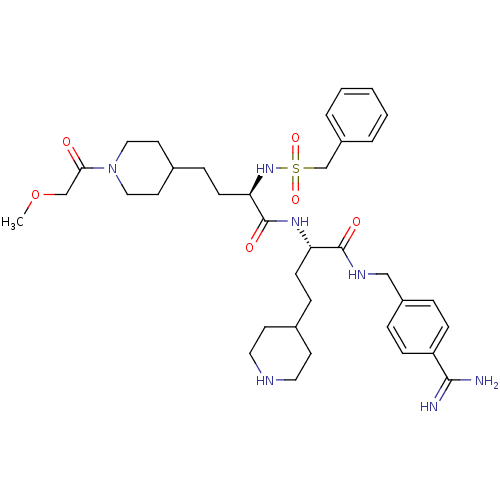 (US8598206, Table 6, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108097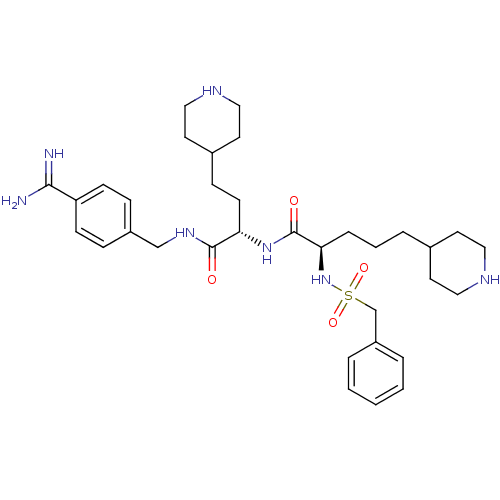 (US8598206, Table 6, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM47177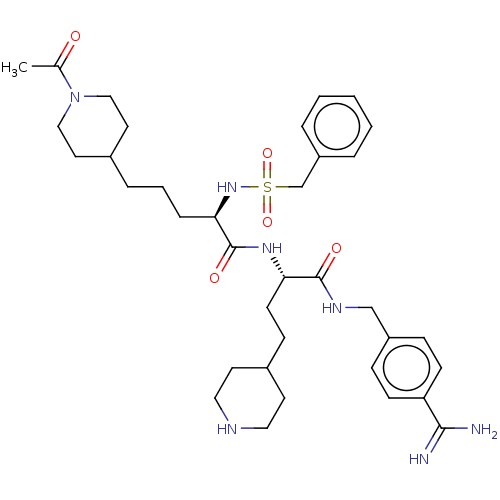 (BDBM108100 | US8598206, Table 6, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108101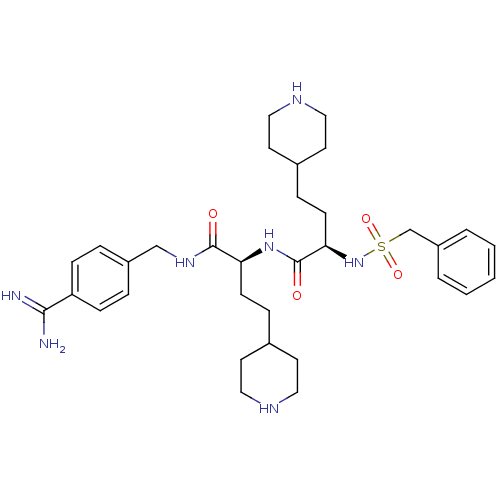 (US8598206, Table 6, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM108115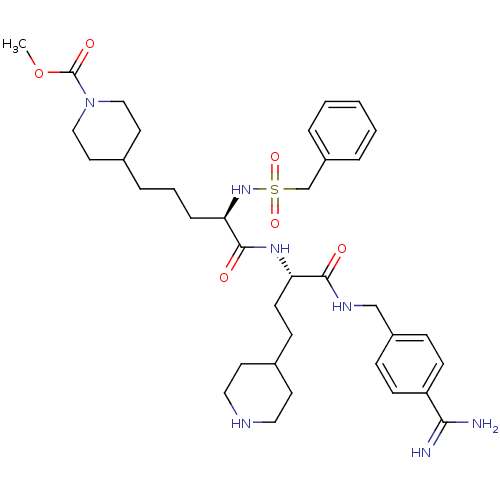 (US8598206, Table 6, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Medicines Company (Leipzig) GmbH US Patent | Assay Description Inhibition of human C1r was determined by the method described in [0092]-[0098] using native human activated C1r complement component from Calbiochem... | US Patent US8598206 (2013) BindingDB Entry DOI: 10.7270/Q25T3J5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063701 (2-(2-Iodo-phenylamino)-naphtho[2,3-d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063745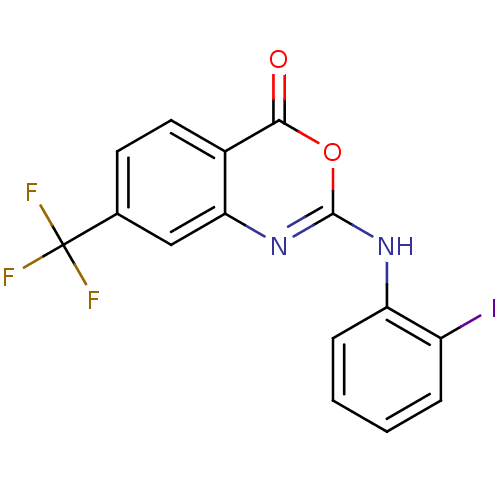 (2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063726 (7-Chloro-2-(2,6-dichloro-phenylamino)-benzo[d][1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063745 (2-(2-Iodo-phenylamino)-7-trifluoromethyl-benzo[d][...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for inhibitory activity against purified human C1r protease protease | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063722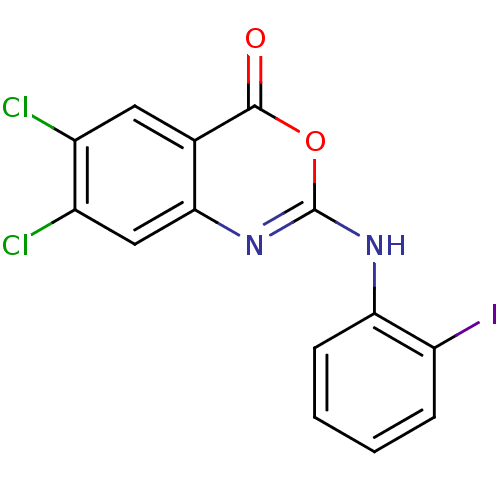 (6,7-Dichloro-2-(2-iodo-phenylamino)-benzo[d][1,3]o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50462100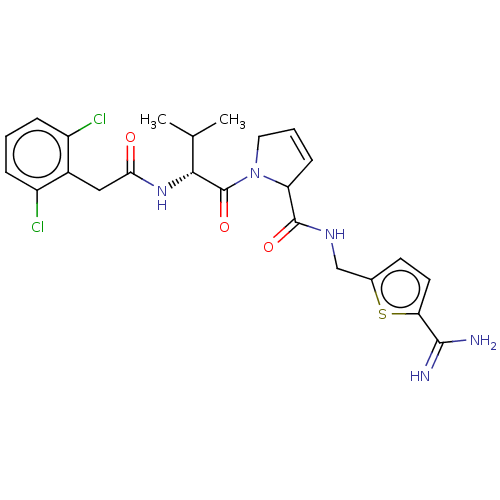 (CHEMBL4247936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasma C1r using Cbz-Gly-Arg-S-Bzl as substrate preincubated for 10 mins followed by substrate addition and measured after 30 min... | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063711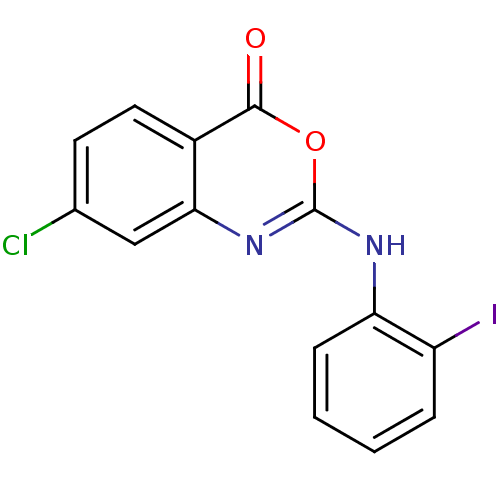 (7-Chloro-2-(2-iodo-phenylamino)-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50289007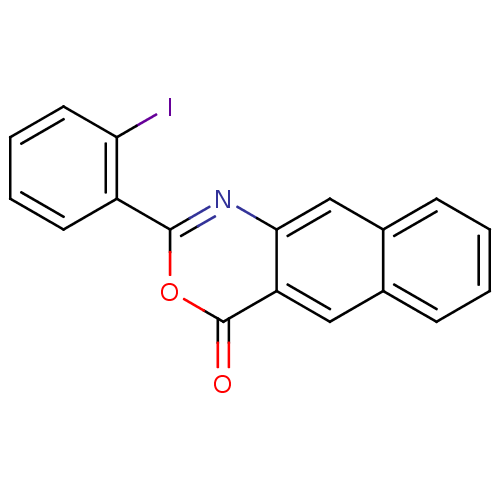 (2-(2-Iodo-phenyl)-naphtho[2,3-d][1,3]oxazin-4-one ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50075982 (4-Methoxy-N-methyl-N-(4-oxo-4H-benzo[d][1,3]oxazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of purified human C1r protease. | Bioorg Med Chem Lett 9: 815-20 (1999) BindingDB Entry DOI: 10.7270/Q29G5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063723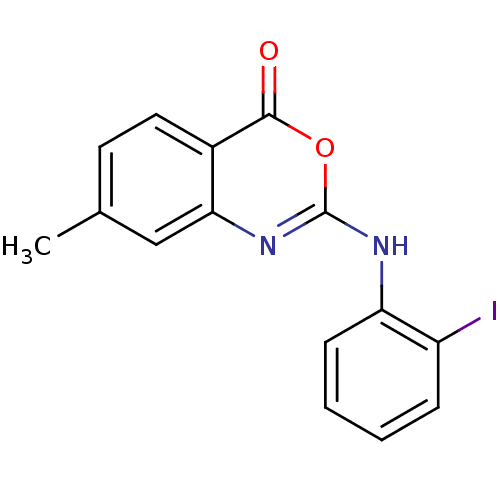 (2-(2-Iodo-phenylamino)-7-methyl-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human serum C1r using AAME as substrate after 30 mins | J Med Chem 61: 3253-3276 (2018) Article DOI: 10.1021/acs.jmedchem.7b00882 BindingDB Entry DOI: 10.7270/Q2MK6GJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063717 (7-Chloro-2-(2-chloro-phenylamino)-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063721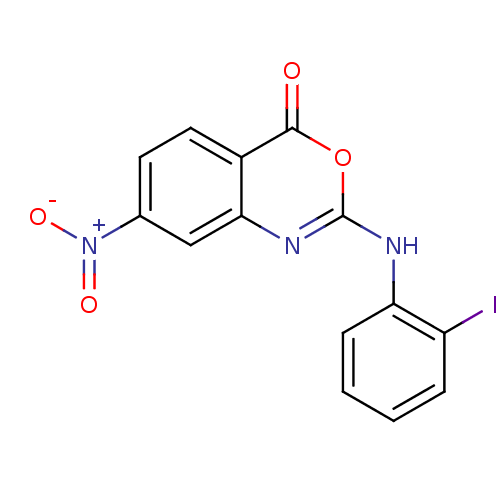 (2-(2-Iodo-phenylamino)-7-nitro-benzo[d][1,3]oxazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063717 (7-Chloro-2-(2-chloro-phenylamino)-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063732 (2-(2-Chloro-phenylamino)-7-methyl-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063724 (2-(2,6-Dichloro-phenylamino)-benzo[d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063711 (7-Chloro-2-(2-iodo-phenylamino)-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063726 (7-Chloro-2-(2,6-dichloro-phenylamino)-benzo[d][1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063724 (2-(2,6-Dichloro-phenylamino)-benzo[d][1,3]oxazin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description 50% inhibition of human C1r serine protease after 60 mins using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063703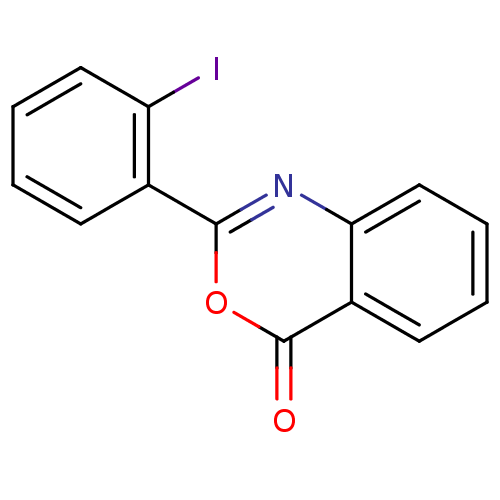 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063703 (2-(2-Iodo-phenyl)-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50063732 (2-(2-Chloro-phenylamino)-7-methyl-benzo[d][1,3]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 50% of human C1r Serine Protease by initially using CbzGly-Arg-S-Bzl as substrate | J Med Chem 41: 1060-7 (1998) Article DOI: 10.1021/jm970394d BindingDB Entry DOI: 10.7270/Q2PZ57ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 212 total ) | Next | Last >> |