

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50427221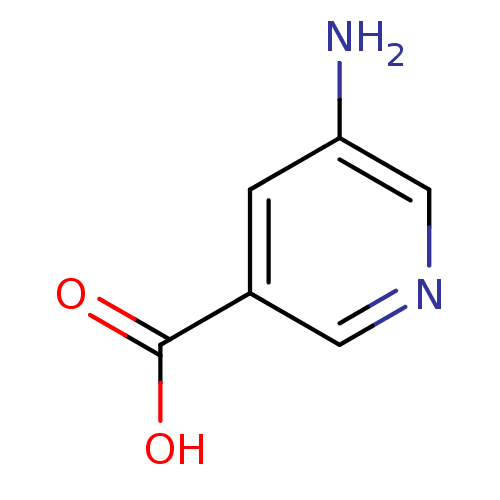 (5-Aminonicotinic Acid | CHEMBL1491941) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 8.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121995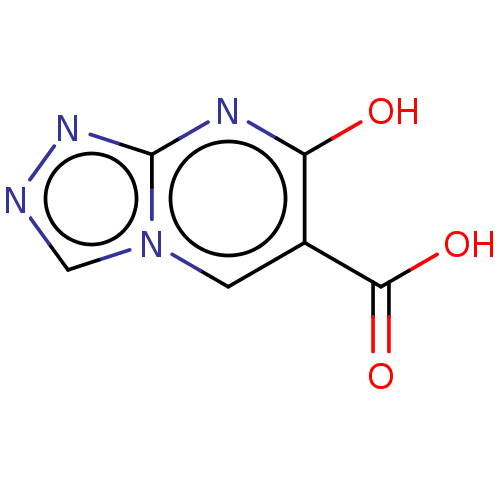 (CHEMBL3617316) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM50121995 (CHEMBL3617316) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM14673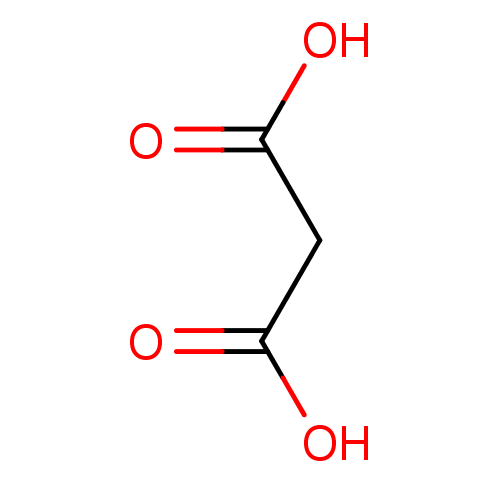 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to human recombinant DDO | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Mus musculus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RCG38204, isoform CRA_d (Rattus norvegicus) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of rat recombinant DDO using D-Asp | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31148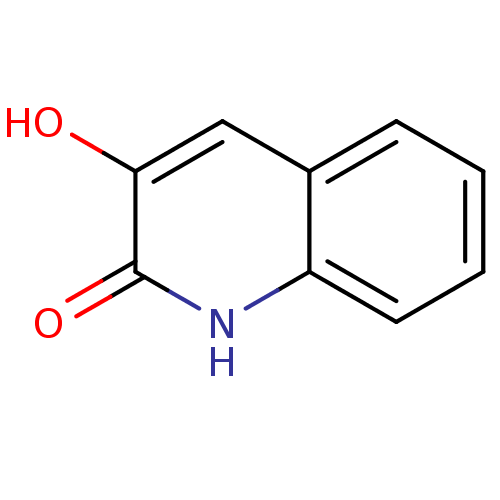 (3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31149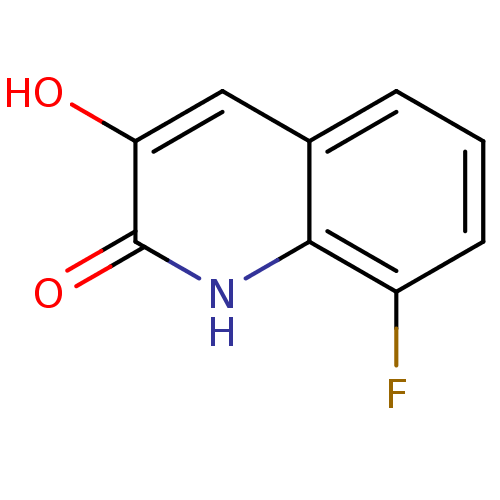 (3-hydroxyquinolin-2(1H)-one, 3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50004955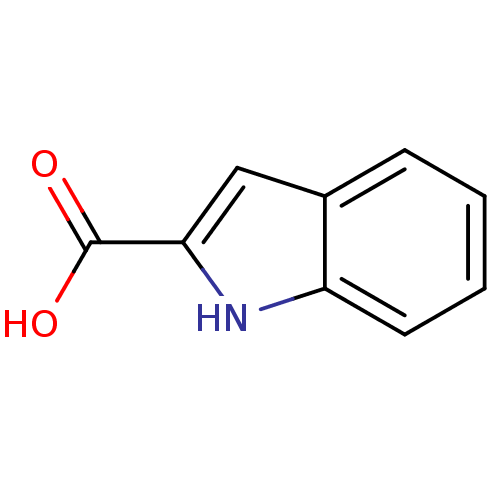 (1H-Indole-2-carboxylic acid | CHEMBL278390 | Indol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Curated by ChEMBL | Assay Description Inhibition of human DDO | Bioorg Med Chem Lett 18: 3386-91 (2008) Article DOI: 10.1016/j.bmcl.2008.04.020 BindingDB Entry DOI: 10.7270/Q2WQ03KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM23174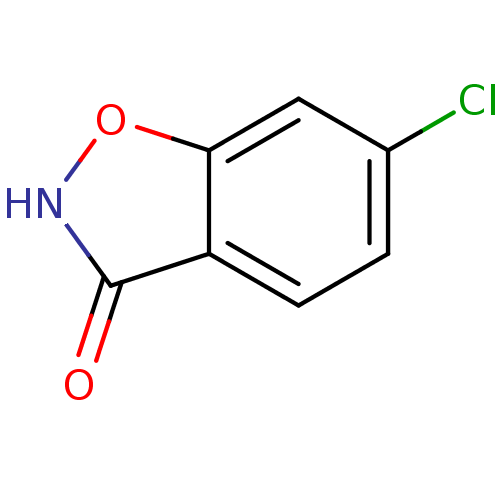 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Curated by ChEMBL | Assay Description Inhibition of human DDO | Bioorg Med Chem Lett 18: 3386-91 (2008) Article DOI: 10.1016/j.bmcl.2008.04.020 BindingDB Entry DOI: 10.7270/Q2WQ03KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50260722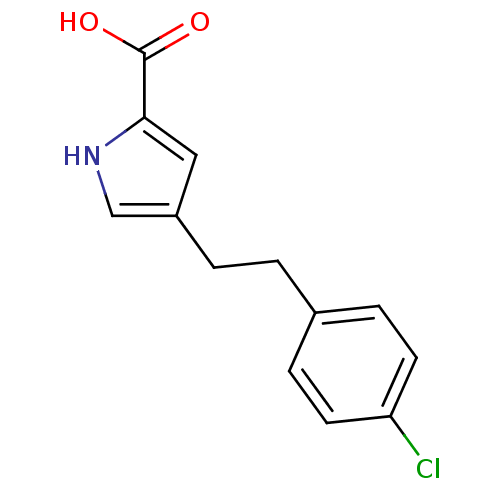 (4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Curated by ChEMBL | Assay Description Inhibition of human DDO | Bioorg Med Chem Lett 18: 3386-91 (2008) Article DOI: 10.1016/j.bmcl.2008.04.020 BindingDB Entry DOI: 10.7270/Q2WQ03KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31147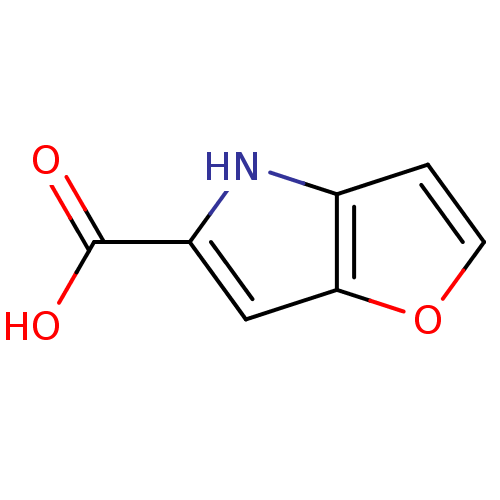 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Curated by ChEMBL | Assay Description Inhibition of human DDO | Bioorg Med Chem Lett 18: 3386-91 (2008) Article DOI: 10.1016/j.bmcl.2008.04.020 BindingDB Entry DOI: 10.7270/Q2WQ03KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50260721 (4,5-dichlorofuran-2-carboxylic acid | CHEMBL511101) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Curated by ChEMBL | Assay Description Inhibition of human DDO | Bioorg Med Chem Lett 18: 3386-91 (2008) Article DOI: 10.1016/j.bmcl.2008.04.020 BindingDB Entry DOI: 10.7270/Q2WQ03KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31164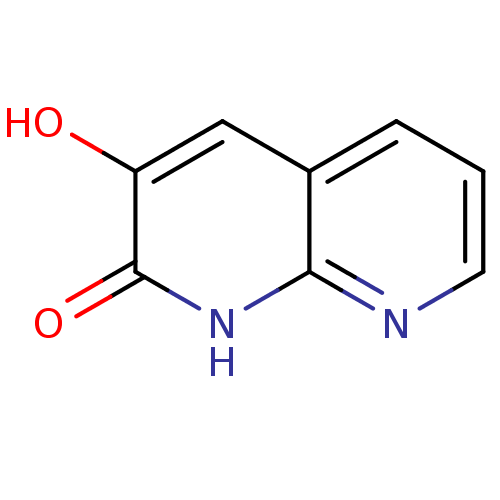 (naphthyridinone analog.,18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >7.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31151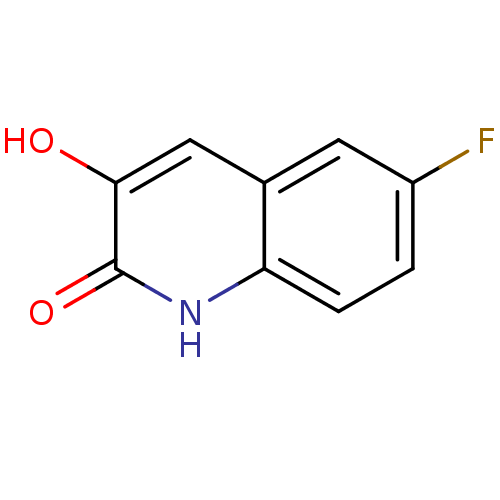 (3-hydroxyquinolin-2(1H)-one, 5) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >8.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31172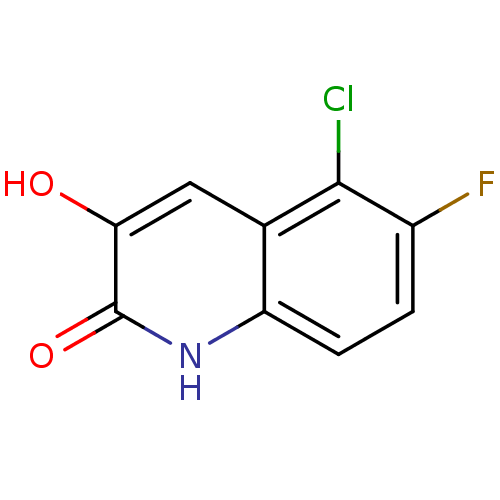 (3-hydroxyquinolin-2(1H)-one, 26) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM50211362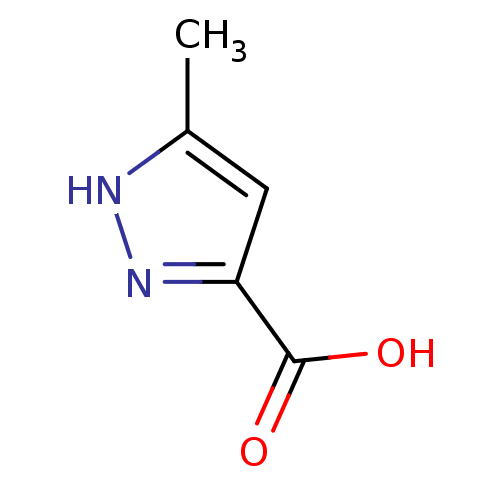 (5-methyl-1H-pyrazole-3-carboxylic acid | 5-methyl-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM23174 (6-chloro-1,2-benzoxazol-3-ol | 6-chlorobenzo[d]iso...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM50260725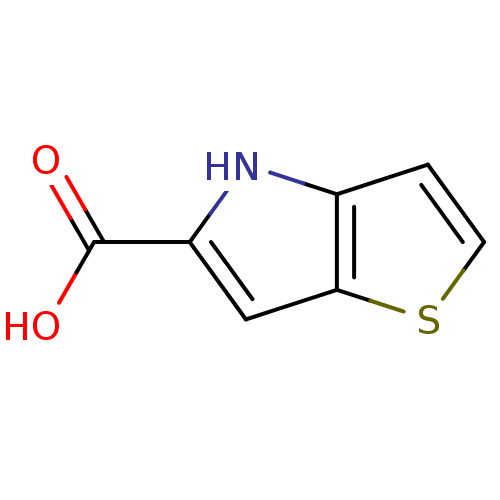 (4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Bos taurus) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Inhibition of bovine recombinant DASPO expressed in Escherichia coli preincubated for 15 mins by fluorescence assay | Eur J Med Chem 46: 4808-19 (2011) Article DOI: 10.1016/j.ejmech.2011.04.023 BindingDB Entry DOI: 10.7270/Q2BK1CRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31173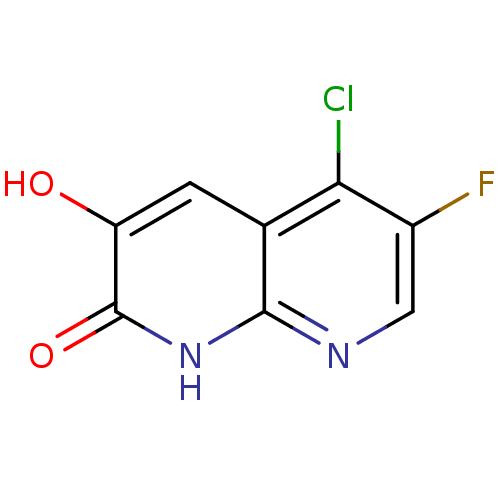 (naphthyridinone analog., 27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31158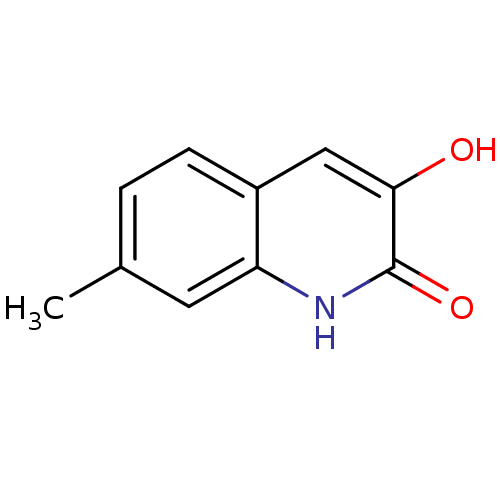 (3-hydroxyquinolin-2(1H)-one, 12) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50427221 (5-Aminonicotinic Acid | CHEMBL1491941) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31150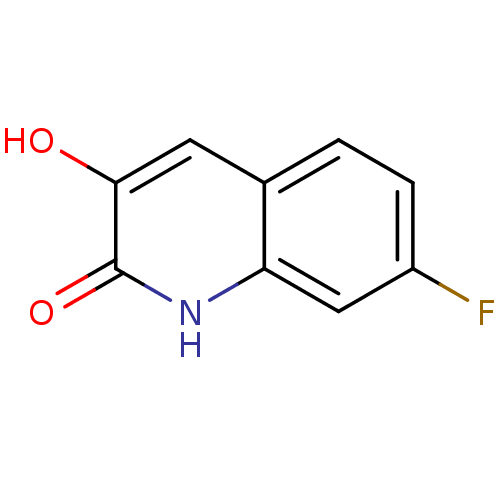 (3-hydroxyquinolin-2(1H)-one, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31157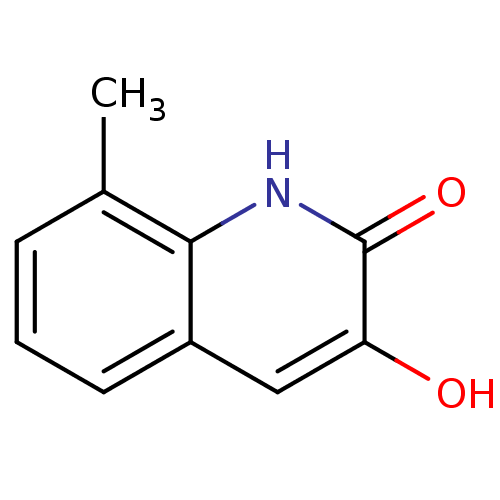 (3-hydroxyquinolin-2(1H)-one, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31169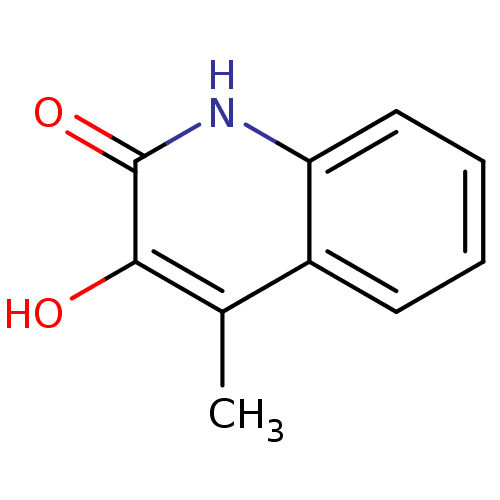 (3-hydroxyquinolin-2(1H)-one, 23 | US9701638, 14) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31165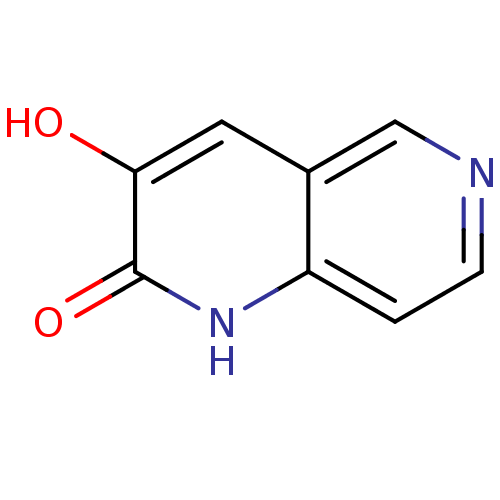 (naphthyridinone analog.,19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31153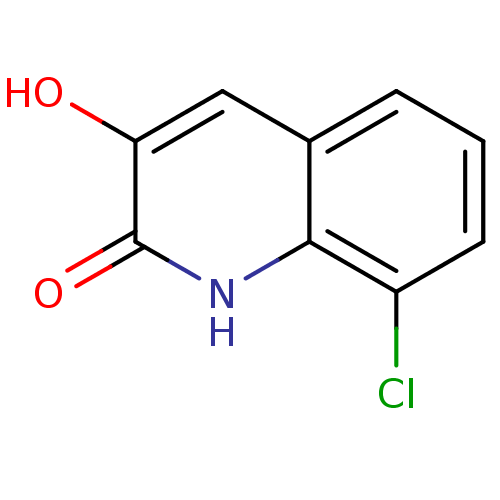 (3-hydroxyquinolin-2(1H)-one, 7) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31155 (3-hydroxyquinolin-2(1H)-one, 9) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31154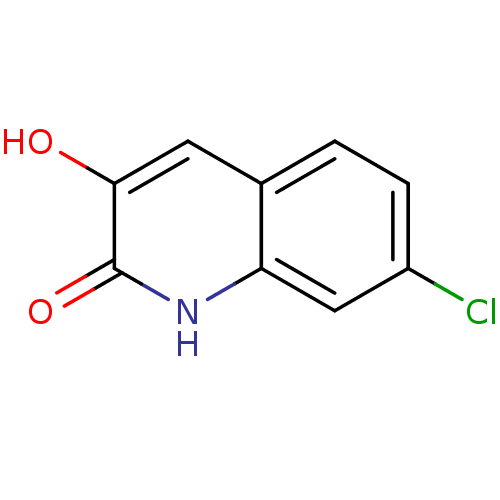 (3-hydroxyquinolin-2(1H)-one, 8) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31167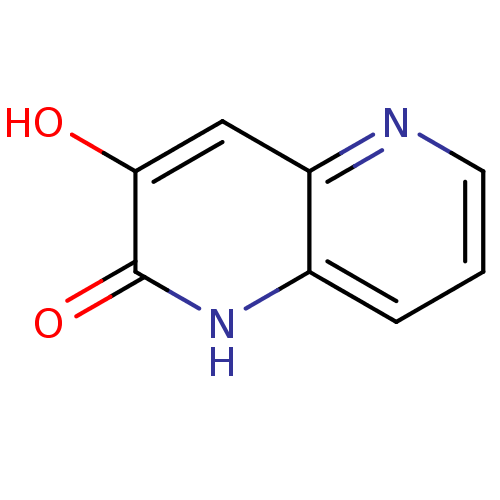 (naphthyridinone analog.,21) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31168 (pyridopyrazinone, 22) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31166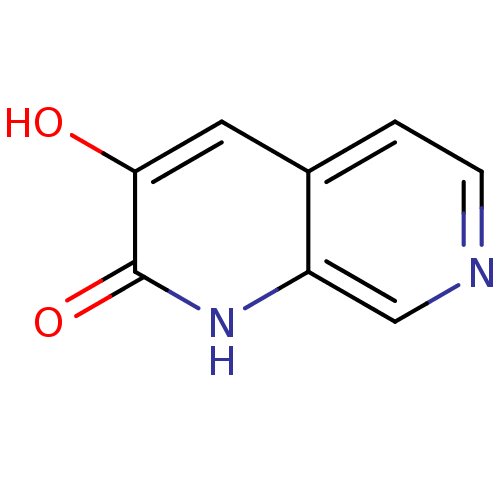 (naphthyridinone analog.,20) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31163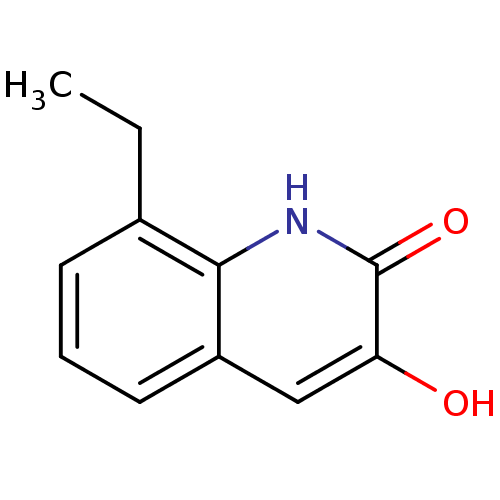 (3-hydroxyquinolin-2(1H)-one, 17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31160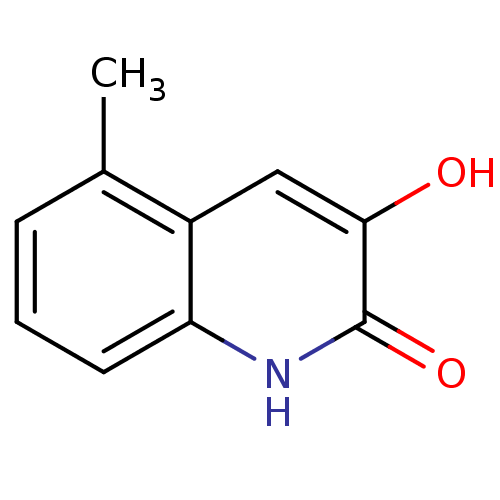 (3-hydroxyquinolin-2(1H)-one, 14) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31161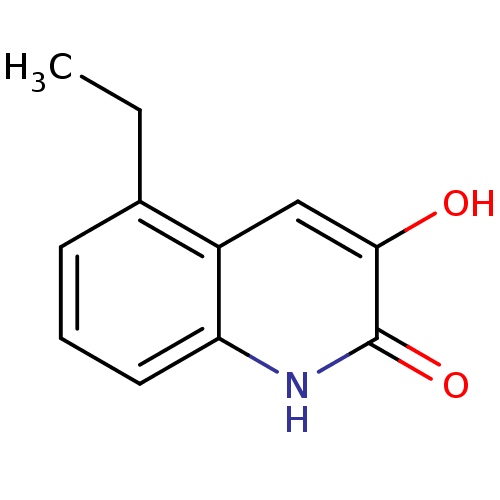 (3-hydroxyquinolin-2(1H)-one, 15) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31152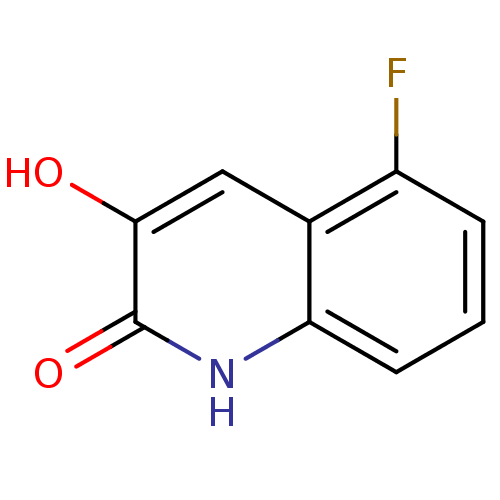 (3-hydroxyquinolin-2(1H)-one, 6) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121995 (CHEMBL3617316) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31147 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged DDO expressed in Escherichia coli BL21(DE3) using D-aspartate as substrate by colorimetric assa... | J Med Chem 56: 1894-907 (2013) Article DOI: 10.1021/jm3017865 BindingDB Entry DOI: 10.7270/Q2H70H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50122001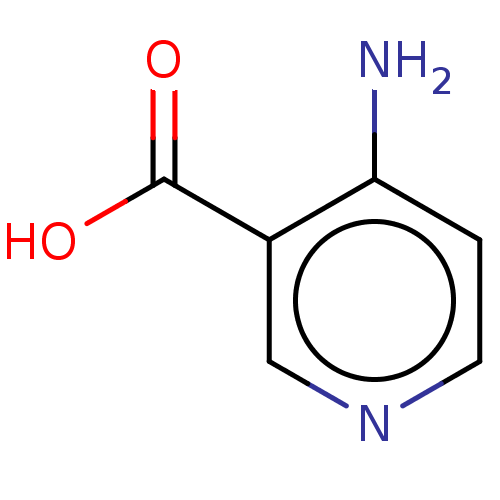 (CHEMBL3617320) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121998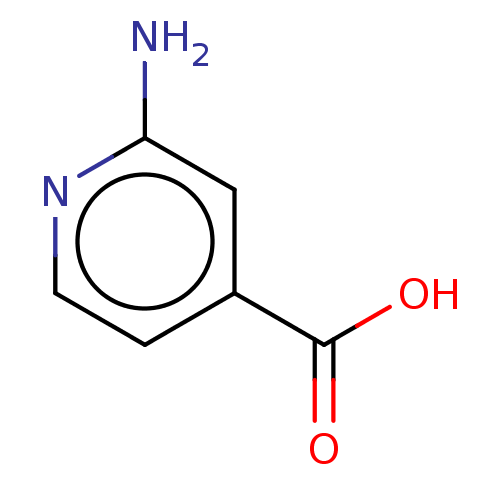 (CHEMBL3322866) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121997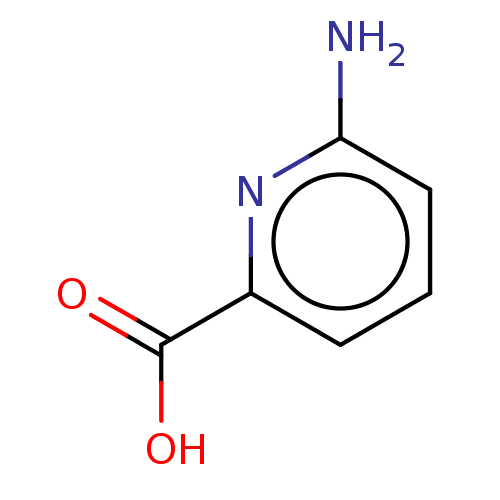 (CHEMBL3322863) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM50121996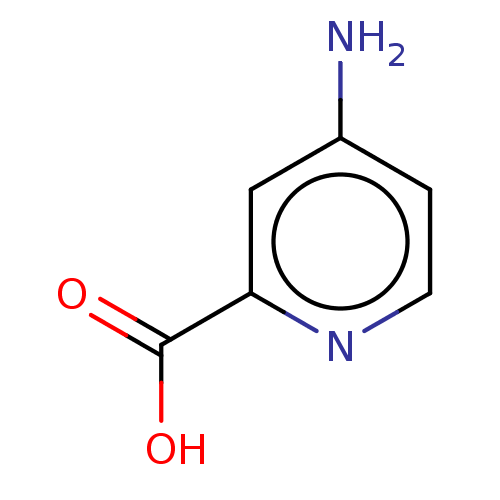 (CHEMBL3617326) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of human recombinant DDO expressed in Escherichia coli BL21(DE3) using D-Asp and D-Ala assessed as 2-oxo acid production after 10 mins by ... | J Med Chem 58: 7328-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00871 BindingDB Entry DOI: 10.7270/Q2WM1G6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-aspartate oxidase (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-aspartic acid was linke... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |