 Found 10107 hits Enz. Inhib. hit(s) with Target = 'Dihydrofolate reductase'
Found 10107 hits Enz. Inhib. hit(s) with Target = 'Dihydrofolate reductase' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082
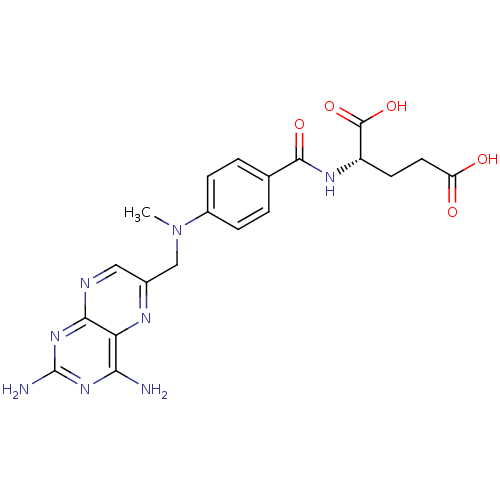
((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
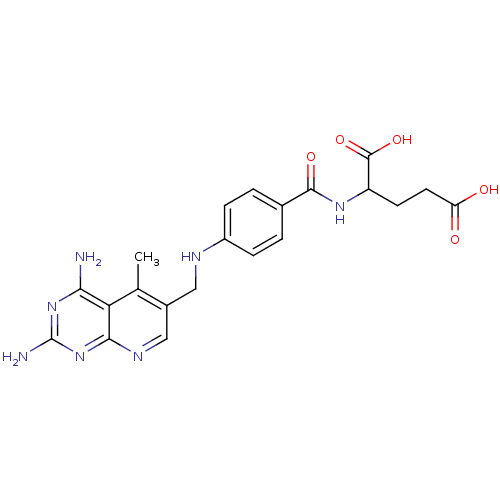
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905
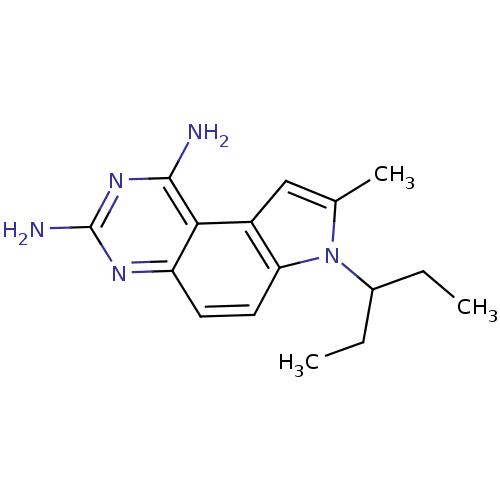
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon Health& Science University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DHFR preincubated for 2 mins followed by substrate addition in presence of dihydrofolate |
Medchemcomm 6: 510-520 (2015)
BindingDB Entry DOI: 10.7270/Q2QN68NS |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320
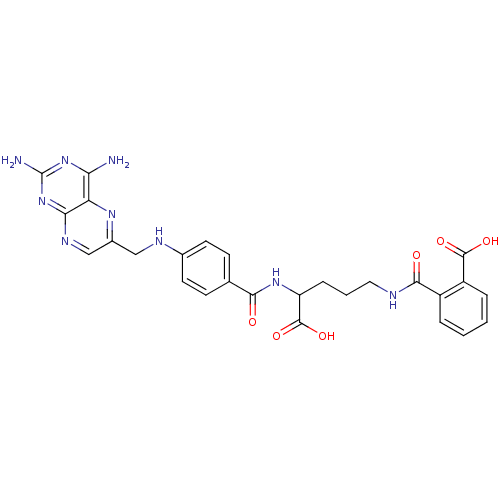
(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068810
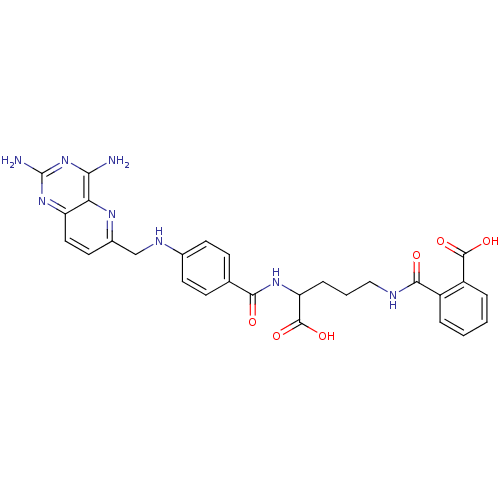
(CHEMBL149164 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C28H28N8O6/c29-23-22-20(35-28(30)36-23)12-11-17(33-22)14-32-16-9-7-15(8-10-16)24(37)34-21(27(41)42)6-3-13-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-12,21,32H,3,6,13-14H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068812
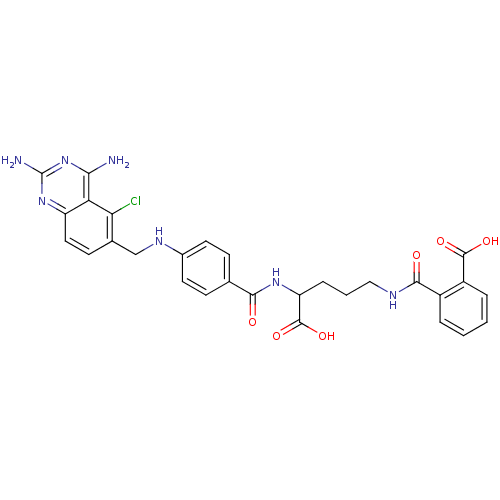
(CHEMBL146917 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-c...)Show SMILES Nc1nc(N)c2c(Cl)c(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H28ClN7O6/c30-23-16(9-12-20-22(23)24(31)37-29(32)36-20)14-34-17-10-7-15(8-11-17)25(38)35-21(28(42)43)6-3-13-33-26(39)18-4-1-2-5-19(18)27(40)41/h1-2,4-5,7-12,21,34H,3,6,13-14H2,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068809
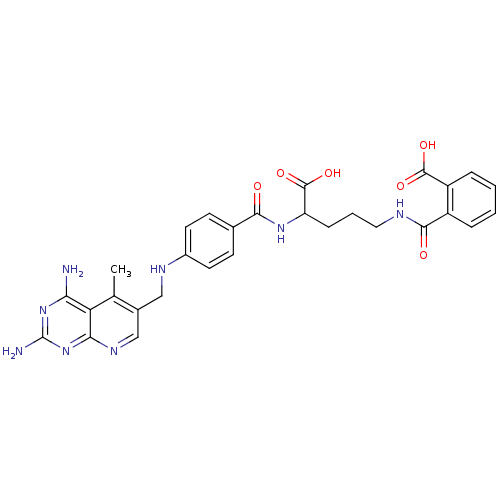
(CHEMBL150607 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C29H30N8O6/c1-15-17(14-34-24-22(15)23(30)36-29(31)37-24)13-33-18-10-8-16(9-11-18)25(38)35-21(28(42)43)7-4-12-32-26(39)19-5-2-3-6-20(19)27(40)41/h2-3,5-6,8-11,14,21,33H,4,7,12-13H2,1H3,(H,32,39)(H,35,38)(H,40,41)(H,42,43)(H4,30,31,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068811
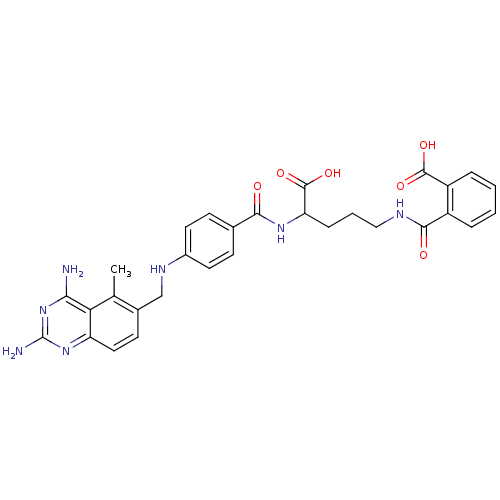
(CHEMBL149218 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)ccc2nc(N)nc(N)c12 Show InChI InChI=1S/C30H31N7O6/c1-16-18(10-13-22-24(16)25(31)37-30(32)36-22)15-34-19-11-8-17(9-12-19)26(38)35-23(29(42)43)7-4-14-33-27(39)20-5-2-3-6-21(20)28(40)41/h2-3,5-6,8-13,23,34H,4,7,14-15H2,1H3,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808
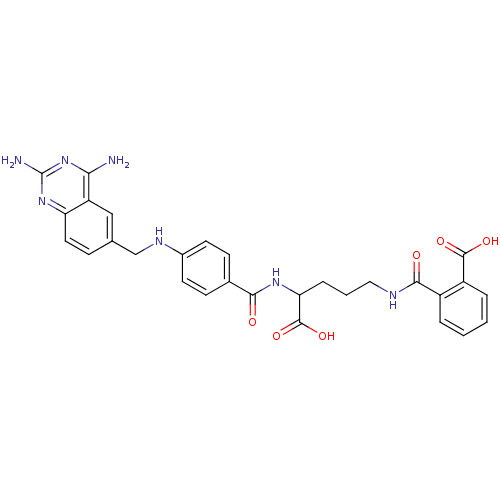
(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068813
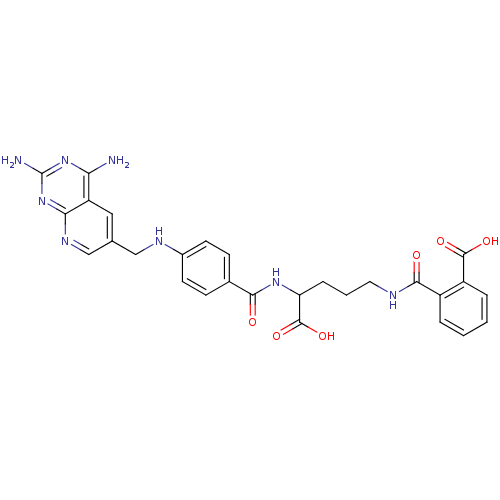
(CHEMBL149962 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H28N8O6/c29-22-20-12-15(14-33-23(20)36-28(30)35-22)13-32-17-9-7-16(8-10-17)24(37)34-21(27(41)42)6-3-11-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-10,12,14,21,32H,3,6,11,13H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808

(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0000140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111552
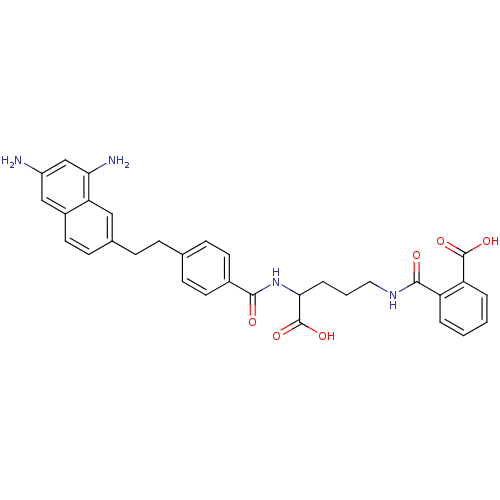
(CHEMBL297558 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-n...)Show SMILES Nc1cc(N)c2cc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2c1 Show InChI InChI=1S/C32H32N4O6/c33-23-17-22-14-11-20(16-26(22)27(34)18-23)8-7-19-9-12-21(13-10-19)29(37)36-28(32(41)42)6-3-15-35-30(38)24-4-1-2-5-25(24)31(39)40/h1-2,4-5,9-14,16-18,28H,3,6-8,15,33-34H2,(H,35,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111550
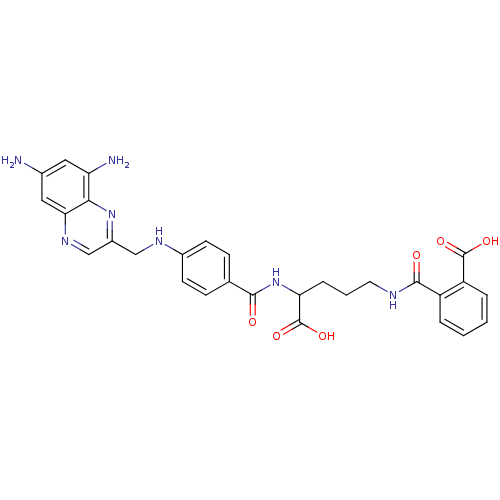
(CHEMBL296545 | N-(4-Carboxy-4-{4-[(6,8-diamino-qui...)Show SMILES Nc1cc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C29H29N7O6/c30-17-12-22(31)25-24(13-17)34-15-19(35-25)14-33-18-9-7-16(8-10-18)26(37)36-23(29(41)42)6-3-11-32-27(38)20-4-1-2-5-21(20)28(39)40/h1-2,4-5,7-10,12-13,15,23,33H,3,6,11,14,30-31H2,(H,32,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.000340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059955

(4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccc(cc2)C(=O)NC)c(c1)[N+]([O-])=O Show InChI InChI=1S/C22H25N7O3/c1-4-16-19(20(23)27-22(24)26-16)15-9-10-17(18(11-15)29(31)32)28(3)12-13-5-7-14(8-6-13)21(30)25-2/h5-11H,4,12H2,1-3H3,(H,25,30)(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111549
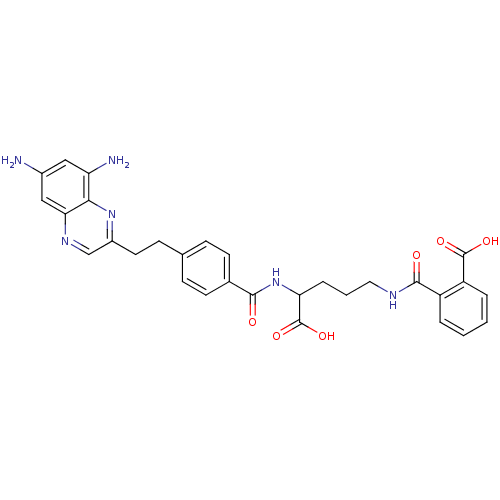
(CHEMBL47689 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C30H30N6O6/c31-19-14-23(32)26-25(15-19)34-16-20(35-26)12-9-17-7-10-18(11-8-17)27(37)36-24(30(41)42)6-3-13-33-28(38)21-4-1-2-5-22(21)29(39)40/h1-2,4-5,7-8,10-11,14-16,24H,3,6,9,12-13,31-32H2,(H,33,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50059948

(4-({[4-(2,4-Diamino-6-ethyl-pyrimidin-5-yl)-2-nitr...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc(N(C)Cc2ccc(cc2)C(O)=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H22N6O4/c1-3-15-18(19(22)25-21(23)24-15)14-8-9-16(17(10-14)27(30)31)26(2)11-12-4-6-13(7-5-12)20(28)29/h4-10H,3,11H2,1-2H3,(H,28,29)(H4,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against rat liver Dihydrofolate reductase |
J Med Chem 40: 3040-8 (1997)
Article DOI: 10.1021/jm970055k
BindingDB Entry DOI: 10.7270/Q2C53JZ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111548

(CHEMBL47919 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CC(CC#C)c3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C33H32N6O6/c1-2-6-21(15-23-18-37-28-17-22(34)16-26(35)29(28)38-23)19-10-12-20(13-11-19)30(40)39-27(33(44)45)9-5-14-36-31(41)24-7-3-4-8-25(24)32(42)43/h1,3-4,7-8,10-13,16-18,21,27H,5-6,9,14-15,34-35H2,(H,36,41)(H,39,40)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111551

(CHEMBL295859 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-q...)Show SMILES CCC(Cc1cnc2cc(N)cc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCNC(=O)c1ccccc1C(O)=O)C(O)=O Show InChI InChI=1S/C32H34N6O6/c1-2-18(14-22-17-36-27-16-21(33)15-25(34)28(27)37-22)19-9-11-20(12-10-19)29(39)38-26(32(43)44)8-5-13-35-30(40)23-6-3-4-7-24(23)31(41)42/h3-4,6-7,9-12,15-18,26H,2,5,8,13-14,33-34H2,1H3,(H,35,40)(H,38,39)(H,41,42)(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli DHFR |
ACS Med Chem Lett 7: 692-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00120
BindingDB Entry DOI: 10.7270/Q2DJ5HKK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50448761
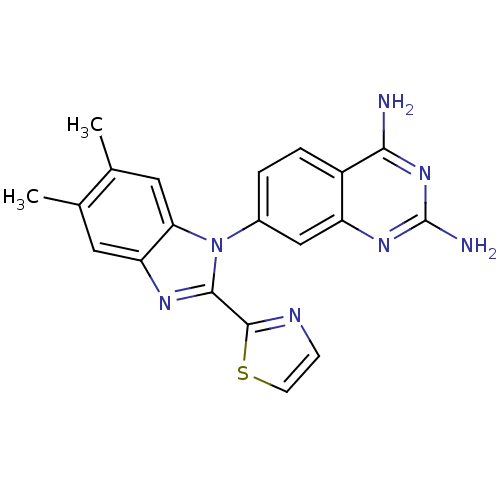
(CHEMBL3128021 | US8835445, 22)Show SMILES Cc1cc2nc(-c3nccs3)n(-c3ccc4c(N)nc(N)nc4c3)c2cc1C Show InChI InChI=1S/C20H17N7S/c1-10-7-15-16(8-11(10)2)27(18(24-15)19-23-5-6-28-19)12-3-4-13-14(9-12)25-20(22)26-17(13)21/h3-9H,1-2H3,(H4,21,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
US Patent
| Assay Description
Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... |
US Patent US8835445 (2014)
BindingDB Entry DOI: 10.7270/Q2DB80J0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM50448761

(CHEMBL3128021 | US8835445, 22)Show SMILES Cc1cc2nc(-c3nccs3)n(-c3ccc4c(N)nc(N)nc4c3)c2cc1C Show InChI InChI=1S/C20H17N7S/c1-10-7-15-16(8-11(10)2)27(18(24-15)19-23-5-6-28-19)12-3-4-13-14(9-12)25-20(22)26-17(13)21/h3-9H,1-2H3,(H4,21,22,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... |
J Med Chem 57: 651-68 (2014)
Article DOI: 10.1021/jm401204g
BindingDB Entry DOI: 10.7270/Q2PN974G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50010932
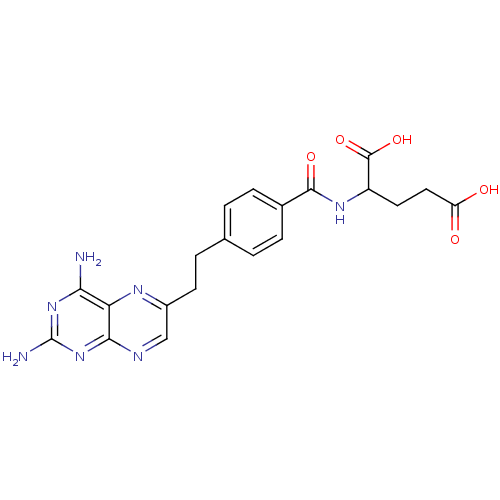
((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...)Show SMILES Nc1nc(N)c2nc(CCc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-15-17(27-20(22)26-16)23-9-12(24-15)6-3-10-1-4-11(5-2-10)18(30)25-13(19(31)32)7-8-14(28)29/h1-2,4-5,9,13H,3,6-8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016323
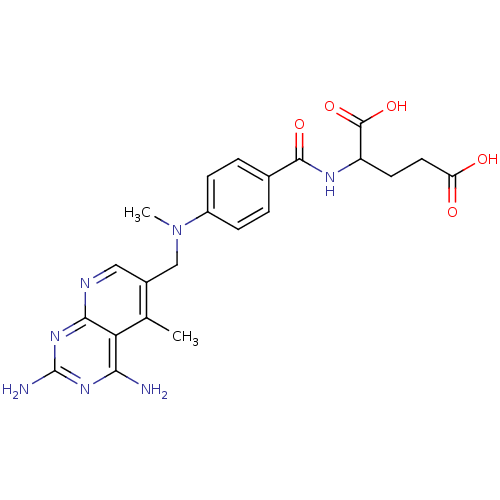
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1C)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-11-13(9-25-19-17(11)18(23)27-22(24)28-19)10-29(2)14-5-3-12(4-6-14)20(32)26-15(21(33)34)7-8-16(30)31/h3-6,9,15H,7-8,10H2,1-2H3,(H,26,32)(H,30,31)(H,33,34)(H4,23,24,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50367055
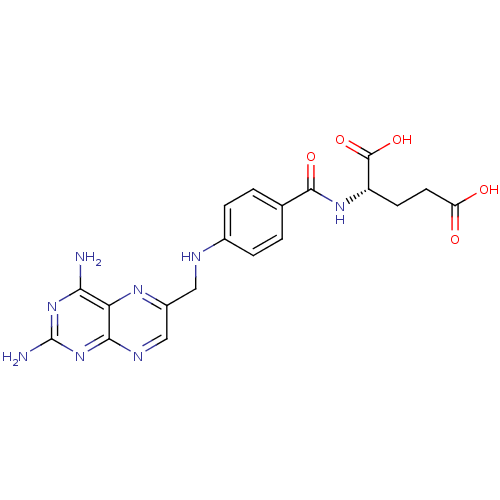
(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme from mouse |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50025009
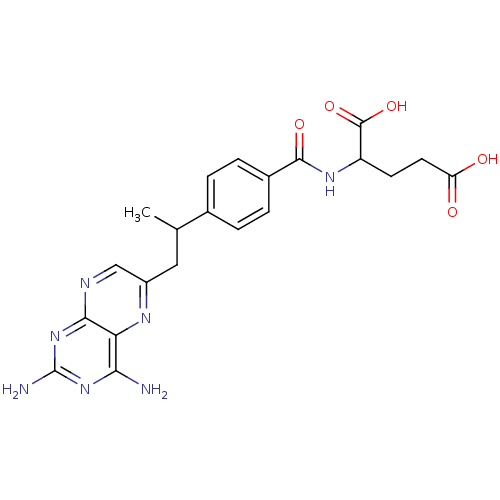
(2-{4-[2-(2,4-Diamino-pteridin-6-yl)-1-methyl-ethyl...)Show SMILES CC(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10(8-13-9-24-18-16(25-13)17(22)27-21(23)28-18)11-2-4-12(5-3-11)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9-10,14H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028641
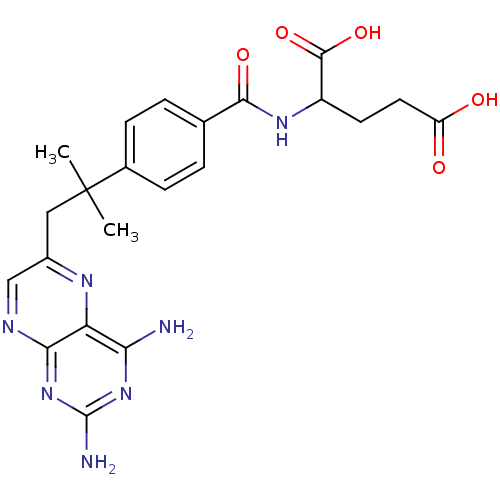
(2-{4-[2-(2,4-Diamino-pteridin-6-yl)-1,1-dimethyl-e...)Show SMILES CC(C)(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-22(2,9-13-10-25-18-16(26-13)17(23)28-21(24)29-18)12-5-3-11(4-6-12)19(32)27-14(20(33)34)7-8-15(30)31/h3-6,10,14H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016324
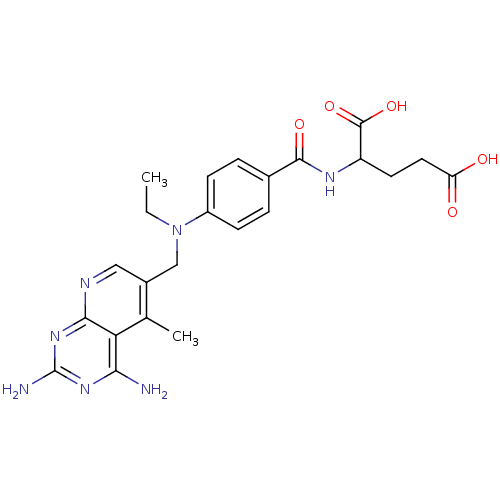
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES CCN(Cc1cnc2nc(N)nc(N)c2c1C)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C23H27N7O5/c1-3-30(11-14-10-26-20-18(12(14)2)19(24)28-23(25)29-20)15-6-4-13(5-7-15)21(33)27-16(22(34)35)8-9-17(31)32/h4-7,10,16H,3,8-9,11H2,1-2H3,(H,27,33)(H,31,32)(H,34,35)(H4,24,25,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028533

(4-Carbamoyl-2-{4-[(2,4-diamino-pteridin-6-ylmethyl...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(N)=O)C(O)=O Show InChI InChI=1S/C20H23N9O4/c1-29(9-11-8-24-17-15(25-11)16(22)27-20(23)28-17)12-4-2-10(3-5-12)18(31)26-13(19(32)33)6-7-14(21)30/h2-5,8,13H,6-7,9H2,1H3,(H2,21,30)(H,26,31)(H,32,33)(H4,22,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028539

(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...)Show SMILES CNC(=O)CCC(NC(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O Show InChI InChI=1S/C21H25N9O4/c1-24-15(31)8-7-14(20(33)34)27-19(32)11-3-5-13(6-4-11)30(2)10-12-9-25-18-16(26-12)17(22)28-21(23)29-18/h3-6,9,14H,7-8,10H2,1-2H3,(H,24,31)(H,27,32)(H,33,34)(H4,22,23,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50004544
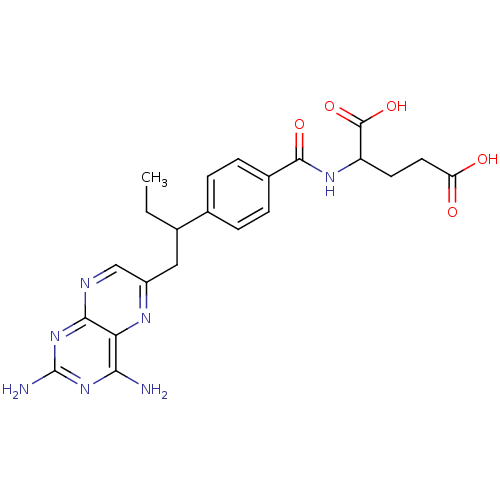
(2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...)Show SMILES CCC(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-2-11(9-14-10-25-19-17(26-14)18(23)28-22(24)29-19)12-3-5-13(6-4-12)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,10-11,15H,2,7-9H2,1H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016460
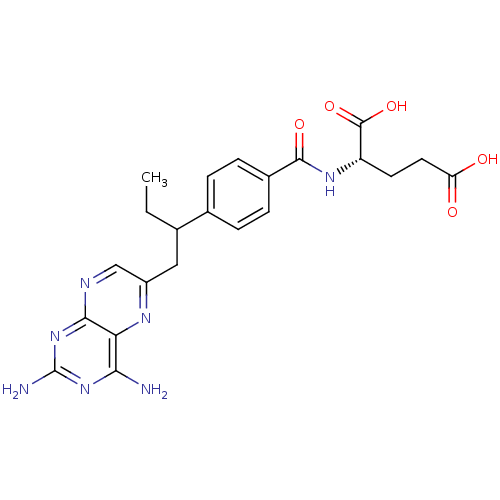
((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...)Show SMILES CCC(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-2-11(9-14-10-25-19-17(26-14)18(23)28-22(24)29-19)12-3-5-13(6-4-12)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,10-11,15H,2,7-9H2,1H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,25,28,29)/t11?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells |
J Med Chem 33: 212-5 (1990)
BindingDB Entry DOI: 10.7270/Q2FX78FR |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028542

(CHEMBL293546 | derivative of methotrexate)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(=O)N[C@@H](CC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C24H27N9O8/c1-33(10-12-9-27-20-18(28-12)19(25)31-24(26)32-20)13-4-2-11(3-5-13)21(37)30-14(22(38)39)6-7-16(34)29-15(23(40)41)8-17(35)36/h2-5,9,14-15H,6-8,10H2,1H3,(H,29,34)(H,30,37)(H,35,36)(H,38,39)(H,40,41)(H4,25,26,27,31,32)/t14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028536

(4-(Carboxymethyl-carbamoyl)-2-{4-[(2,4-diamino-pte...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N9O6/c1-31(10-12-8-26-19-17(27-12)18(23)29-22(24)30-19)13-4-2-11(3-5-13)20(35)28-14(21(36)37)6-7-15(32)25-9-16(33)34/h2-5,8,14H,6-7,9-10H2,1H3,(H,25,32)(H,28,35)(H,33,34)(H,36,37)(H4,23,24,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326

(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50405400
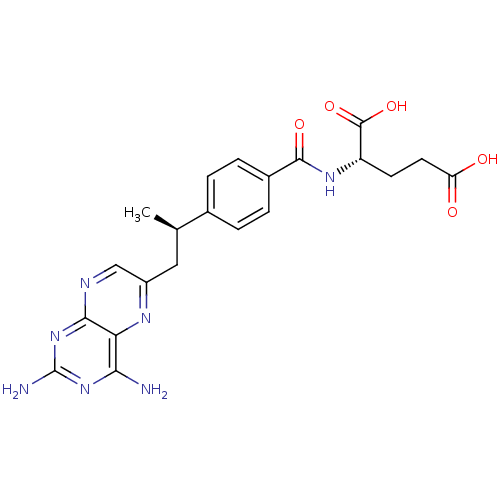
(CHEMBL2051987)Show SMILES C[C@H](Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H23N7O5/c1-10(8-13-9-24-18-16(25-13)17(22)27-21(23)28-18)11-2-4-12(5-3-11)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9-10,14H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,24,27,28)/t10-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells |
J Med Chem 29: 1056-61 (1986)
BindingDB Entry DOI: 10.7270/Q2P55MHM |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028605
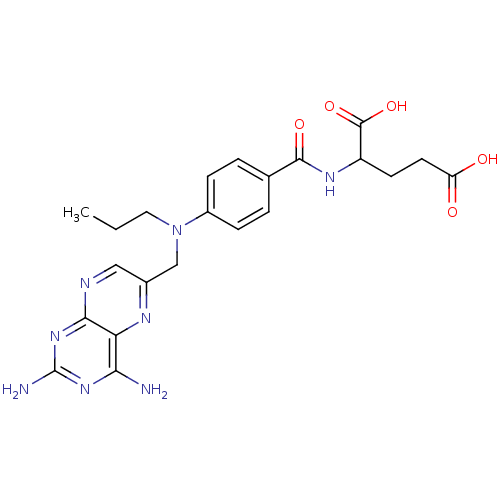
(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-propyl-ami...)Show SMILES CCCN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H26N8O5/c1-2-9-30(11-13-10-25-19-17(26-13)18(23)28-22(24)29-19)14-5-3-12(4-6-14)20(33)27-15(21(34)35)7-8-16(31)32/h3-6,10,15H,2,7-9,11H2,1H3,(H,27,33)(H,31,32)(H,34,35)(H4,23,24,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells |
J Med Chem 25: 877-80 (1982)
BindingDB Entry DOI: 10.7270/Q23F4Q6X |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50405400

(CHEMBL2051987)Show SMILES C[C@H](Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H23N7O5/c1-10(8-13-9-24-18-16(25-13)17(22)27-21(23)28-18)11-2-4-12(5-3-11)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9-10,14H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,24,27,28)/t10-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells |
J Med Chem 29: 1056-61 (1986)
BindingDB Entry DOI: 10.7270/Q2P55MHM |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... |
ACS Med Chem Lett 7: 692-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00120
BindingDB Entry DOI: 10.7270/Q2DJ5HKK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of dihydrofolate reductase enzyme |
J Med Chem 25: 1227-30 (1983)
BindingDB Entry DOI: 10.7270/Q2WW7J72 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50010932

((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...)Show SMILES Nc1nc(N)c2nc(CCc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-15-17(27-20(22)26-16)23-9-12(24-15)6-3-10-1-4-11(5-2-10)18(30)25-13(19(31)32)7-8-14(28)29/h1-2,4-5,9,13H,3,6-8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells |
J Med Chem 33: 212-5 (1990)
BindingDB Entry DOI: 10.7270/Q2FX78FR |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028541

(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...)Show SMILES CCCCCNC(=O)CCC(NC(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O Show InChI InChI=1S/C25H33N9O4/c1-3-4-5-12-28-19(35)11-10-18(24(37)38)31-23(36)15-6-8-17(9-7-15)34(2)14-16-13-29-22-20(30-16)21(26)32-25(27)33-22/h6-9,13,18H,3-5,10-12,14H2,1-2H3,(H,28,35)(H,31,36)(H,37,38)(H4,26,27,29,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.00355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028531

(4-Benzylcarbamoyl-2-{4-[(2,4-diamino-pteridin-6-yl...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(=O)NCc1ccccc1)C(O)=O Show InChI InChI=1S/C27H29N9O4/c1-36(15-18-14-31-24-22(32-18)23(28)34-27(29)35-24)19-9-7-17(8-10-19)25(38)33-20(26(39)40)11-12-21(37)30-13-16-5-3-2-4-6-16/h2-10,14,20H,11-13,15H2,1H3,(H,30,37)(H,33,38)(H,39,40)(H4,28,29,31,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016325
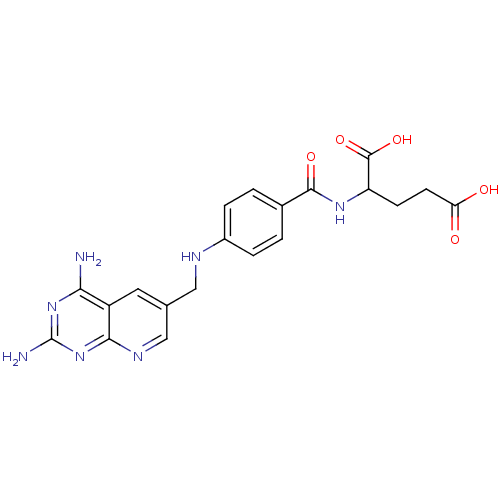
(2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H21N7O5/c21-16-13-7-10(9-24-17(13)27-20(22)26-16)8-23-12-3-1-11(2-4-12)18(30)25-14(19(31)32)5-6-15(28)29/h1-4,7,9,14,23H,5-6,8H2,(H,25,30)(H,28,29)(H,31,32)(H4,21,22,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) |
J Med Chem 29: 1080-7 (1986)
BindingDB Entry DOI: 10.7270/Q2416XMQ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043393
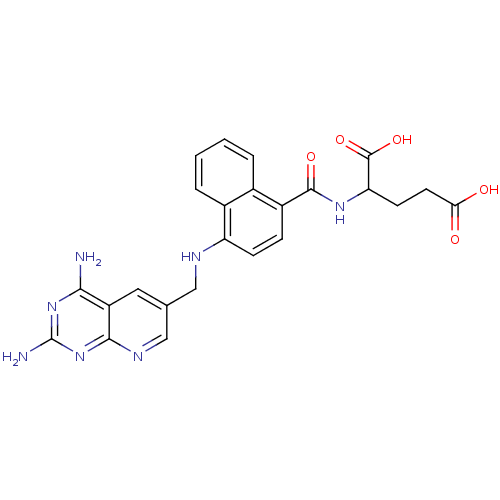
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028538
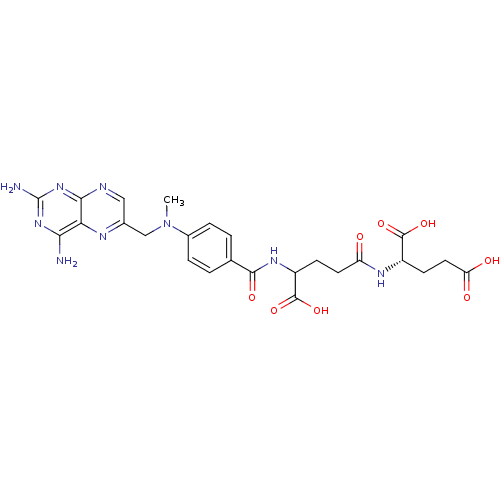
(CHEMBL293147 | derivative of methotrexate)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C25H29N9O8/c1-34(11-13-10-28-21-19(29-13)20(26)32-25(27)33-21)14-4-2-12(3-5-14)22(38)31-16(24(41)42)6-8-17(35)30-15(23(39)40)7-9-18(36)37/h2-5,10,15-16H,6-9,11H2,1H3,(H,30,35)(H,31,38)(H,36,37)(H,39,40)(H,41,42)(H4,26,27,28,32,33)/t15-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50028540

(2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...)Show SMILES CN(C)C(=O)CCC(NC(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O Show InChI InChI=1S/C22H27N9O4/c1-30(2)16(32)9-8-15(21(34)35)27-20(33)12-4-6-14(7-5-12)31(3)11-13-10-25-19-17(26-13)18(23)28-22(24)29-19/h4-7,10,15H,8-9,11H2,1-3H3,(H,27,33)(H,34,35)(H4,23,24,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Dihydrofolate reductase in mouse L1210 cells |
J Med Chem 25: 182-7 (1982)
BindingDB Entry DOI: 10.7270/Q2KS6QJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data