 Found 1256 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EHMT1'
Found 1256 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EHMT1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041
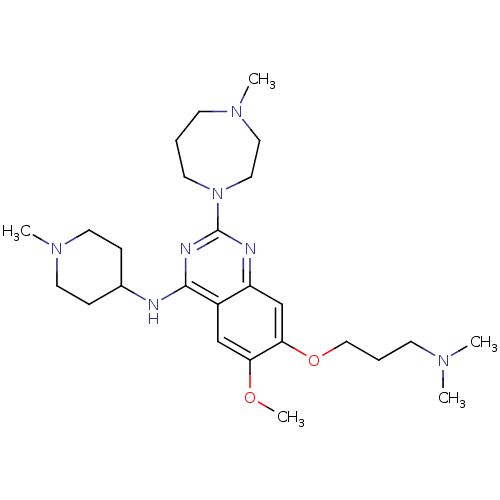
(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f... |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041

(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant G9a catalytic domain amino acid 913 to 1193 expressed in Escherichia coli BL21 (DE3) by isothermal titration ca... |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221
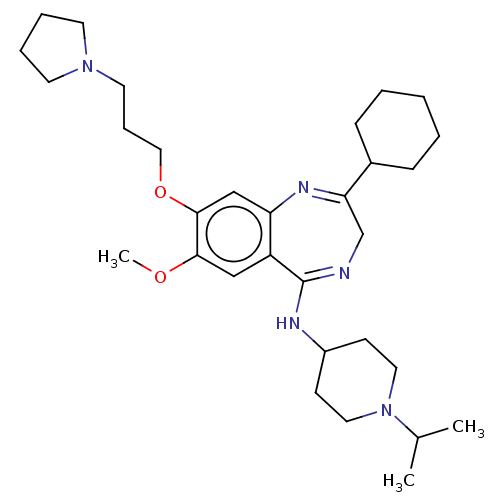
(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate after 30 mins in presence of va... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50442103
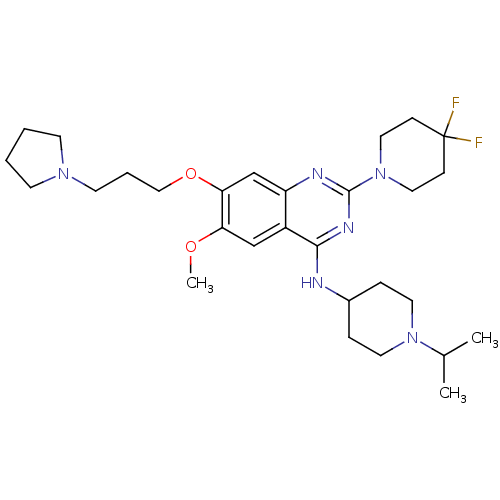
(CHEMBL2441082)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C29H44F2N6O2/c1-21(2)36-14-7-22(8-15-36)32-27-23-19-25(38-3)26(39-18-6-13-35-11-4-5-12-35)20-24(23)33-28(34-27)37-16-9-29(30,31)10-17-37/h19-22H,4-18H2,1-3H3,(H,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of lysine methyltransferase G9a (unknown origin) using SAM as substrate by Michaelis-Menten kinetic assay |
J Med Chem 56: 8931-42 (2013)
Article DOI: 10.1021/jm401480r
BindingDB Entry DOI: 10.7270/Q2NZ892T |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a by fluorescence polarization assay in presence of fluorescein-labeled H3 peptide |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a (unknown origin) by Morrison plot analysis in presence of histone H3 (1 to 25 residues) |
Bioorg Med Chem 24: 6102-6108 (2016)
Article DOI: 10.1016/j.bmc.2016.09.071
BindingDB Entry DOI: 10.7270/Q29C70D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221

(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate at varying concentration af... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50507295
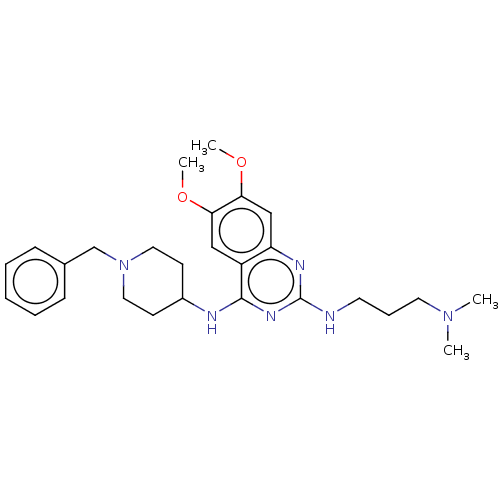
(CHEMBL1232432)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C27H38N6O2/c1-32(2)14-8-13-28-27-30-23-18-25(35-4)24(34-3)17-22(23)26(31-27)29-21-11-15-33(16-12-21)19-20-9-6-5-7-10-20/h5-7,9-10,17-18,21H,8,11-16,19H2,1-4H3,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594083
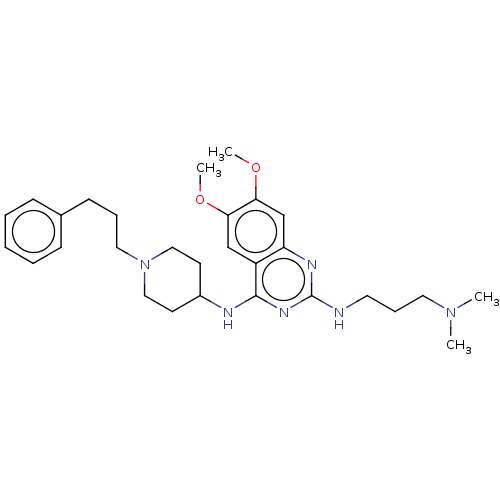
(CHEMBL5179091)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594086

(CHEMBL5170197)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594108
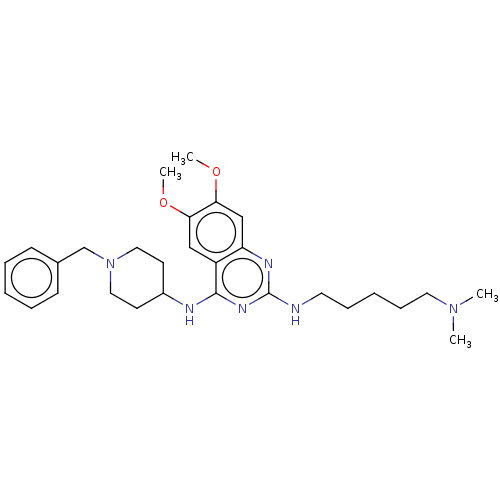
(CHEMBL5175660)Show SMILES COc1cc2nc(NCCCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594085
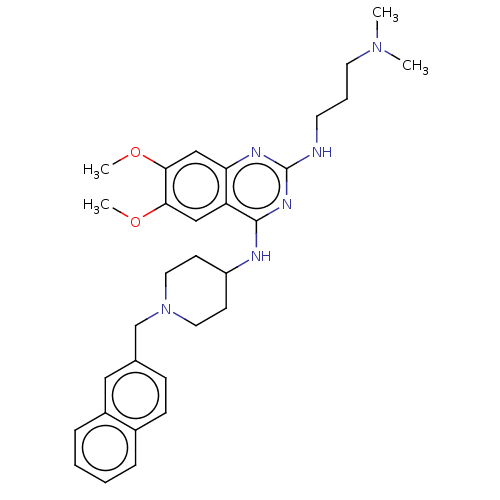
(CHEMBL5178765)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594084
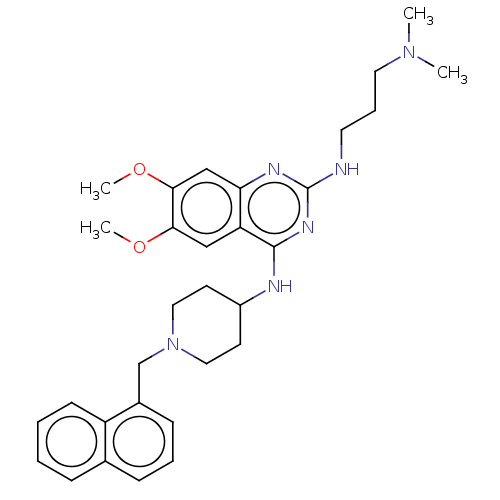
(CHEMBL5204694)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4cccc5ccccc45)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594082
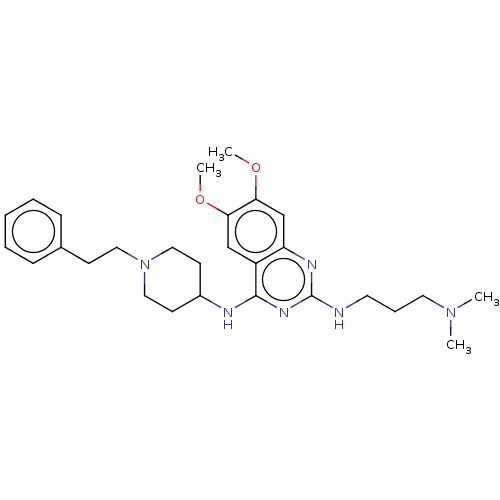
(CHEMBL5197685)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594101
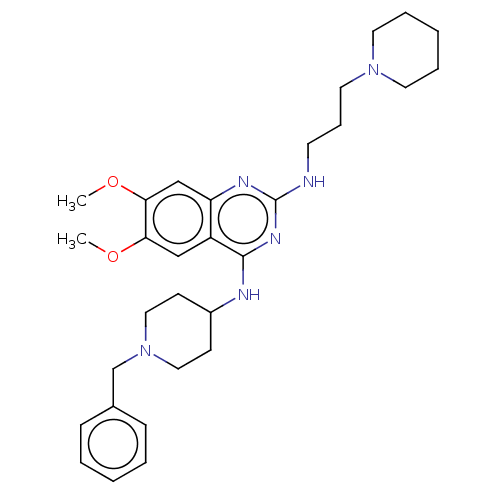
(CHEMBL5175171)Show SMILES COc1cc2nc(NCCCN3CCCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594099
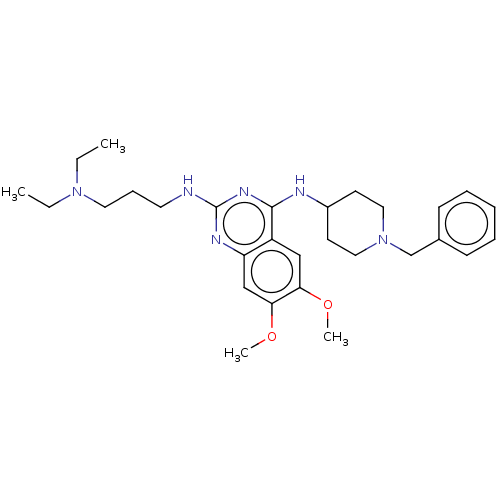
(CHEMBL5186174)Show SMILES CCN(CC)CCCNc1nc(NC2CCN(Cc3ccccc3)CC2)c2cc(OC)c(OC)cc2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594100

(CHEMBL5181017)Show SMILES COc1cc2nc(NCCCN3CCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594081
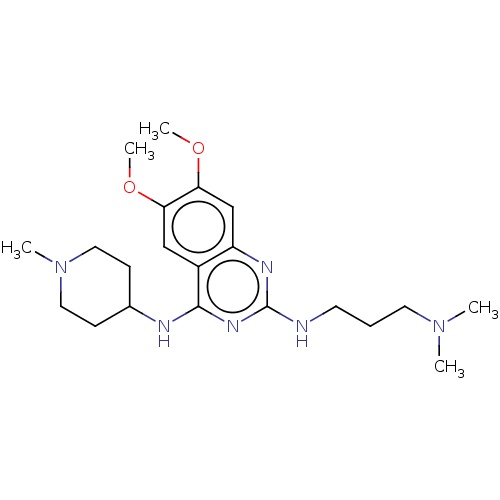
(CHEMBL5199513)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(C)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594096

(CHEMBL5189695)Show SMILES COc1cc2nc(NCCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594080
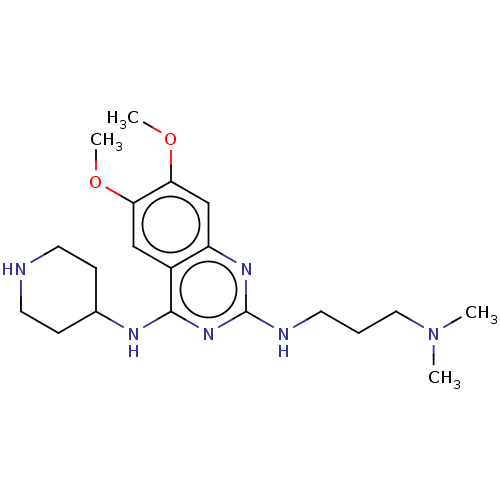
(CHEMBL5208341) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594095
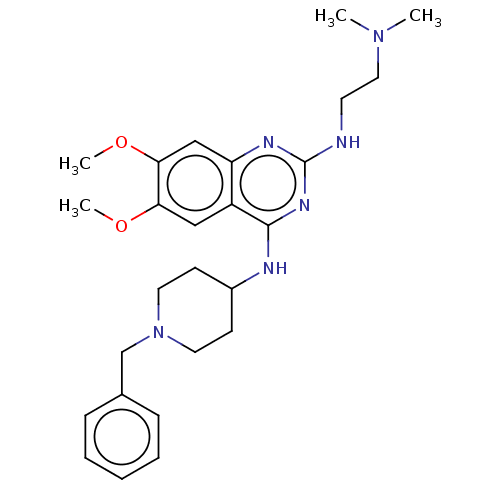
(CHEMBL5178617)Show SMILES COc1cc2nc(NCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594105
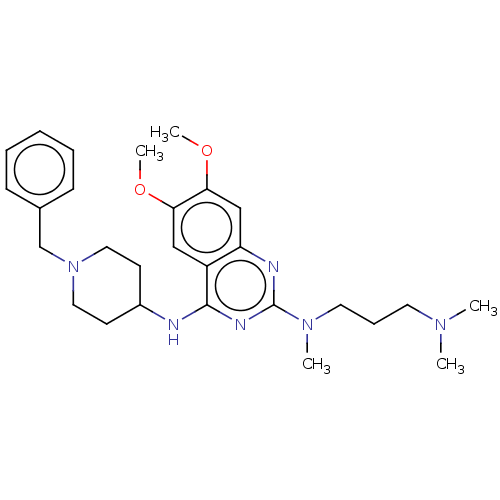
(CHEMBL5189661)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N(C)CCCN(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594104

(CHEMBL5200394)Show SMILES COc1cc2nc(NC3CCN(C)CC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594091

(CHEMBL5183341)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)C3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594097

(CHEMBL5175173)Show SMILES COc1cc2nc(NCCCN(C)Cc3ccccc3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594103

(CHEMBL5194210)Show SMILES COc1cc2nc(NCCCN3CCN(C)CC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396981
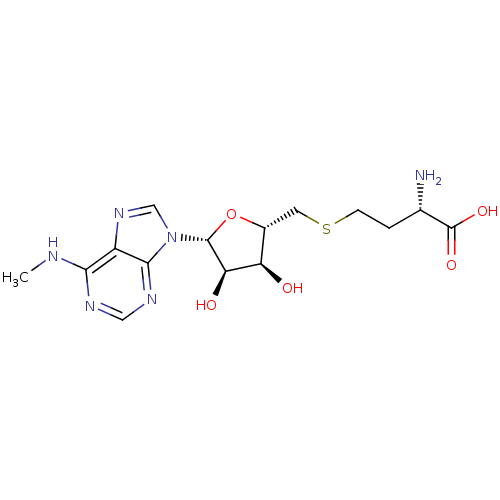
(CHEMBL2171174)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CSCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22N6O5S/c1-17-12-9-13(19-5-18-12)21(6-20-9)14-11(23)10(22)8(26-14)4-27-3-2-7(16)15(24)25/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H,24,25)(H,17,18,19)/t7-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Competitive inhibition of G9a (unknown origin) using histone-H3 (1 to 21) as substrate preincubated for 10 mins followed by substrate addition measur... |
J Med Chem 58: 1596-629 (2015)
Article DOI: 10.1021/jm501234a
BindingDB Entry DOI: 10.7270/Q28K7BS2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594107

(CHEMBL5189756)Show SMILES COc1cc2c(NCCNc3cccc4ccccc34)nc(NCCCN(C)C)nc2cc1OCc1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594102

(CHEMBL5207435)Show SMILES COc1cc2nc(NCCCN3CCOCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594092
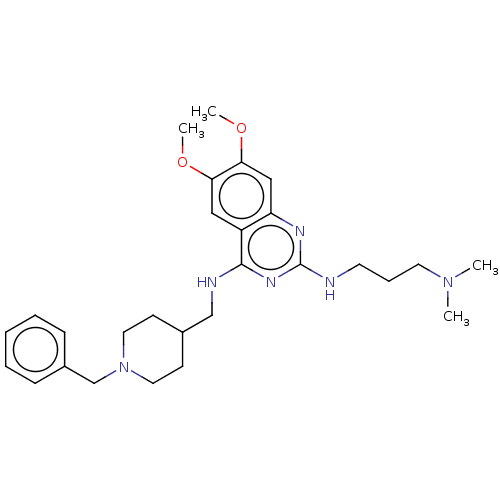
(CHEMBL5171992)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NCC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594098
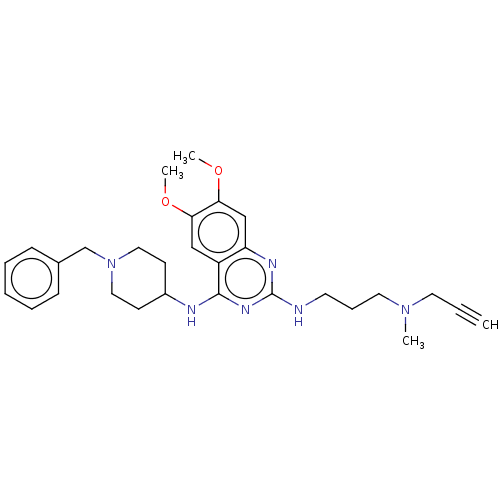
(CHEMBL5198334)Show SMILES COc1cc2nc(NCCCN(C)CC#C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594089

(CHEMBL5181174)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CC3)C(=O)c3ccccc3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594106
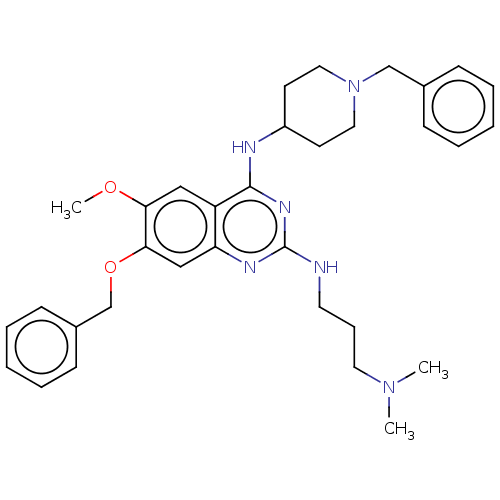
(CHEMBL5202637)Show SMILES COc1cc2c(NC3CCN(Cc4ccccc4)CC3)nc(NCCCN(C)C)nc2cc1OCc1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594094

(CHEMBL5194934)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NCCNc3cccc4ccccc34)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594090
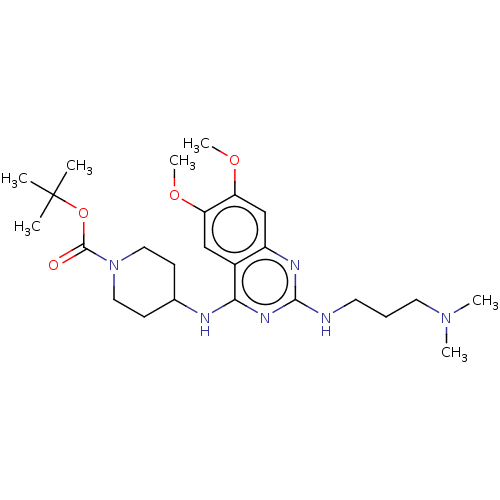
(CHEMBL5205888)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CC3)C(=O)OC(C)(C)C)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594093
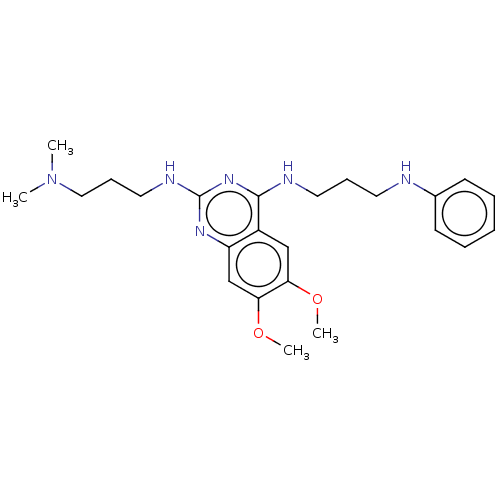
(CHEMBL5191114)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NCCCNc3ccccc3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594087
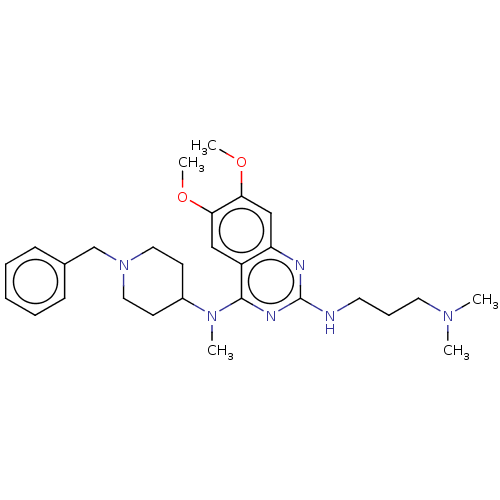
(CHEMBL5189687)Show SMILES COc1cc2nc(NCCCN(C)C)nc(N(C)C3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50594088

(CHEMBL5172748)Show SMILES COc1cc2nc(NCCCN(C)C)nc(OC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50281305
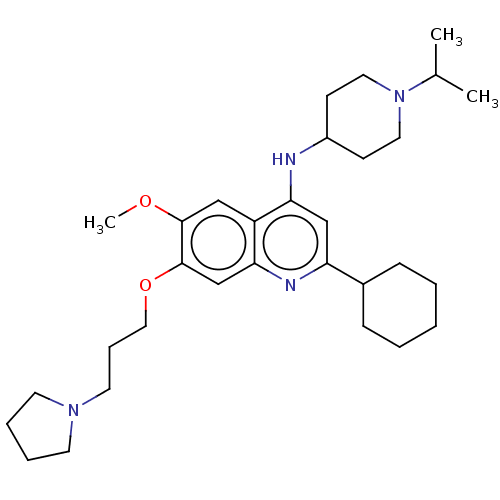
(CHEMBL4162206)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)cc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C31H48N4O2/c1-23(2)35-17-12-25(13-18-35)32-28-21-27(24-10-5-4-6-11-24)33-29-22-31(30(36-3)20-26(28)29)37-19-9-16-34-14-7-8-15-34/h20-25H,4-19H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Oviedo-Principado de Asturias
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human G9a using biotinylated-H3K9 peptide as substrate after 1 hr in presence of 2 to 640 uM SAM by TR-FRET assay |
J Med Chem 61: 6518-6545 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01926
BindingDB Entry DOI: 10.7270/Q23X895V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50281300

(CHEMBL4170225)Show SMILES COc1cc2c(NC3CCN(C)CC3)cc(nc2cc1OCCCN1CCCC1)-c1ccc(C)[nH]1 Show InChI InChI=1S/C28H39N5O2/c1-20-7-8-23(29-20)26-18-24(30-21-9-14-32(2)15-10-21)22-17-27(34-3)28(19-25(22)31-26)35-16-6-13-33-11-4-5-12-33/h7-8,17-19,21,29H,4-6,9-16H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Oviedo-Principado de Asturias
Curated by ChEMBL
| Assay Description
Inhibition of human G9a using biotinylated-H3K9 peptide as substrate after 1 hr in presence of SAM by TR-FRET assay |
J Med Chem 61: 6518-6545 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01926
BindingDB Entry DOI: 10.7270/Q23X895V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50281305

(CHEMBL4162206)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)cc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C31H48N4O2/c1-23(2)35-17-12-25(13-18-35)32-28-21-27(24-10-5-4-6-11-24)33-29-22-31(30(36-3)20-26(28)29)37-19-9-16-34-14-7-8-15-34/h20-25H,4-19H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Oviedo-Principado de Asturias
Curated by ChEMBL
| Assay Description
Competitive inhibition of human G9a using 40 to 2560 nM biotinylated-H3K9 peptide as substrate after 1 hr in presence of SAM by TR-FRET assay |
J Med Chem 61: 6518-6545 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01926
BindingDB Entry DOI: 10.7270/Q23X895V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50281305

(CHEMBL4162206)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)cc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C31H48N4O2/c1-23(2)35-17-12-25(13-18-35)32-28-21-27(24-10-5-4-6-11-24)33-29-22-31(30(36-3)20-26(28)29)37-19-9-16-34-14-7-8-15-34/h20-25H,4-19H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Oviedo-Principado de Asturias
Curated by ChEMBL
| Assay Description
Inhibition of human G9a using biotinylated-H3K9 peptide as substrate after 1 hr in presence of SAM by TR-FRET assay |
J Med Chem 61: 6518-6545 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01926
BindingDB Entry DOI: 10.7270/Q23X895V |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446378
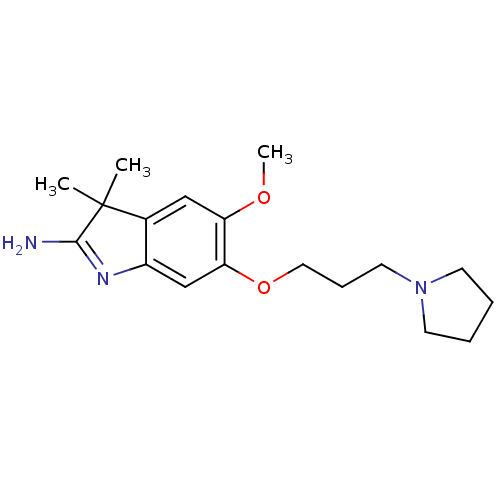
(CHEMBL3109639)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C2(C)C |t:19| Show InChI InChI=1S/C18H27N3O2/c1-18(2)13-11-15(22-3)16(12-14(13)20-17(18)19)23-10-6-9-21-7-4-5-8-21/h11-12H,4-10H2,1-3H3,(H2,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay |
ACS Med Chem Lett 5: 205-9 (2014)
Article DOI: 10.1021/ml400496h
BindingDB Entry DOI: 10.7270/Q2FT8NJ0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446386
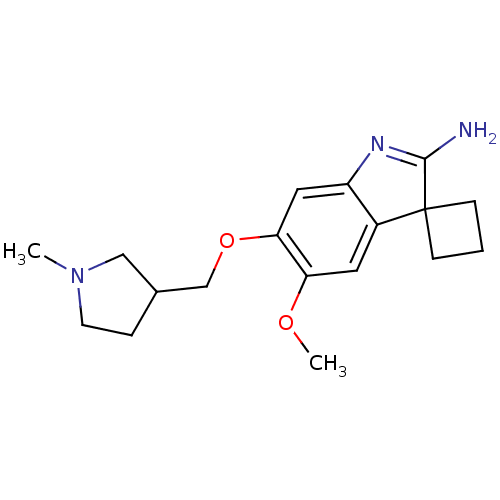
(CHEMBL3109631)Show SMILES COc1cc2c(cc1OCC1CCN(C)C1)N=C(N)C21CCC1 |t:18| Show InChI InChI=1S/C18H25N3O2/c1-21-7-4-12(10-21)11-23-16-9-14-13(8-15(16)22-2)18(5-3-6-18)17(19)20-14/h8-9,12H,3-7,10-11H2,1-2H3,(H2,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay |
ACS Med Chem Lett 5: 205-9 (2014)
Article DOI: 10.1021/ml400496h
BindingDB Entry DOI: 10.7270/Q2FT8NJ0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50599565
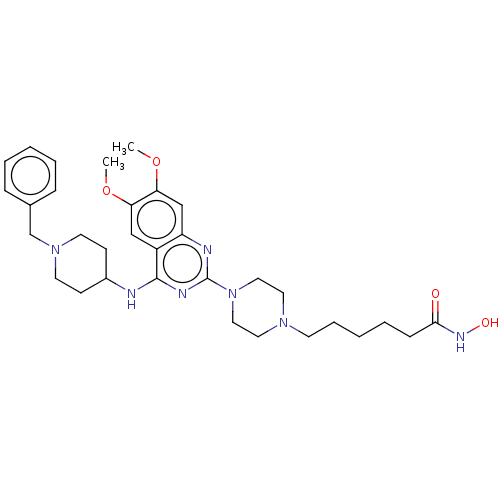
(CHEMBL5191361)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N1CCN(CCCCCC(=O)NO)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116524
BindingDB Entry DOI: 10.7270/Q2W09B07 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592391
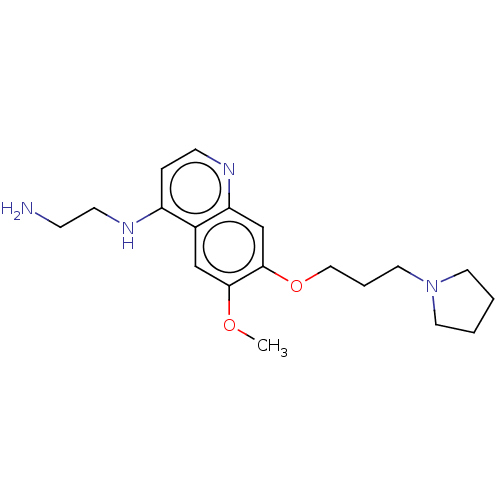
(CHEMBL5187766) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50599563
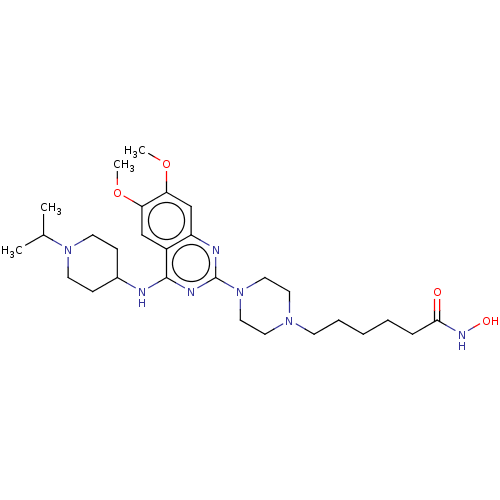
(CHEMBL5171820)Show SMILES COc1cc2nc(nc(NC3CCN(CC3)C(C)C)c2cc1OC)N1CCN(CCCCCC(=O)NO)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116524
BindingDB Entry DOI: 10.7270/Q2W09B07 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353121
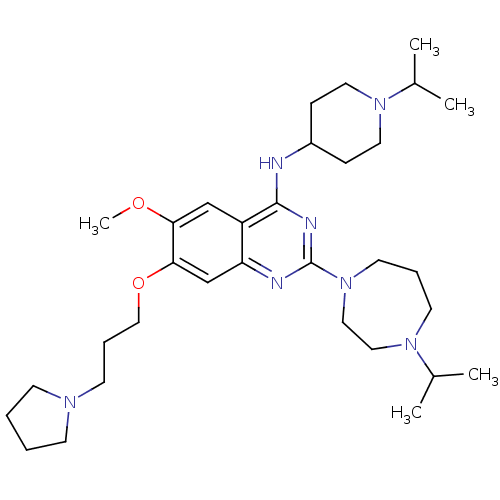
(CHEMBL1829295)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C32H53N7O2/c1-24(2)37-15-8-16-39(20-19-37)32-34-28-23-30(41-21-9-14-36-12-6-7-13-36)29(40-5)22-27(28)31(35-32)33-26-10-17-38(18-11-26)25(3)4/h22-26H,6-21H2,1-5H3,(H,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of G9a assessed as hydrolysis of S-adenosyl-L-homocysteine after 2 mins by SAHH-coupled fluorescence assay |
J Med Chem 54: 6139-50 (2011)
Article DOI: 10.1021/jm200903z
BindingDB Entry DOI: 10.7270/Q237793P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data