 Found 37 hits Enz. Inhib. hit(s) with Target = 'Erythropoietin receptor'
Found 37 hits Enz. Inhib. hit(s) with Target = 'Erythropoietin receptor' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091861
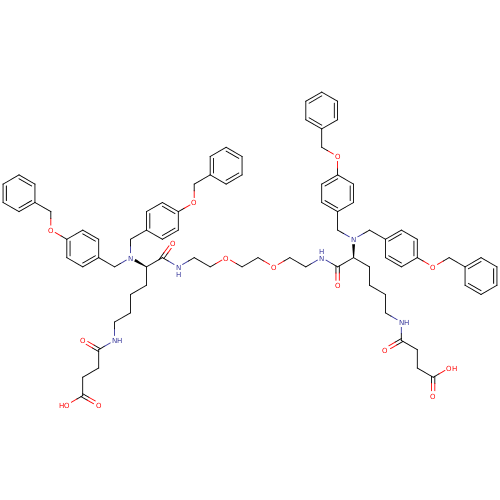
(CHEMBL269188 | N-{(R)-5-[Bis-(4-benzyloxy-benzyl)-...)Show SMILES OC(=O)CCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCOCCOCCNC(=O)[C@H](CCCCNC(=O)CCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C82H96N6O14/c89-77(43-45-79(91)92)83-47-15-13-25-75(87(55-63-27-35-71(36-28-63)99-59-67-17-5-1-6-18-67)56-64-29-37-72(38-30-64)100-60-68-19-7-2-8-20-68)81(95)85-49-51-97-53-54-98-52-50-86-82(96)76(26-14-16-48-84-78(90)44-46-80(93)94)88(57-65-31-39-73(40-32-65)101-61-69-21-9-3-10-22-69)58-66-33-41-74(42-34-66)102-62-70-23-11-4-12-24-70/h1-12,17-24,27-42,75-76H,13-16,25-26,43-62H2,(H,83,89)(H,84,90)(H,85,95)(H,86,96)(H,91,92)(H,93,94)/t75-,76+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091849

(4-{(R)-5-[Bis-(4-benzyloxy-benzyl)-amino]-5-[2-(2-...)Show SMILES OC(=O)CCCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCOCCOCCNC(=O)[C@H](CCCCNC(=O)CCCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C84H100N6O14/c91-79(29-17-31-81(93)94)85-49-15-13-27-77(89(57-65-33-41-73(42-34-65)101-61-69-19-5-1-6-20-69)58-66-35-43-74(44-36-66)102-62-70-21-7-2-8-22-70)83(97)87-51-53-99-55-56-100-54-52-88-84(98)78(28-14-16-50-86-80(92)30-18-32-82(95)96)90(59-67-37-45-75(46-38-67)103-63-71-23-9-3-10-24-71)60-68-39-47-76(48-40-68)104-64-72-25-11-4-12-26-72/h1-12,19-26,33-48,77-78H,13-18,27-32,49-64H2,(H,85,91)(H,86,92)(H,87,97)(H,88,98)(H,93,94)(H,95,96)/t77-,78+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091841

((S)-6-Amino-2-[bis-(4-benzyloxy-benzyl)-amino]-hex...)Show SMILES NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCOCCOCCNC(=O)[C@H](CCCCN)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C74H88N6O8/c75-43-15-13-25-71(79(51-59-27-35-67(36-28-59)85-55-63-17-5-1-6-18-63)52-60-29-37-68(38-30-60)86-56-64-19-7-2-8-20-64)73(81)77-45-47-83-49-50-84-48-46-78-74(82)72(26-14-16-44-76)80(53-61-31-39-69(40-32-61)87-57-65-21-9-3-10-22-65)54-62-33-41-70(42-34-62)88-58-66-23-11-4-12-24-66/h1-12,17-24,27-42,71-72H,13-16,25-26,43-58,75-76H2,(H,77,81)(H,78,82)/t71-,72+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091862
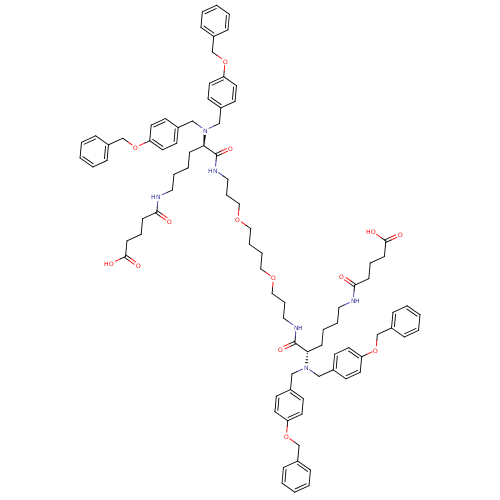
(4-{(S)-5-[Bis-(4-benzyloxy-benzyl)-amino]-5-[3-(4-...)Show SMILES OC(=O)CCCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCCCOCCCNC(=O)[C@H](CCCCNC(=O)CCCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C88H108N6O14/c95-83(33-19-35-85(97)98)89-53-15-13-31-81(93(61-69-37-45-77(46-38-69)105-65-73-23-5-1-6-24-73)62-70-39-47-78(48-40-70)106-66-74-25-7-2-8-26-74)87(101)91-55-21-59-103-57-17-18-58-104-60-22-56-92-88(102)82(32-14-16-54-90-84(96)34-20-36-86(99)100)94(63-71-41-49-79(50-42-71)107-67-75-27-9-3-10-28-75)64-72-43-51-80(52-44-72)108-68-76-29-11-4-12-30-76/h1-12,23-30,37-52,81-82H,13-22,31-36,53-68H2,(H,89,95)(H,90,96)(H,91,101)(H,92,102)(H,97,98)(H,99,100)/t81-,82+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091858
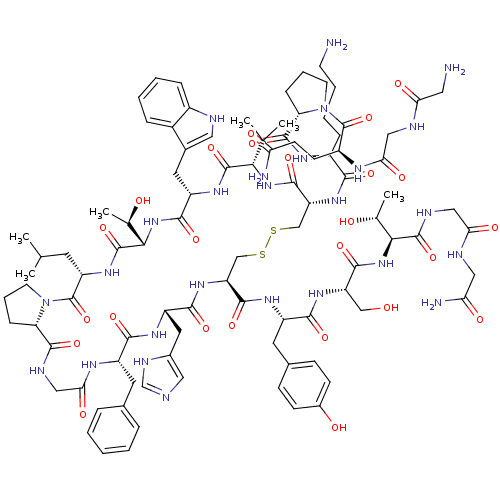
(CHEMBL440818 | GGQPK-cyclic-(CVWTLPGFHC)-YSTGG-NH2)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]2CCCN2C1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)CN)C(C)C)[C@@H](C)O Show InChI InChI=1S/C94H134N26O25S2/c1-48(2)32-65-94(145)119-30-14-21-69(119)88(139)103-43-76(130)107-61(33-52-16-8-7-9-17-52)81(132)110-64(36-55-39-99-47-105-55)83(134)115-67(86(137)109-62(34-53-23-25-56(124)26-24-53)82(133)113-66(44-121)85(136)117-78(50(5)122)90(141)104-41-74(128)101-40-72(98)126)45-146-147-46-68(87(138)116-77(49(3)4)91(142)111-63(84(135)118-79(51(6)123)92(143)112-65)35-54-38-100-58-19-11-10-18-57(54)58)114-80(131)59(20-12-13-29-95)108-89(140)70-22-15-31-120(70)93(144)60(27-28-71(97)125)106-75(129)42-102-73(127)37-96/h7-11,16-19,23-26,38-39,47-51,59-70,77-79,100,121-124H,12-15,20-22,27-37,40-46,95-96H2,1-6H3,(H2,97,125)(H2,98,126)(H,99,105)(H,101,128)(H,102,127)(H,103,139)(H,104,141)(H,106,129)(H,107,130)(H,108,140)(H,109,137)(H,110,132)(H,111,142)(H,112,143)(H,113,133)(H,114,131)(H,115,134)(H,116,138)(H,117,136)(H,118,135)/t50-,51-,59+,60+,61+,62+,63+,64+,65+,66+,67+,68-,69+,70+,77+,78+,79+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091854

(CHEMBL217589 | N-{(S)-5-[Bis-(4-benzyloxy-benzyl)-...)Show SMILES OC(=O)CCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCCCOCCCNC(=O)[C@H](CCCCNC(=O)CCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C86H104N6O14/c93-81(47-49-83(95)96)87-51-15-13-29-79(91(59-67-31-39-75(40-32-67)103-63-71-21-5-1-6-22-71)60-68-33-41-76(42-34-68)104-64-72-23-7-2-8-24-72)85(99)89-53-19-57-101-55-17-18-56-102-58-20-54-90-86(100)80(30-14-16-52-88-82(94)48-50-84(97)98)92(61-69-35-43-77(44-36-69)105-65-73-25-9-3-10-26-73)62-70-37-45-78(46-38-70)106-66-74-27-11-4-12-28-74/h1-12,21-28,31-46,79-80H,13-20,29-30,47-66H2,(H,87,93)(H,88,94)(H,89,99)(H,90,100)(H,95,96)(H,97,98)/t79-,80+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091850

(CHEMBL415681 | N-((S)-5-[Bis-(4-benzyloxy-benzyl)-...)Show SMILES OC(=O)CCC(=O)NCCCC[C@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCOCCOCCCNC(=O)[C@H](CCCCNC(=O)CCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C86H104N6O15/c93-81(45-47-83(95)96)87-49-15-13-27-79(91(59-67-29-37-75(38-30-67)104-63-71-19-5-1-6-20-71)60-68-31-39-76(40-32-68)105-64-72-21-7-2-8-22-72)85(99)89-51-17-53-101-55-57-103-58-56-102-54-18-52-90-86(100)80(28-14-16-50-88-82(94)46-48-84(97)98)92(61-69-33-41-77(42-34-69)106-65-73-23-9-3-10-24-73)62-70-35-43-78(44-36-70)107-66-74-25-11-4-12-26-74/h1-12,19-26,29-44,79-80H,13-18,27-28,45-66H2,(H,87,93)(H,88,94)(H,89,99)(H,90,100)(H,95,96)(H,97,98)/t79-,80-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091843
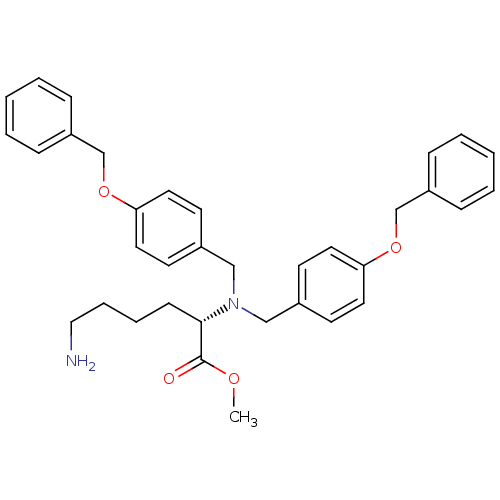
((S)-6-Amino-2-[bis-(4-benzyloxy-benzyl)-amino]-hex...)Show SMILES COC(=O)[C@H](CCCCN)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C35H40N2O4/c1-39-35(38)34(14-8-9-23-36)37(24-28-15-19-32(20-16-28)40-26-30-10-4-2-5-11-30)25-29-17-21-33(22-18-29)41-27-31-12-6-3-7-13-31/h2-7,10-13,15-22,34H,8-9,14,23-27,36H2,1H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091855

((S)-6-Amino-2-[bis-(3-benzyloxy-benzyl)-amino]-hex...)Show SMILES COC(=O)[C@H](CCCCN)N(Cc1cccc(OCc2ccccc2)c1)Cc1cccc(OCc2ccccc2)c1 Show InChI InChI=1S/C35H40N2O4/c1-39-35(38)34(20-8-9-21-36)37(24-30-16-10-18-32(22-30)40-26-28-12-4-2-5-13-28)25-31-17-11-19-33(23-31)41-27-29-14-6-3-7-15-29/h2-7,10-19,22-23,34H,8-9,20-21,24-27,36H2,1H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091851

((S)-6-Amino-2-((3-benzyloxy-benzyl)-{(E)-3-[3-(4-t...)Show SMILES COC(=O)[C@H](CCCCN)N(Cc1cccc(OCc2ccccc2)c1)\C=C\Cc1cccc(Oc2ccc(cc2)C(C)(C)C)c1 Show InChI InChI=1S/C40H48N2O4/c1-40(2,3)34-21-23-35(24-22-34)46-37-19-10-15-31(27-37)17-12-26-42(38(39(43)44-4)20-8-9-25-41)29-33-16-11-18-36(28-33)45-30-32-13-6-5-7-14-32/h5-7,10-16,18-19,21-24,26-28,38H,8-9,17,20,25,29-30,41H2,1-4H3/b26-12+/t38-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091840

(4-((S)-5-[Bis-(4-benzyloxy-benzyl)-amino]-5-{3-[2-...)Show SMILES OC(=O)CCCC(=O)NCCCC[C@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCOCCOCCCNC(=O)[C@H](CCCCNC(=O)CCCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C88H108N6O15/c95-83(31-17-33-85(97)98)89-51-15-13-29-81(93(61-69-35-43-77(44-36-69)106-65-73-21-5-1-6-22-73)62-70-37-45-78(46-38-70)107-66-74-23-7-2-8-24-74)87(101)91-53-19-55-103-57-59-105-60-58-104-56-20-54-92-88(102)82(30-14-16-52-90-84(96)32-18-34-86(99)100)94(63-71-39-47-79(48-40-71)108-67-75-25-9-3-10-26-75)64-72-41-49-80(50-42-72)109-68-76-27-11-4-12-28-76/h1-12,21-28,35-50,81-82H,13-20,29-34,51-68H2,(H,89,95)(H,90,96)(H,91,101)(H,92,102)(H,97,98)(H,99,100)/t81-,82-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091837

((R)-3-[Bis-(4-benzyloxy-benzyl)-amino]-N-{3-[4-(3-...)Show SMILES OC(=O)C[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCCCOCCCNC(=O)[C@H](CC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C74H82N4O12/c79-71(80)47-69(77(49-57-25-33-65(34-26-57)87-53-61-17-5-1-6-18-61)50-58-27-35-66(36-28-58)88-54-62-19-7-2-8-20-62)73(83)75-41-15-45-85-43-13-14-44-86-46-16-42-76-74(84)70(48-72(81)82)78(51-59-29-37-67(38-30-59)89-55-63-21-9-3-10-22-63)52-60-31-39-68(40-32-60)90-56-64-23-11-4-12-24-64/h1-12,17-40,69-70H,13-16,41-56H2,(H,75,83)(H,76,84)(H,79,80)(H,81,82)/t69-,70+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091838

((S)-3-[Bis-(4-benzyloxy-benzyl)-amino]-N-(3-{2-[2-...)Show SMILES OC(=O)C[C@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCOCCOCCOCCCNC(=O)[C@H](CC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C74H82N4O13/c79-71(80)47-69(77(49-57-23-31-65(32-24-57)88-53-61-15-5-1-6-16-61)50-58-25-33-66(34-26-58)89-54-62-17-7-2-8-18-62)73(83)75-39-13-41-85-43-45-87-46-44-86-42-14-40-76-74(84)70(48-72(81)82)78(51-59-27-35-67(36-28-59)90-55-63-19-9-3-10-20-63)52-60-29-37-68(38-30-60)91-56-64-21-11-4-12-22-64/h1-12,15-38,69-70H,13-14,39-56H2,(H,75,83)(H,76,84)(H,79,80)(H,81,82)/t69-,70-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091844

((S)-2-(Bis-{(E)-3-[3-(4-tert-butyl-phenoxy)-phenyl...)Show SMILES CC(C)(C)c1ccc(Oc2cccc(\C=C\CN(C\C=C\c3cccc(Oc4ccc(cc4)C(C)(C)C)c3)[C@@H](CCC(O)=O)C(O)=O)c2)cc1 Show InChI InChI=1S/C43H49NO6/c1-42(2,3)33-17-21-35(22-18-33)49-37-15-7-11-31(29-37)13-9-27-44(39(41(47)48)25-26-40(45)46)28-10-14-32-12-8-16-38(30-32)50-36-23-19-34(20-24-36)43(4,5)6/h7-24,29-30,39H,25-28H2,1-6H3,(H,45,46)(H,47,48)/b13-9+,14-10+/t39-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091846
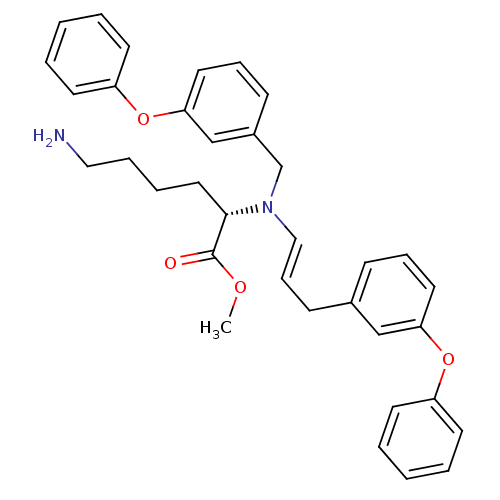
((S)-6-Amino-2-{(3-phenoxy-benzyl)-[(E)-3-(3-phenox...)Show SMILES COC(=O)[C@H](CCCCN)N(Cc1cccc(Oc2ccccc2)c1)\C=C\Cc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C35H38N2O4/c1-39-35(38)34(22-8-9-23-36)37(27-29-14-11-21-33(26-29)41-31-18-6-3-7-19-31)24-12-15-28-13-10-20-32(25-28)40-30-16-4-2-5-17-30/h2-7,10-14,16-21,24-26,34H,8-9,15,22-23,27,36H2,1H3/b24-12+/t34-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091859

((S)-2-(Bis-{(E)-3-[3-(3-trifluoromethyl-phenoxy)-p...)Show SMILES OC(=O)CC[C@H](N(C\C=C\c1cccc(Oc2cccc(c2)C(F)(F)F)c1)C\C=C\c1cccc(Oc2cccc(c2)C(F)(F)F)c1)C(O)=O Show InChI InChI=1S/C37H31F6NO6/c38-36(39,40)27-11-3-15-31(23-27)49-29-13-1-7-25(21-29)9-5-19-44(33(35(47)48)17-18-34(45)46)20-6-10-26-8-2-14-30(22-26)50-32-16-4-12-28(24-32)37(41,42)43/h1-16,21-24,33H,17-20H2,(H,45,46)(H,47,48)/b9-5+,10-6+/t33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091842
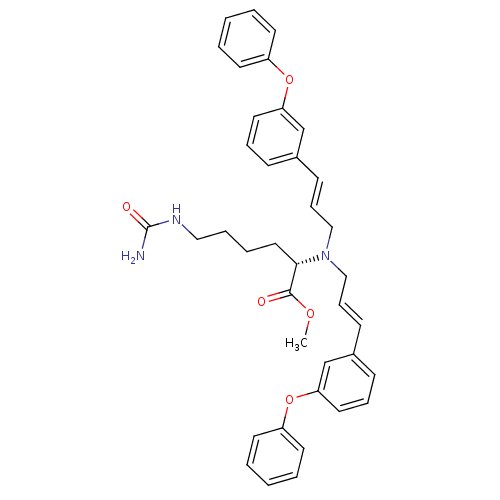
((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES COC(=O)[C@H](CCCCNC(N)=O)N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C38H41N3O5/c1-44-37(42)36(24-8-9-25-40-38(39)43)41(26-12-16-30-14-10-22-34(28-30)45-32-18-4-2-5-19-32)27-13-17-31-15-11-23-35(29-31)46-33-20-6-3-7-21-33/h2-7,10-23,28-29,36H,8-9,24-27H2,1H3,(H3,39,40,43)/b16-12+,17-13+/t36-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091857

((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES COC(=O)[C@H](CCCCNS(C)(=O)=O)N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C38H42N2O6S/c1-44-38(41)37(25-9-10-26-39-47(2,42)43)40(27-13-17-31-15-11-23-35(29-31)45-33-19-5-3-6-20-33)28-14-18-32-16-12-24-36(30-32)46-34-21-7-4-8-22-34/h3-8,11-24,29-30,37,39H,9-10,25-28H2,1-2H3/b17-13+,18-14+/t37-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091856

((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES OC(=O)C[C@H](N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1)C(O)=O Show InChI InChI=1S/C34H31NO6/c36-33(37)25-32(34(38)39)35(21-9-13-26-11-7-19-30(23-26)40-28-15-3-1-4-16-28)22-10-14-27-12-8-20-31(24-27)41-29-17-5-2-6-18-29/h1-20,23-24,32H,21-22,25H2,(H,36,37)(H,38,39)/b13-9+,14-10+/t32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091860

((S)-2-{(3-Phenoxy-benzyl)-[(E)-3-(3-phenoxy-phenyl...)Show SMILES COC(=O)[C@H](CC(O)=O)N(Cc1cccc(Oc2ccccc2)c1)\C=C\Cc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C33H31NO6/c1-38-33(37)31(23-32(35)36)34(24-26-12-9-19-30(22-26)40-28-16-6-3-7-17-28)20-10-13-25-11-8-18-29(21-25)39-27-14-4-2-5-15-27/h2-12,14-22,31H,13,23-24H2,1H3,(H,35,36)/b20-10+/t31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091839

((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES C[C@H](N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1)C(O)=O Show InChI InChI=1S/C33H31NO4/c1-26(33(35)36)34(22-10-14-27-12-8-20-31(24-27)37-29-16-4-2-5-17-29)23-11-15-28-13-9-21-32(25-28)38-30-18-6-3-7-19-30/h2-21,24-26H,22-23H2,1H3,(H,35,36)/b14-10+,15-11+/t26-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091836

((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES OC(=O)CC[C@H](N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1)C(O)=O Show InChI InChI=1S/C35H33NO6/c37-34(38)22-21-33(35(39)40)36(23-9-13-27-11-7-19-31(25-27)41-29-15-3-1-4-16-29)24-10-14-28-12-8-20-32(26-28)42-30-17-5-2-6-18-30/h1-20,25-26,33H,21-24H2,(H,37,38)(H,39,40)/b13-9+,14-10+/t33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091845

((S)-4-[Bis-(4-benzyloxy-benzyl)-amino]-4-{2-[2-(2-...)Show SMILES OC(=O)CC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCOCCOCCNC(=O)[C@H](CCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C72H78N4O12/c77-69(78)39-37-67(75(47-55-21-29-63(30-22-55)85-51-59-13-5-1-6-14-59)48-56-23-31-64(32-24-56)86-52-60-15-7-2-8-16-60)71(81)73-41-43-83-45-46-84-44-42-74-72(82)68(38-40-70(79)80)76(49-57-25-33-65(34-26-57)87-53-61-17-9-3-10-18-61)50-58-27-35-66(36-28-58)88-54-62-19-11-4-12-20-62/h1-36,67-68H,37-54H2,(H,73,81)(H,74,82)(H,77,78)(H,79,80)/t67-,68+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091847

(4-((S)-5-[Bis-(4-benzyloxy-benzyl)-amino]-5-{12-[(...)Show SMILES OC(=O)CCCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCCCCCCCCCCNC(=O)[C@H](CCCCNC(=O)CCCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C90H112N6O12/c97-85(39-27-41-87(99)100)91-59-25-21-37-83(95(63-71-43-51-79(52-44-71)105-67-75-29-13-9-14-30-75)64-72-45-53-80(54-46-72)106-68-76-31-15-10-16-32-76)89(103)93-61-23-7-5-3-1-2-4-6-8-24-62-94-90(104)84(38-22-26-60-92-86(98)40-28-42-88(101)102)96(65-73-47-55-81(56-48-73)107-69-77-33-17-11-18-34-77)66-74-49-57-82(58-50-74)108-70-78-35-19-12-20-36-78/h9-20,29-36,43-58,83-84H,1-8,21-28,37-42,59-70H2,(H,91,97)(H,92,98)(H,93,103)(H,94,104)(H,99,100)(H,101,102)/t83-,84+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091848

(CHEMBL59107 | {Bis-[(E)-3-(3-phenoxy-phenyl)-allyl...)Show SMILES OC(=O)CN(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C32H29NO4/c34-32(35)25-33(21-9-13-26-11-7-19-30(23-26)36-28-15-3-1-4-16-28)22-10-14-27-12-8-20-31(24-27)37-29-17-5-2-6-18-29/h1-20,23-24H,21-22,25H2,(H,34,35)/b13-9+,14-10+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091852

(CHEMBL268782 | N-((S)-5-[Bis-(4-benzyloxy-benzyl)-...)Show SMILES OC(=O)CCC(=O)NCCCC[C@@H](N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1)C(=O)NCCCCCCCCCCCCNC(=O)[C@H](CCCCNC(=O)CCC(O)=O)N(Cc1ccc(OCc2ccccc2)cc1)Cc1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C88H108N6O12/c95-83(53-55-85(97)98)89-57-25-21-35-81(93(61-69-37-45-77(46-38-69)103-65-73-27-13-9-14-28-73)62-70-39-47-78(48-40-70)104-66-74-29-15-10-16-30-74)87(101)91-59-23-7-5-3-1-2-4-6-8-24-60-92-88(102)82(36-22-26-58-90-84(96)54-56-86(99)100)94(63-71-41-49-79(50-42-71)105-67-75-31-17-11-18-32-75)64-72-43-51-80(52-44-72)106-68-76-33-19-12-20-34-76/h9-20,27-34,37-52,81-82H,1-8,21-26,35-36,53-68H2,(H,89,95)(H,90,96)(H,91,101)(H,92,102)(H,97,98)(H,99,100)/t81-,82+ | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50091853

((S)-2-{Bis-[(E)-3-(3-phenoxy-phenyl)-allyl]-amino}...)Show SMILES COC(=O)[C@H](CO)N(C\C=C\c1cccc(Oc2ccccc2)c1)C\C=C\c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C34H33NO5/c1-38-34(37)33(26-36)35(22-10-14-27-12-8-20-31(24-27)39-29-16-4-2-5-17-29)23-11-15-28-13-9-21-32(25-28)40-30-18-6-3-7-19-30/h2-21,24-25,33,36H,22-23,26H2,1H3/b14-10+,15-11+/t33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [125I]-EPO binding to Erythropoietin receptor (EBP) |
Bioorg Med Chem Lett 10: 1995-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8KXD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463572

(CHEMBL4244951)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(Br)cc2)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C103H160BrN29O25S3/c1-14-56(8)84-99(155)130-85(58(10)134)100(156)125-72(42-60-24-28-62(104)29-25-60)93(149)128-83(55(6)7)98(154)127-75(94(150)121-68(32-33-78(106)137)102(158)133-38-19-23-77(133)96(152)124-70(41-54(4)5)91(147)120-67(21-17-36-111-103(108)109)101(157)131(12)49-81(140)117-65(86(107)142)20-15-16-35-105)51-161-160-50-74(95(151)123-73(44-63-45-110-52-115-63)92(148)119-66(34-39-159-13)88(144)114-48-82(141)132-37-18-22-76(132)97(153)129-84)126-87(143)57(9)116-89(145)71(43-61-26-30-64(136)31-27-61)122-90(146)69(40-53(2)3)118-80(139)47-113-79(138)46-112-59(11)135/h24-31,45,52-58,65-77,83-85,134,136H,14-23,32-44,46-51,105H2,1-13H3,(H2,106,137)(H2,107,142)(H,110,115)(H,112,135)(H,113,138)(H,114,144)(H,116,145)(H,117,140)(H,118,139)(H,119,148)(H,120,147)(H,121,150)(H,122,146)(H,123,151)(H,124,152)(H,125,156)(H,126,143)(H,127,154)(H,128,149)(H,129,153)(H,130,155)(H4,108,109,111)/t56-,57-,58+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,83-,84-,85-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463573

(CHEMBL4239773)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C106H163N29O25S3/c1-16-58(8)87-102(157)132-88(61(11)136)103(158)127-76(45-65-26-21-25-64-24-17-18-27-68(64)65)97(152)130-86(57(6)7)101(156)129-79(98(153)123-72(35-36-81(108)139)105(160)134(14)60(10)91(146)124-74(43-56(4)5)95(150)122-71(29-22-39-113-106(110)111)104(159)133(13)51-84(142)119-69(89(109)144)28-19-20-38-107)53-163-162-52-78(99(154)126-77(46-66-47-112-54-117-66)96(151)121-70(37-41-161-15)92(147)116-50-85(143)135-40-23-30-80(135)100(155)131-87)128-90(145)59(9)118-93(148)75(44-63-31-33-67(138)34-32-63)125-94(149)73(42-55(2)3)120-83(141)49-115-82(140)48-114-62(12)137/h17-18,21,24-27,31-34,47,54-61,69-80,86-88,136,138H,16,19-20,22-23,28-30,35-46,48-53,107H2,1-15H3,(H2,108,139)(H2,109,144)(H,112,117)(H,114,137)(H,115,140)(H,116,147)(H,118,148)(H,119,142)(H,120,141)(H,121,151)(H,122,150)(H,123,153)(H,124,146)(H,125,149)(H,126,154)(H,127,158)(H,128,145)(H,129,156)(H,130,152)(H,131,155)(H,132,157)(H4,110,111,113)/t58-,59-,60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,86-,87-,88-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463574
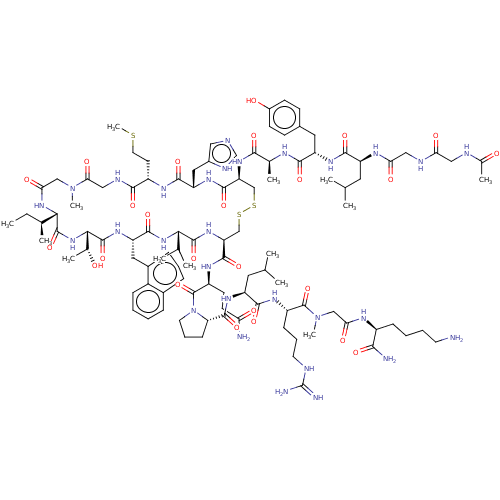
(CHEMBL4239220)Show SMILES CC[C@H](C)[C@@H]1NC(=O)CN(C)C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C105H161N29O25S3/c1-15-58(8)87-101(156)131-88(60(10)135)102(157)126-75(44-64-25-20-24-63-23-16-17-26-67(63)64)96(151)130-86(57(6)7)100(155)128-78(97(152)122-71(34-35-80(107)138)104(159)134-39-22-29-79(134)99(154)125-73(42-56(4)5)94(149)121-70(28-21-38-112-105(109)110)103(158)133(13)51-83(141)118-68(89(108)144)27-18-19-37-106)53-162-161-52-77(98(153)124-76(45-65-46-111-54-116-65)95(150)120-69(36-40-160-14)91(146)115-49-85(143)132(12)50-84(142)129-87)127-90(145)59(9)117-92(147)74(43-62-30-32-66(137)33-31-62)123-93(148)72(41-55(2)3)119-82(140)48-114-81(139)47-113-61(11)136/h16-17,20,23-26,30-33,46,54-60,68-79,86-88,135,137H,15,18-19,21-22,27-29,34-45,47-53,106H2,1-14H3,(H2,107,138)(H2,108,144)(H,111,116)(H,113,136)(H,114,139)(H,115,146)(H,117,147)(H,118,141)(H,119,140)(H,120,150)(H,121,149)(H,122,152)(H,123,148)(H,124,153)(H,125,154)(H,126,157)(H,127,145)(H,128,155)(H,129,142)(H,130,151)(H,131,156)(H4,109,110,112)/t58-,59-,60+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,86-,87-,88-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463575

(CHEMBL4244759)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C107H163N29O25S3/c1-14-59(8)88-103(158)133-89(61(10)137)104(159)128-76(46-65-25-19-24-64-23-15-16-26-68(64)65)97(152)131-87(58(6)7)102(157)130-79(98(153)124-72(35-36-82(109)140)106(161)136-41-22-30-81(136)100(155)127-74(44-57(4)5)95(150)123-71(28-20-39-114-107(111)112)105(160)134(12)52-85(143)120-69(90(110)145)27-17-18-38-108)54-164-163-53-78(99(154)126-77(47-66-48-113-55-118-66)96(151)122-70(37-42-162-13)92(147)117-51-86(144)135-40-21-29-80(135)101(156)132-88)129-91(146)60(9)119-93(148)75(45-63-31-33-67(139)34-32-63)125-94(149)73(43-56(2)3)121-84(142)50-116-83(141)49-115-62(11)138/h15-16,19,23-26,31-34,48,55-61,69-81,87-89,137,139H,14,17-18,20-22,27-30,35-47,49-54,108H2,1-13H3,(H2,109,140)(H2,110,145)(H,113,118)(H,115,138)(H,116,141)(H,117,147)(H,119,148)(H,120,143)(H,121,142)(H,122,151)(H,123,150)(H,124,153)(H,125,149)(H,126,154)(H,127,155)(H,128,159)(H,129,146)(H,130,157)(H,131,152)(H,132,156)(H,133,158)(H4,111,112,114)/t59-,60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463571
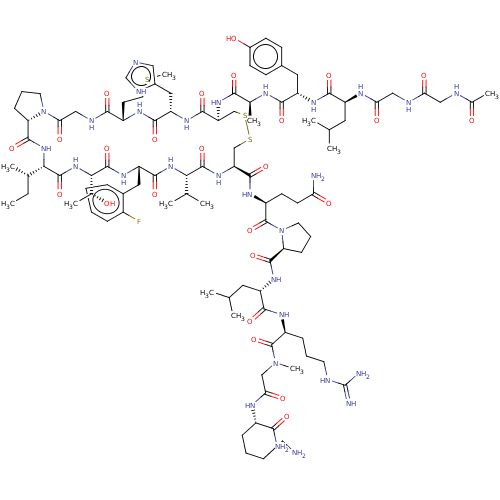
(CHEMBL4244160)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2F)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C103H160FN29O25S3/c1-14-56(8)84-99(155)130-85(58(10)134)100(156)125-72(43-61-22-15-16-23-64(61)104)93(149)128-83(55(6)7)98(154)127-75(94(150)121-68(32-33-78(106)137)102(158)133-38-21-27-77(133)96(152)124-70(41-54(4)5)91(147)120-67(25-19-36-111-103(108)109)101(157)131(12)49-81(140)117-65(86(107)142)24-17-18-35-105)51-161-160-50-74(95(151)123-73(44-62-45-110-52-115-62)92(148)119-66(34-39-159-13)88(144)114-48-82(141)132-37-20-26-76(132)97(153)129-84)126-87(143)57(9)116-89(145)71(42-60-28-30-63(136)31-29-60)122-90(146)69(40-53(2)3)118-80(139)47-113-79(138)46-112-59(11)135/h15-16,22-23,28-31,45,52-58,65-77,83-85,134,136H,14,17-21,24-27,32-44,46-51,105H2,1-13H3,(H2,106,137)(H2,107,142)(H,110,115)(H,112,135)(H,113,138)(H,114,144)(H,116,145)(H,117,140)(H,118,139)(H,119,148)(H,120,147)(H,121,150)(H,122,146)(H,123,151)(H,124,152)(H,125,156)(H,126,143)(H,127,154)(H,128,149)(H,129,153)(H,130,155)(H4,108,109,111)/t56-,57-,58+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,83-,84-,85-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463570

(CHEMBL4243549)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C107H162N28O26S3/c1-14-59(8)88-103(157)132-89(61(10)136)104(158)127-76(46-65-25-19-24-64-23-15-16-26-68(64)65)97(151)130-87(58(6)7)102(156)129-79(98(152)123-72(35-36-82(109)139)106(160)135-41-22-30-81(135)100(154)126-74(44-57(4)5)95(149)122-71(28-20-39-113-107(111)161)105(159)133(12)52-85(142)119-69(90(110)144)27-17-18-38-108)54-164-163-53-78(99(153)125-77(47-66-48-112-55-117-66)96(150)121-70(37-42-162-13)92(146)116-51-86(143)134-40-21-29-80(134)101(155)131-88)128-91(145)60(9)118-93(147)75(45-63-31-33-67(138)34-32-63)124-94(148)73(43-56(2)3)120-84(141)50-115-83(140)49-114-62(11)137/h15-16,19,23-26,31-34,48,55-61,69-81,87-89,136,138H,14,17-18,20-22,27-30,35-47,49-54,108H2,1-13H3,(H2,109,139)(H2,110,144)(H,112,117)(H,114,137)(H,115,140)(H,116,146)(H,118,147)(H,119,142)(H,120,141)(H,121,150)(H,122,149)(H,123,152)(H,124,148)(H,125,153)(H,126,154)(H,127,158)(H,128,145)(H,129,156)(H,130,151)(H,131,155)(H,132,157)(H3,111,113,161)/t59-,60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463568

(CHEMBL4246983)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C103H160ClN29O25S3/c1-14-56(8)84-99(155)130-85(58(10)134)100(156)125-72(42-60-24-28-62(104)29-25-60)93(149)128-83(55(6)7)98(154)127-75(94(150)121-68(32-33-78(106)137)102(158)133-38-19-23-77(133)96(152)124-70(41-54(4)5)91(147)120-67(21-17-36-111-103(108)109)101(157)131(12)49-81(140)117-65(86(107)142)20-15-16-35-105)51-161-160-50-74(95(151)123-73(44-63-45-110-52-115-63)92(148)119-66(34-39-159-13)88(144)114-48-82(141)132-37-18-22-76(132)97(153)129-84)126-87(143)57(9)116-89(145)71(43-61-26-30-64(136)31-27-61)122-90(146)69(40-53(2)3)118-80(139)47-113-79(138)46-112-59(11)135/h24-31,45,52-58,65-77,83-85,134,136H,14-23,32-44,46-51,105H2,1-13H3,(H2,106,137)(H2,107,142)(H,110,115)(H,112,135)(H,113,138)(H,114,144)(H,116,145)(H,117,140)(H,118,139)(H,119,148)(H,120,147)(H,121,150)(H,122,146)(H,123,151)(H,124,152)(H,125,156)(H,126,143)(H,127,154)(H,128,149)(H,129,153)(H,130,155)(H4,108,109,111)/t56-,57-,58+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,83-,84-,85-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463567
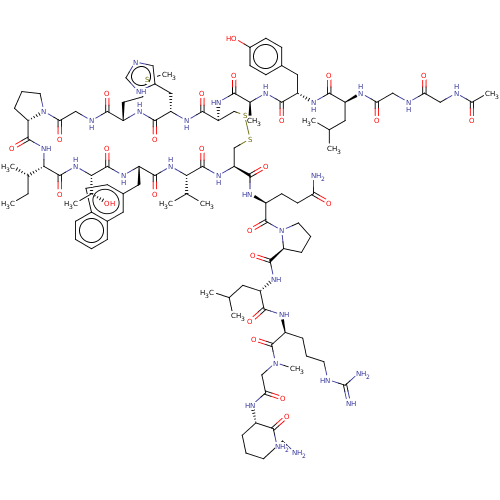
(CHEMBL4240318)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C107H163N29O25S3/c1-14-59(8)88-103(158)133-89(61(10)137)104(159)128-76(46-64-28-31-65-22-15-16-23-66(65)44-64)97(152)131-87(58(6)7)102(157)130-79(98(153)124-72(34-35-82(109)140)106(161)136-40-21-27-81(136)100(155)127-74(43-57(4)5)95(150)123-71(25-19-38-114-107(111)112)105(160)134(12)52-85(143)120-69(90(110)145)24-17-18-37-108)54-164-163-53-78(99(154)126-77(47-67-48-113-55-118-67)96(151)122-70(36-41-162-13)92(147)117-51-86(144)135-39-20-26-80(135)101(156)132-88)129-91(146)60(9)119-93(148)75(45-63-29-32-68(139)33-30-63)125-94(149)73(42-56(2)3)121-84(142)50-116-83(141)49-115-62(11)138/h15-16,22-23,28-33,44,48,55-61,69-81,87-89,137,139H,14,17-21,24-27,34-43,45-47,49-54,108H2,1-13H3,(H2,109,140)(H2,110,145)(H,113,118)(H,115,138)(H,116,141)(H,117,147)(H,119,148)(H,120,143)(H,121,142)(H,122,151)(H,123,150)(H,124,153)(H,125,149)(H,126,154)(H,127,155)(H,128,159)(H,129,146)(H,130,157)(H,131,152)(H,132,156)(H,133,158)(H4,111,112,114)/t59-,60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463576
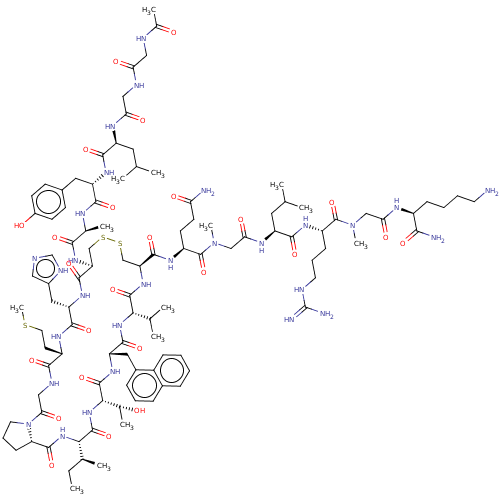
(CHEMBL4249192)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N(C)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C105H161N29O25S3/c1-15-58(8)87-101(156)131-88(60(10)135)102(157)126-75(44-64-25-20-24-63-23-16-17-26-67(63)64)96(151)129-86(57(6)7)100(155)128-78(97(152)123-71(34-35-80(107)138)104(159)133(13)51-84(142)120-73(42-56(4)5)93(148)122-70(28-21-38-112-105(109)110)103(158)132(12)50-83(141)118-68(89(108)144)27-18-19-37-106)53-162-161-52-77(98(153)125-76(45-65-46-111-54-116-65)95(150)121-69(36-40-160-14)91(146)115-49-85(143)134-39-22-29-79(134)99(154)130-87)127-90(145)59(9)117-92(147)74(43-62-30-32-66(137)33-31-62)124-94(149)72(41-55(2)3)119-82(140)48-114-81(139)47-113-61(11)136/h16-17,20,23-26,30-33,46,54-60,68-79,86-88,135,137H,15,18-19,21-22,27-29,34-45,47-53,106H2,1-14H3,(H2,107,138)(H2,108,144)(H,111,116)(H,113,136)(H,114,139)(H,115,146)(H,117,147)(H,118,141)(H,119,140)(H,120,142)(H,121,150)(H,122,148)(H,123,152)(H,124,149)(H,125,153)(H,126,157)(H,127,145)(H,128,155)(H,129,151)(H,130,154)(H,131,156)(H4,109,110,112)/t58-,59-,60+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,86-,87-,88-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |
Erythropoietin receptor
(Homo sapiens (Human)) | BDBM50463569

(CHEMBL4243291)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](C)N(C)C(=O)CNC(=O)[C@H](CCSC)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](NC1=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)CC(=O)N[C@@H](CCCCN)C(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)CNC(C)=O |r| Show InChI InChI=1S/C106H163N29O25S3/c1-16-58(8)87-102(157)132-88(61(11)136)103(158)127-76(45-65-26-21-25-64-24-17-18-27-68(64)65)97(152)130-86(57(6)7)101(156)129-79(98(153)123-72(35-36-81(108)139)105(160)135-40-23-30-80(135)100(155)126-74(43-56(4)5)95(150)122-71(29-22-39-113-106(110)111)104(159)133(13)51-84(142)119-69(89(109)144)28-19-20-38-107)53-163-162-52-78(99(154)125-77(46-66-47-112-54-117-66)96(151)121-70(37-41-161-15)92(147)116-50-85(143)134(14)60(10)91(146)131-87)128-90(145)59(9)118-93(148)75(44-63-31-33-67(138)34-32-63)124-94(149)73(42-55(2)3)120-83(141)49-115-82(140)48-114-62(12)137/h17-18,21,24-27,31-34,47,54-61,69-80,86-88,136,138H,16,19-20,22-23,28-30,35-46,48-53,107H2,1-15H3,(H2,108,139)(H2,109,144)(H,112,117)(H,114,137)(H,115,140)(H,116,147)(H,118,148)(H,119,142)(H,120,141)(H,121,151)(H,122,150)(H,123,153)(H,124,149)(H,125,154)(H,126,155)(H,127,158)(H,128,145)(H,129,156)(H,130,152)(H,131,146)(H,132,157)(H4,110,111,113)/t58-,59-,60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,86-,87-,88-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Agonist activity at EPO receptor in human TF1 cells assessed as induction of cell proliferation after 48 hrs by MTT assay |
Bioorg Med Chem Lett 28: 3038-3041 (2018)
Article DOI: 10.1016/j.bmcl.2018.08.004
BindingDB Entry DOI: 10.7270/Q2J67KMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




































