

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecdysone receptor/Protein ultraspiracle homolog (Choristoneura fumiferana) | BDBM50178988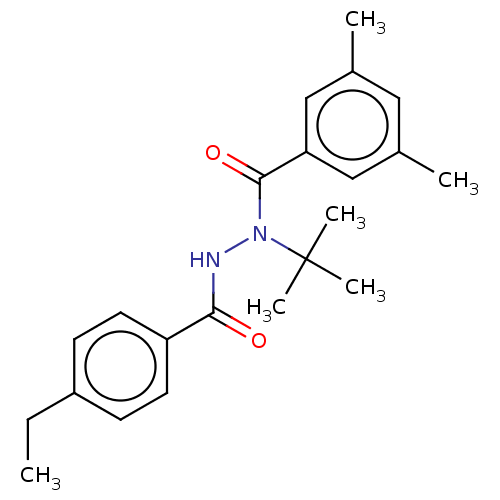 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Choristoneura fumiferana EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle (Chilo suppressalis) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Chilo suppressalis (rice stem borer) EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/USP (Plodia interpunctella) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Plodia interpunctella (Indian meal moth ) EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Gene regulation protein (Heliothis virescens) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Heliothis virescens (tobacco budworm) EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle (Chironomus tentans) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Chironomus tentans EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle protein (Lucilia cuprina) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle (Anthonomus grandis-Leptinotarsa decemlineata) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Anthonomus grandis ecdysone receptor (EcR) | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Nuclear receptor RXR-l (Locusta migratoria) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Locusta migratoria ecdysone receptor (EcR) | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle protein (Myzus persicae) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | <1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Myzus persicae EcR (green peach aphid) | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle protein (Bemisia tabaci) | BDBM50178988 (CHEBI:38452 | Tebufenozide) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | <5.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Bemisia tabaci (sweet potato whitefly) ecdysone receptor (EcR) | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50326776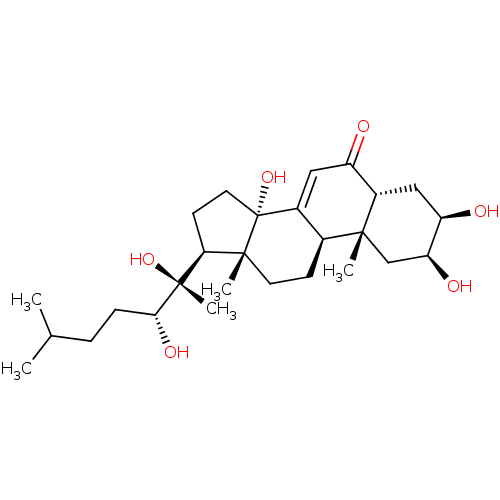 (2,3,14,20,22-PENTAHYDROXYCHOLEST-7-EN-6-ONE | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50326777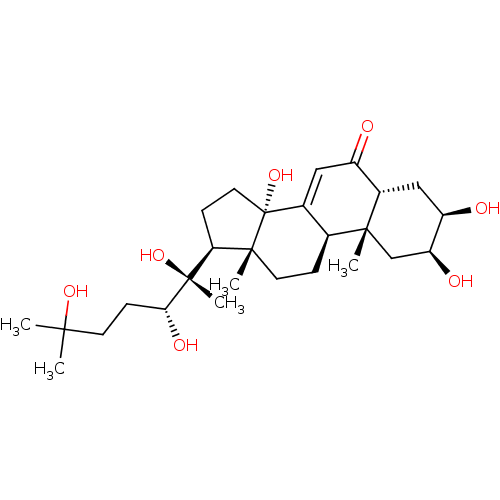 ((2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414658 (CHEMBL559048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414645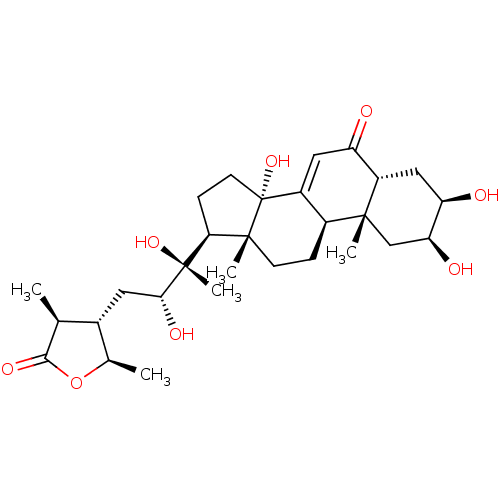 (CYASTERONE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414646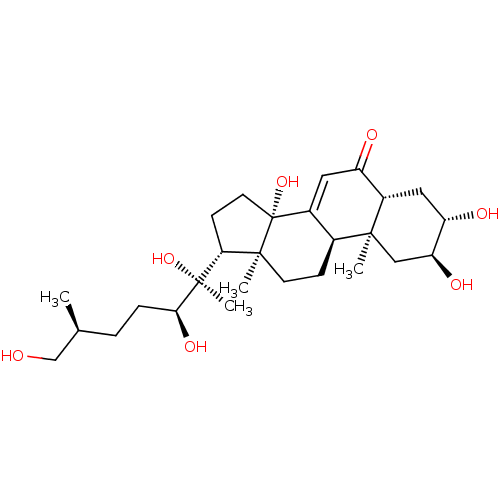 (INOKOSTERONE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50226672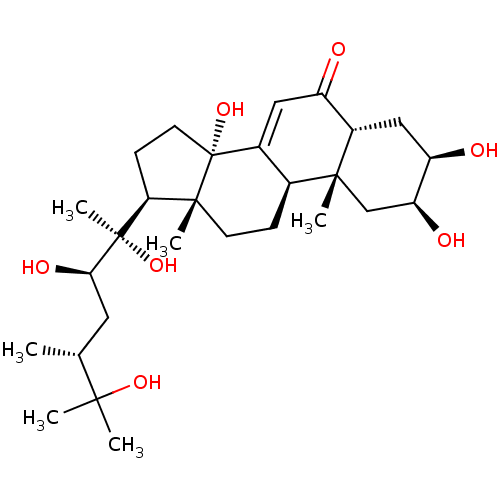 (CHEMBL255034 | makisterone A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414659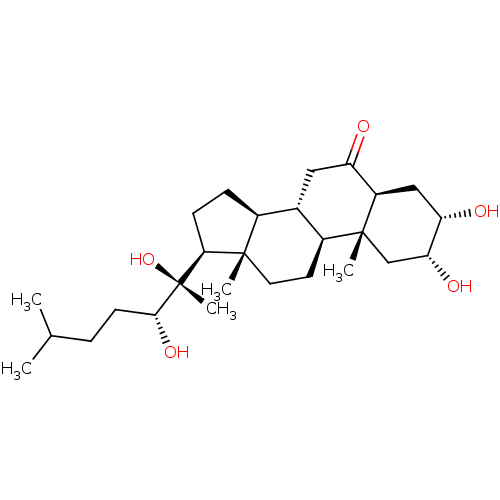 (CHEMBL564892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle protein (Spodoptera frugiperda) | BDBM50226672 (CHEMBL255034 | makisterone A) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Spodoptera frugiperda (fall armyworm) EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor/Ultraspiracle (Leptinotarsa decemlineata) | BDBM50326777 ((2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydrox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Leptinotarsa decemlineata EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414652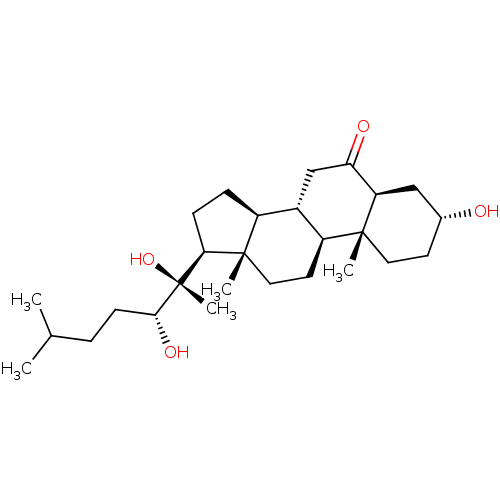 (CHEMBL559941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326776 (2,3,14,20,22-PENTAHYDROXYCHOLEST-7-EN-6-ONE | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ecdysone receptor/Ultraspiracle (Nezara viridula) | BDBM50226672 (CHEMBL255034 | makisterone A) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences Curated by ChEMBL | Assay Description Binding affinity to Nezara viridula EcR | Pest Manag Sci 67: 1457-67 (2011) Article DOI: 10.1002/ps.2200 BindingDB Entry DOI: 10.7270/Q2B27Z6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488465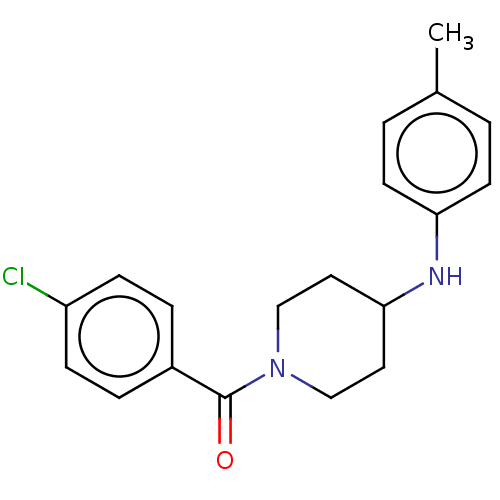 (CHEMBL2286413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414651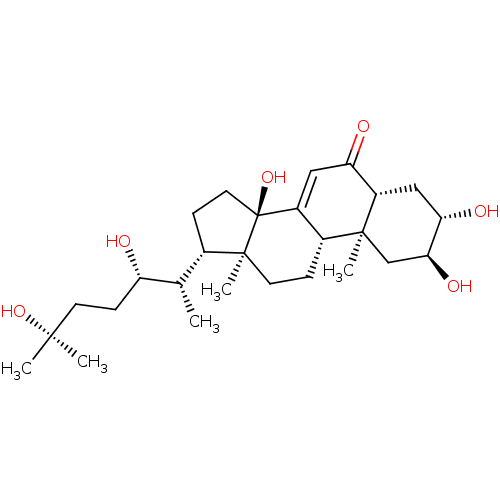 (ECDYSONE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326767 (4-butyl-1-(2-tert-butyl-5-chlorophenyl)-5-methylen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326777 ((2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326770 (4-isobutyl-5-methylene-2-oxo-1-(2,4,5-trichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326755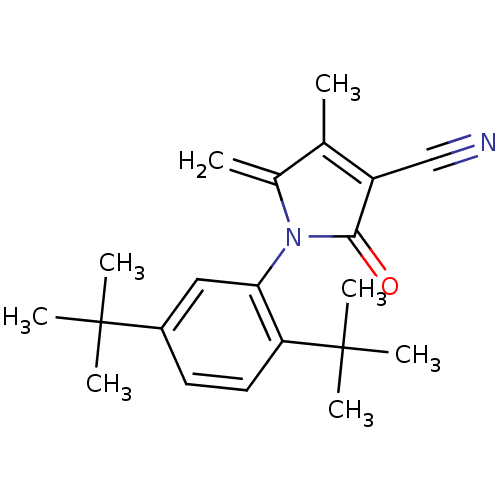 (1-(2,5-di-tert-butylphenyl)-4-methyl-5-methylene-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326760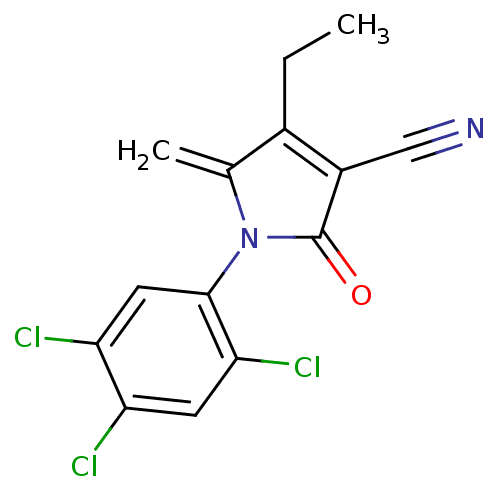 (4-ethyl-5-methylene-2-oxo-1-(2,4,5-trichlorophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326765 (4-butyl-1-(3-chloro-2-methylphenyl)-5-methylene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326758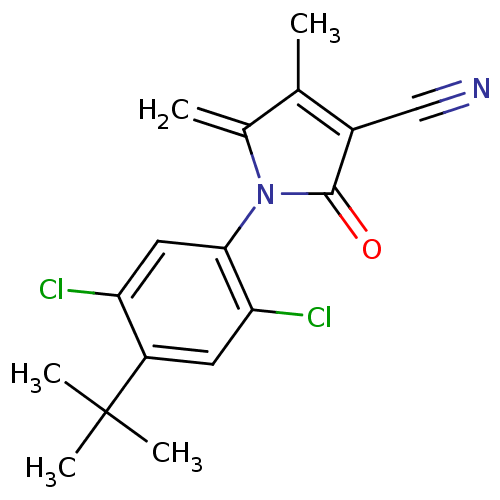 (1-(4-tert-butyl-2,5-dichlorophenyl)-4-methyl-5-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488464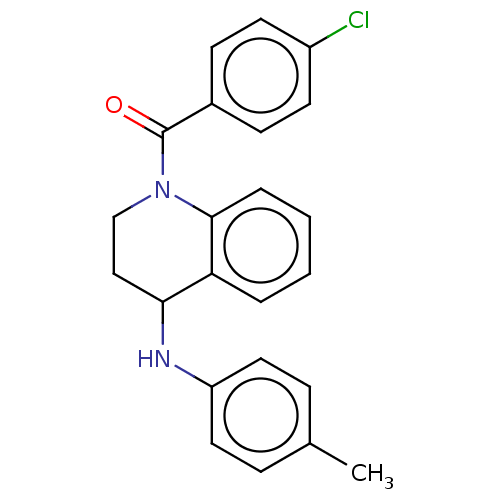 (CHEMBL2286419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414655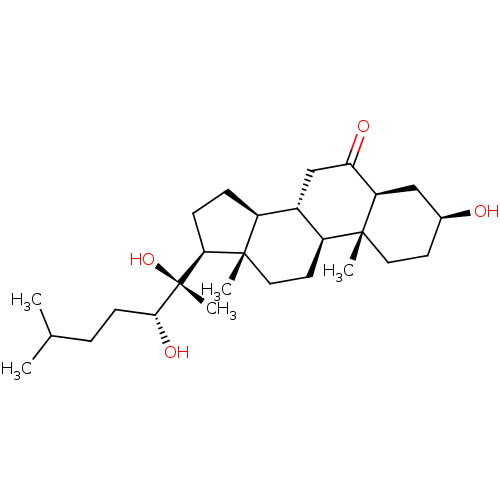 (CHEMBL560796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326759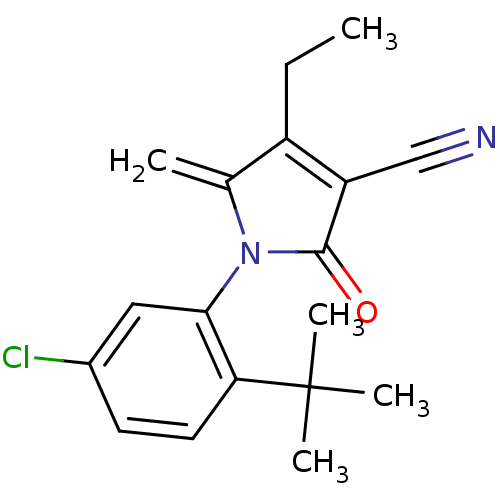 (1-(2-tert-butyl-5-chlorophenyl)-4-ethyl-5-methylen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326766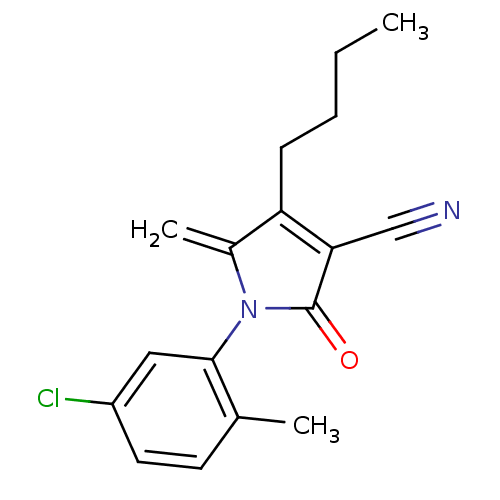 (4-butyl-1-(5-chloro-2-methylphenyl)-5-methylene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326773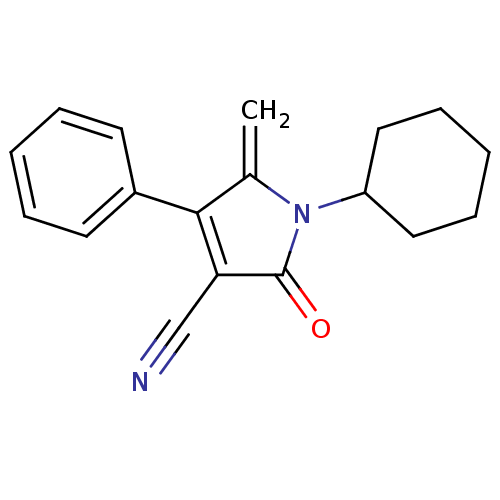 (1-cyclohexyl-5-methylene-2-oxo-4-phenyl-2,5-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Spodoptera littoralis) | BDBM50488463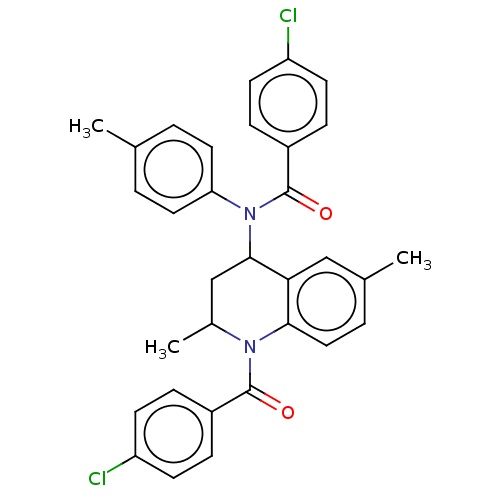 (CHEMBL2285681) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488463 (CHEMBL2285681) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Drosophila melanogaster) | BDBM50414657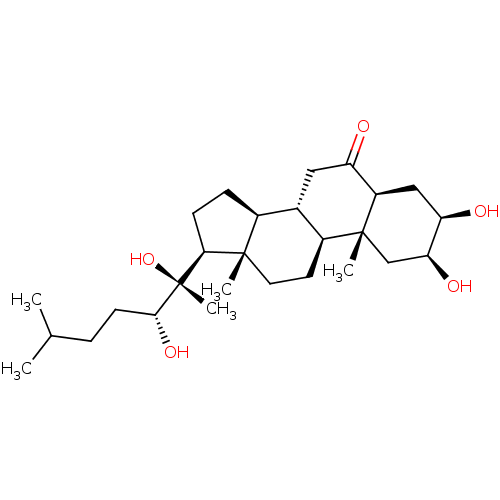 (CHEMBL562711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Binding affinity to ecdysone receptor ligand binding domain in Drosophila melanogaster assessed as number of hydrogen bonds formed | Bioorg Med Chem 17: 5868-73 (2009) Article DOI: 10.1016/j.bmc.2009.07.011 BindingDB Entry DOI: 10.7270/Q2H70H2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488437 (CHEMBL2286416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326761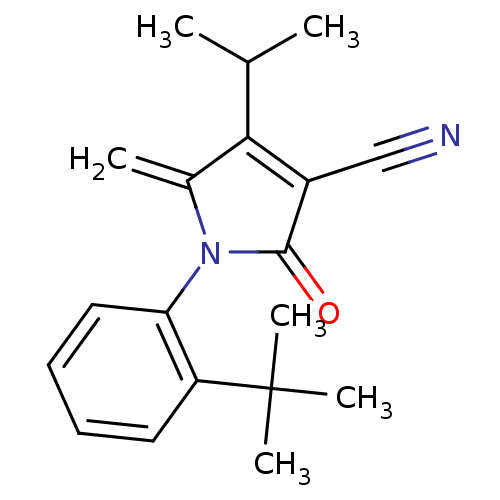 (1-(2-tert-butylphenyl)-4-isopropyl-5-methylene-2-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Spodoptera littoralis) | BDBM50488465 (CHEMBL2286413) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Spodoptera littoralis) | BDBM50488447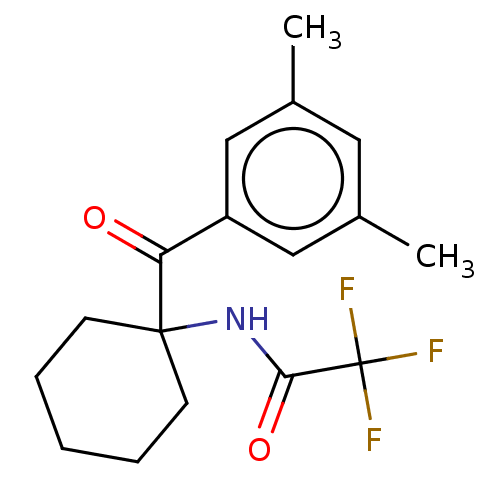 (CHEMBL2286412) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326774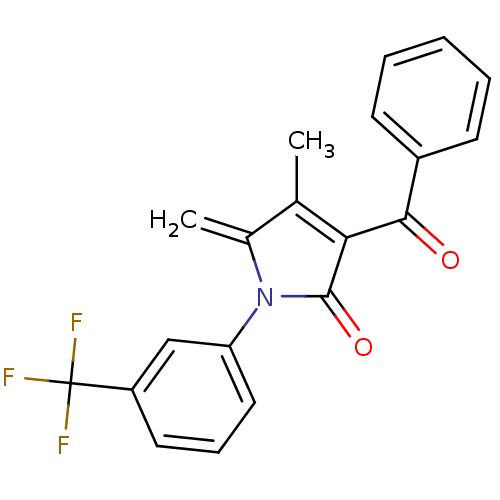 (3-benzoyl-4-methyl-5-methylene-1-(3-(trifluorometh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326756 (1-(2,6-diisopropylphenyl)-4-methyl-5-methylene-2-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488438 (CHEMBL2286414) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Spodoptera littoralis) | BDBM50488448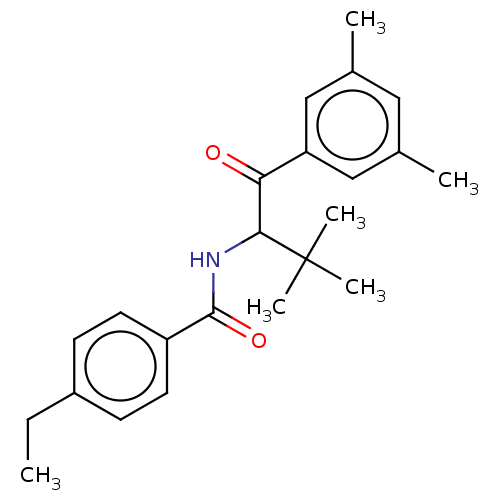 (CHEMBL2286411) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Spodoptera littoralis) | BDBM50488438 (CHEMBL2286414) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Spodoptera littoralis Sl2 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50488477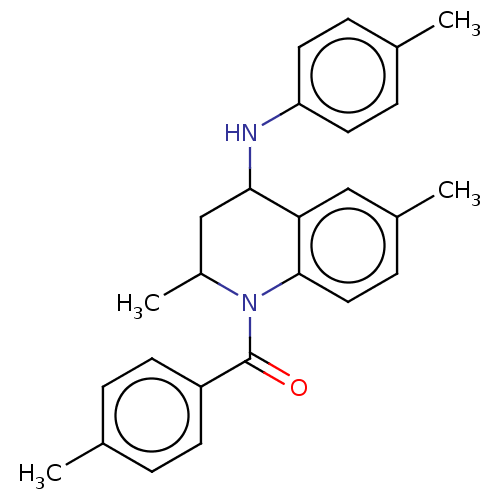 (CHEMBL2286417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Antagonist activity at ecdysone receptor in Bombyx mori Bm5 cells assessed as tebufenozide-induced effect treated 24 hr before tebufenozide challenge... | Pest Manag Sci 66: 526-35 (2010) Article DOI: 10.1002/ps.1903 BindingDB Entry DOI: 10.7270/Q2KK9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326757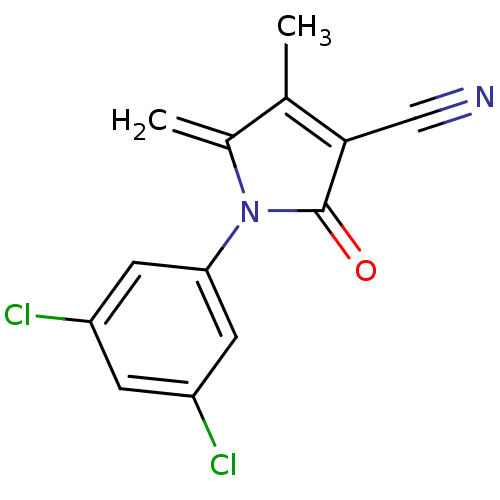 (1-(3,5-dichlorophenyl)-4-methyl-5-methylene-2-oxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 307 total ) | Next | Last >> |