 Found 2367 hits Enz. Inhib. hit(s) with Target = 'Endothelin receptor type B'
Found 2367 hits Enz. Inhib. hit(s) with Target = 'Endothelin receptor type B' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin receptor type B
(Sus scrofa) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368606
(Sarafotoxin S6B)Show SMILES [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@]([H])(NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C110H159N27O34S5/c1-9-55(6)88(108(168)130-77(110(170)171)40-59-45-116-64-22-14-13-21-62(59)64)136-107(167)87(54(4)5)135-102(162)76(44-86(148)149)128-93(153)67(29-31-82(114)141)121-99(159)73(41-60-46-115-52-117-60)126-106(166)81-49-174-173-48-63(113)90(150)131-78(47-138)103(163)134-79-50-175-176-51-80(105(165)123-70(37-53(2)3)96(156)124-72(39-58-25-27-61(140)28-26-58)97(157)125-71(98(158)133-81)38-57-19-11-10-12-20-57)132-94(154)68(30-32-83(142)143)120-91(151)65(23-15-17-34-111)118-101(161)75(43-85(146)147)129-109(169)89(56(7)139)137-95(155)69(33-36-172-8)122-100(160)74(42-84(144)145)127-92(152)66(119-104(79)164)24-16-18-35-112/h10-14,19-22,25-28,45-46,52-56,63,65-81,87-89,116,138-140H,9,15-18,23-24,29-44,47-51,111-113H2,1-8H3,(H2,114,141)(H,115,117)(H,118,161)(H,119,164)(H,120,151)(H,121,159)(H,122,160)(H,123,165)(H,124,156)(H,125,157)(H,126,166)(H,127,152)(H,128,153)(H,129,169)(H,130,168)(H,131,150)(H,132,154)(H,133,158)(H,134,163)(H,135,162)(H,136,167)(H,137,155)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,170,171)/t55-,56+,63+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368606

(Sarafotoxin S6B)Show SMILES [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@]([H])(NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C110H159N27O34S5/c1-9-55(6)88(108(168)130-77(110(170)171)40-59-45-116-64-22-14-13-21-62(59)64)136-107(167)87(54(4)5)135-102(162)76(44-86(148)149)128-93(153)67(29-31-82(114)141)121-99(159)73(41-60-46-115-52-117-60)126-106(166)81-49-174-173-48-63(113)90(150)131-78(47-138)103(163)134-79-50-175-176-51-80(105(165)123-70(37-53(2)3)96(156)124-72(39-58-25-27-61(140)28-26-58)97(157)125-71(98(158)133-81)38-57-19-11-10-12-20-57)132-94(154)68(30-32-83(142)143)120-91(151)65(23-15-17-34-111)118-101(161)75(43-85(146)147)129-109(169)89(56(7)139)137-95(155)69(33-36-172-8)122-100(160)74(42-84(144)145)127-92(152)66(119-104(79)164)24-16-18-35-112/h10-14,19-22,25-28,45-46,52-56,63,65-81,87-89,116,138-140H,9,15-18,23-24,29-44,47-51,111-113H2,1-8H3,(H2,114,141)(H,115,117)(H,118,161)(H,119,164)(H,120,151)(H,121,159)(H,122,160)(H,123,165)(H,124,156)(H,125,157)(H,126,166)(H,127,152)(H,128,153)(H,129,169)(H,130,168)(H,131,150)(H,132,154)(H,133,158)(H,134,163)(H,135,162)(H,136,167)(H,137,155)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,170,171)/t55-,56+,63+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368605
(CHEMBL1790178)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C103H147N27O37S5/c1-10-46(6)80(100(163)123-67(103(166)167)30-50-37-109-54-19-15-14-18-52(50)54)128-99(162)79(45(4)5)127-95(158)66(36-78(144)145)120-85(148)55(20-23-71(105)133)112-90(153)61(31-51-38-108-43-110-51)117-97(160)69-40-170-169-39-53(104)83(146)129-81(47(7)131)102(165)126-70-42-172-171-41-68(96(159)115-59(28-44(2)3)88(151)118-62(32-72(106)134)91(154)116-60(89(152)125-69)29-49-16-12-11-13-17-49)124-86(149)57(22-25-75(138)139)111-84(147)56(21-24-74(136)137)113-94(157)65(35-77(142)143)122-101(164)82(48(8)132)130-87(150)58(26-27-168-9)114-93(156)64(34-76(140)141)121-92(155)63(33-73(107)135)119-98(70)161/h11-19,37-38,43-48,53,55-70,79-82,109,131-132H,10,20-36,39-42,104H2,1-9H3,(H2,105,133)(H2,106,134)(H2,107,135)(H,108,110)(H,111,147)(H,112,153)(H,113,157)(H,114,156)(H,115,159)(H,116,154)(H,117,160)(H,118,151)(H,119,161)(H,120,148)(H,121,155)(H,122,164)(H,123,163)(H,124,149)(H,125,152)(H,126,165)(H,127,158)(H,128,162)(H,129,146)(H,130,150)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,144,145)(H,166,167)/t46-,47+,48+,53+,55-,56-,57-,58+,59-,60-,61-,62+,63+,64-,65+,66-,67-,68+,69-,70-,79-,80-,81+,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368607
(CHEMBL1790180)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CCc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)[C@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C122H170N26O33S4/c1-11-63(7)98(118(176)140-90(122(180)181)50-71-54-127-78-25-17-16-24-76(71)78)146-119(177)99(64(8)12-2)145-113(171)89(53-96(158)159)138-107(165)83(46-61(3)4)133-110(168)87(51-72-55-126-60-128-72)136-114(172)91-57-183-182-56-77(125)102(160)147-100(65(9)149)121(179)143-92-58-184-185-59-93(116(174)144-97(62(5)6)117(175)139-85(49-70-32-39-75(153)40-33-70)108(166)134-84(109(167)142-91)48-69-30-37-74(152)38-31-69)141-106(164)82(42-43-94(154)155)131-103(161)79(26-18-20-44-123)130-111(169)88(52-95(156)157)137-104(162)80(27-19-21-45-124)129-105(163)81(41-34-67-28-35-73(151)36-29-67)132-120(178)101(66(10)150)148-112(170)86(135-115(92)173)47-68-22-14-13-15-23-68/h13-17,22-25,28-33,35-40,54-55,60-66,77,79-93,97-101,127,149-153H,11-12,18-21,26-27,34,41-53,56-59,123-125H2,1-10H3,(H,126,128)(H,129,163)(H,130,169)(H,131,161)(H,132,178)(H,133,168)(H,134,166)(H,135,173)(H,136,172)(H,137,162)(H,138,165)(H,139,175)(H,140,176)(H,141,164)(H,142,167)(H,143,179)(H,144,174)(H,145,171)(H,146,177)(H,147,160)(H,148,170)(H,154,155)(H,156,157)(H,158,159)(H,180,181)/t63-,64-,65+,66-,77+,79-,80-,81+,82-,83-,84-,85+,86+,87-,88+,89-,90-,91-,92-,93+,97-,98-,99-,100+,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368605

(CHEMBL1790178)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C103H147N27O37S5/c1-10-46(6)80(100(163)123-67(103(166)167)30-50-37-109-54-19-15-14-18-52(50)54)128-99(162)79(45(4)5)127-95(158)66(36-78(144)145)120-85(148)55(20-23-71(105)133)112-90(153)61(31-51-38-108-43-110-51)117-97(160)69-40-170-169-39-53(104)83(146)129-81(47(7)131)102(165)126-70-42-172-171-41-68(96(159)115-59(28-44(2)3)88(151)118-62(32-72(106)134)91(154)116-60(89(152)125-69)29-49-16-12-11-13-17-49)124-86(149)57(22-25-75(138)139)111-84(147)56(21-24-74(136)137)113-94(157)65(35-77(142)143)122-101(164)82(48(8)132)130-87(150)58(26-27-168-9)114-93(156)64(34-76(140)141)121-92(155)63(33-73(107)135)119-98(70)161/h11-19,37-38,43-48,53,55-70,79-82,109,131-132H,10,20-36,39-42,104H2,1-9H3,(H2,105,133)(H2,106,134)(H2,107,135)(H,108,110)(H,111,147)(H,112,153)(H,113,157)(H,114,156)(H,115,159)(H,116,154)(H,117,160)(H,118,151)(H,119,161)(H,120,148)(H,121,155)(H,122,164)(H,123,163)(H,124,149)(H,125,152)(H,126,165)(H,127,158)(H,128,162)(H,129,146)(H,130,150)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,144,145)(H,166,167)/t46-,47+,48+,53+,55-,56-,57-,58+,59-,60-,61-,62+,63+,64-,65+,66-,67-,68+,69-,70-,79-,80-,81+,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368607

(CHEMBL1790180)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CCc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)[C@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C122H170N26O33S4/c1-11-63(7)98(118(176)140-90(122(180)181)50-71-54-127-78-25-17-16-24-76(71)78)146-119(177)99(64(8)12-2)145-113(171)89(53-96(158)159)138-107(165)83(46-61(3)4)133-110(168)87(51-72-55-126-60-128-72)136-114(172)91-57-183-182-56-77(125)102(160)147-100(65(9)149)121(179)143-92-58-184-185-59-93(116(174)144-97(62(5)6)117(175)139-85(49-70-32-39-75(153)40-33-70)108(166)134-84(109(167)142-91)48-69-30-37-74(152)38-31-69)141-106(164)82(42-43-94(154)155)131-103(161)79(26-18-20-44-123)130-111(169)88(52-95(156)157)137-104(162)80(27-19-21-45-124)129-105(163)81(41-34-67-28-35-73(151)36-29-67)132-120(178)101(66(10)150)148-112(170)86(135-115(92)173)47-68-22-14-13-15-23-68/h13-17,22-25,28-33,35-40,54-55,60-66,77,79-93,97-101,127,149-153H,11-12,18-21,26-27,34,41-53,56-59,123-125H2,1-10H3,(H,126,128)(H,129,163)(H,130,169)(H,131,161)(H,132,178)(H,133,168)(H,134,166)(H,135,173)(H,136,172)(H,137,162)(H,138,165)(H,139,175)(H,140,176)(H,141,164)(H,142,167)(H,143,179)(H,144,174)(H,145,171)(H,146,177)(H,147,160)(H,148,170)(H,154,155)(H,156,157)(H,158,159)(H,180,181)/t63-,64-,65+,66-,77+,79-,80-,81+,82-,83-,84-,85+,86+,87-,88+,89-,90-,91-,92-,93+,97-,98-,99-,100+,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287890
(CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C77H105N15O21/c1-11-41(7)64(75(110)89-58(77(112)113)33-47-36-79-51-21-17-16-20-50(47)51)92-76(111)65(42(8)12-2)91-73(108)57(35-62(99)100)87-70(105)53(30-39(3)4)85-72(107)56(34-48-37-78-38-80-48)84-66(101)43(9)82-69(104)54(31-45-18-14-13-15-19-45)86-71(106)55(32-46-22-24-49(93)25-23-46)88-74(109)63(40(5)6)90-67(102)44(10)81-68(103)52(26-28-60(95)96)83-59(94)27-29-61(97)98/h13-25,36-44,52-58,63-65,79,93H,11-12,26-35H2,1-10H3,(H,78,80)(H,81,103)(H,82,104)(H,83,94)(H,84,101)(H,85,107)(H,86,106)(H,87,105)(H,88,109)(H,89,110)(H,90,102)(H,91,108)(H,92,111)(H,95,96)(H,97,98)(H,99,100)(H,112,113)/t41-,42-,43-,44-,52-,53-,54-,55-,56-,57-,58-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50143784
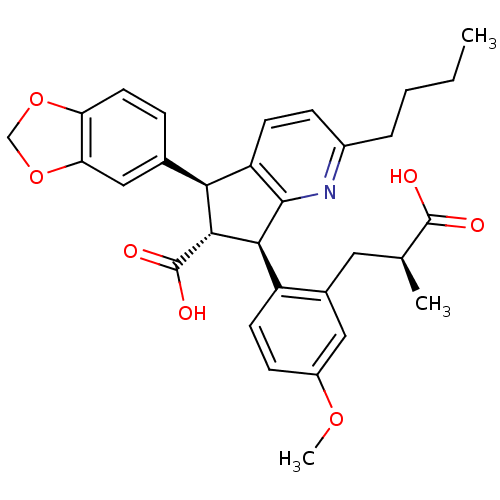
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1262-70 (1999)
BindingDB Entry DOI: 10.7270/Q2Q52N5Q |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071445
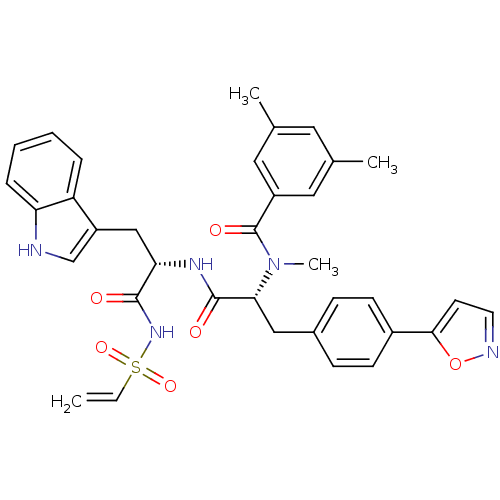
(CHEMBL310039 | N-[(R)-1-[(S)-2-Ethenesulfonylamino...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccno1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NS(=O)(=O)C=C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C35H35N5O6S/c1-5-47(44,45)39-33(41)30(20-27-21-36-29-9-7-6-8-28(27)29)38-34(42)31(40(4)35(43)26-17-22(2)16-23(3)18-26)19-24-10-12-25(13-11-24)32-14-15-37-46-32/h5-18,21,30-31,36H,1,19-20H2,2-4H3,(H,38,42)(H,39,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071438
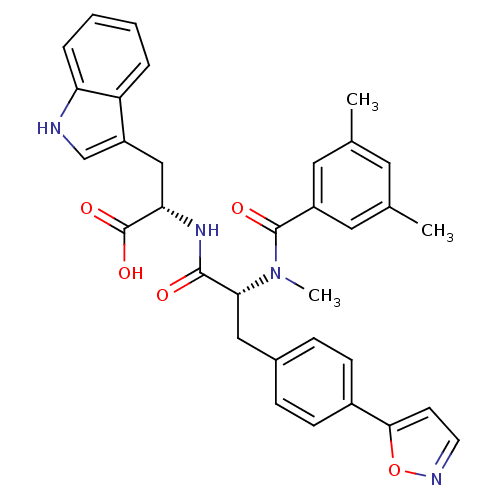
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccno1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C33H32N4O5/c1-20-14-21(2)16-24(15-20)32(39)37(3)29(17-22-8-10-23(11-9-22)30-12-13-35-42-30)31(38)36-28(33(40)41)18-25-19-34-27-7-5-4-6-26(25)27/h4-16,19,28-29,34H,17-18H2,1-3H3,(H,36,38)(H,40,41)/t28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071435
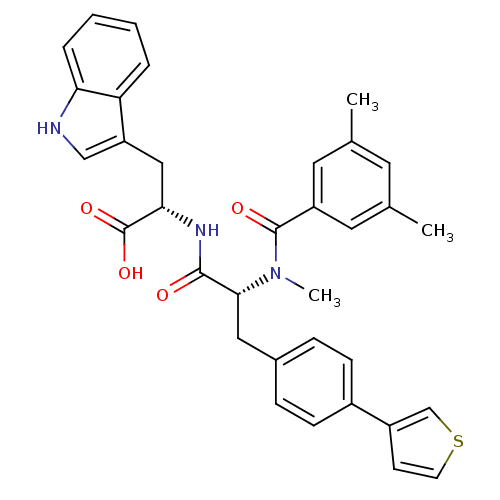
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccsc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C34H33N3O4S/c1-21-14-22(2)16-26(15-21)33(39)37(3)31(17-23-8-10-24(11-9-23)25-12-13-42-20-25)32(38)36-30(34(40)41)18-27-19-35-29-7-5-4-6-28(27)29/h4-16,19-20,30-31,35H,17-18H2,1-3H3,(H,36,38)(H,40,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071437

(CHEMBL308102 | N-[(R)-1-[(S)-1-(1H-Indol-3-ylmethy...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccno1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NS(=O)(=O)CC=C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H37N5O6S/c1-5-16-48(45,46)40-34(42)31(21-28-22-37-30-9-7-6-8-29(28)30)39-35(43)32(41(4)36(44)27-18-23(2)17-24(3)19-27)20-25-10-12-26(13-11-25)33-14-15-38-47-33/h5-15,17-19,22,31-32,37H,1,16,20-21H2,2-4H3,(H,39,43)(H,40,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071423
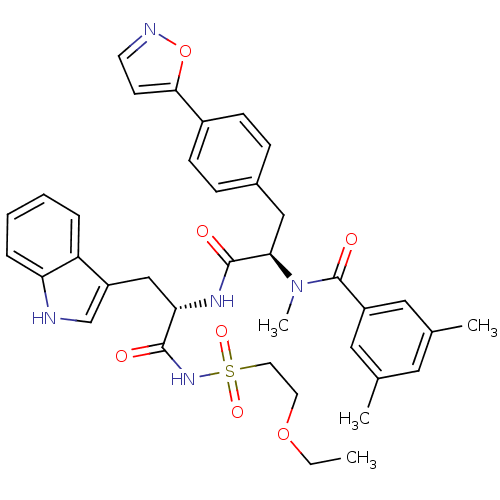
(CHEMBL72924 | N-[(R)-1-[(S)-2-(2-Ethoxy-ethanesulf...)Show SMILES CCOCCS(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C37H41N5O7S/c1-5-48-16-17-50(46,47)41-35(43)32(22-29-23-38-31-9-7-6-8-30(29)31)40-36(44)33(42(4)37(45)28-19-24(2)18-25(3)20-28)21-26-10-12-27(13-11-26)34-14-15-39-49-34/h6-15,18-20,23,32-33,38H,5,16-17,21-22H2,1-4H3,(H,40,44)(H,41,43)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071420
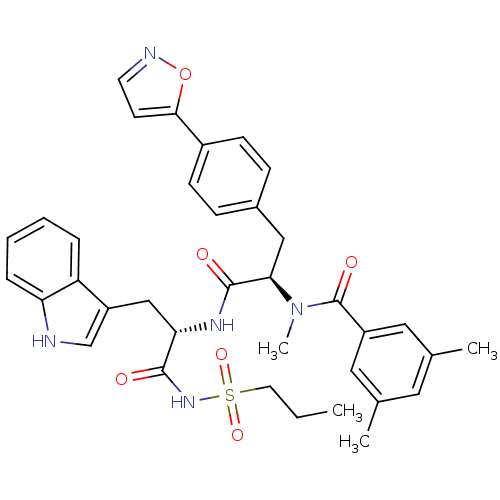
(CHEMBL72348 | N-[(R)-1-[(S)-1-(1H-Indol-3-ylmethyl...)Show SMILES CCCS(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H39N5O6S/c1-5-16-48(45,46)40-34(42)31(21-28-22-37-30-9-7-6-8-29(28)30)39-35(43)32(41(4)36(44)27-18-23(2)17-24(3)19-27)20-25-10-12-26(13-11-25)33-14-15-38-47-33/h6-15,17-19,22,31-32,37H,5,16,20-21H2,1-4H3,(H,39,43)(H,40,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071436
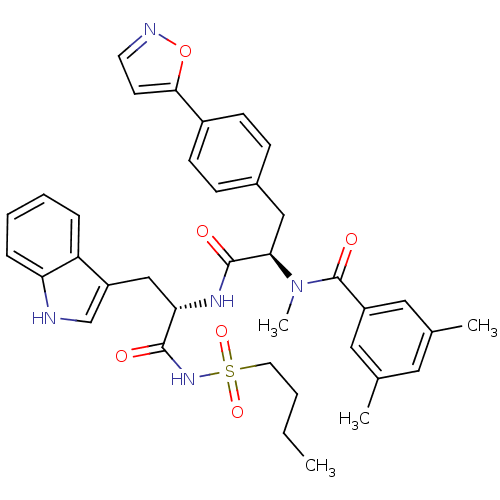
(CHEMBL306950 | N-[(R)-1-[(S)-2-(Butane-1-sulfonyla...)Show SMILES CCCCS(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C37H41N5O6S/c1-5-6-17-49(46,47)41-35(43)32(22-29-23-38-31-10-8-7-9-30(29)31)40-36(44)33(42(4)37(45)28-19-24(2)18-25(3)20-28)21-26-11-13-27(14-12-26)34-15-16-39-48-34/h7-16,18-20,23,32-33,38H,5-6,17,21-22H2,1-4H3,(H,40,44)(H,41,43)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287885
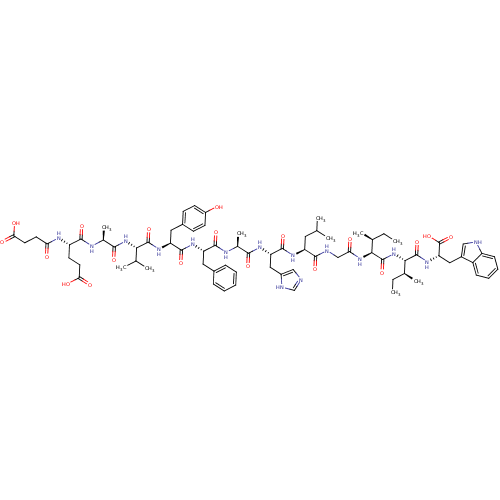
(CHEMBL405377 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C75H103N15O19/c1-11-41(7)63(74(107)90-64(42(8)12-2)73(106)87-57(75(108)109)33-47-35-77-51-21-17-16-20-50(47)51)88-59(93)37-78-67(100)53(30-39(3)4)84-71(104)56(34-48-36-76-38-79-48)83-65(98)43(9)81-69(102)54(31-45-18-14-13-15-19-45)85-70(103)55(32-46-22-24-49(91)25-23-46)86-72(105)62(40(5)6)89-66(99)44(10)80-68(101)52(26-28-60(94)95)82-58(92)27-29-61(96)97/h13-25,35-36,38-44,52-57,62-64,77,91H,11-12,26-34,37H2,1-10H3,(H,76,79)(H,78,100)(H,80,101)(H,81,102)(H,82,92)(H,83,98)(H,84,104)(H,85,103)(H,86,105)(H,87,106)(H,88,93)(H,89,99)(H,90,107)(H,94,95)(H,96,97)(H,108,109)/t41-,42-,43-,44-,52-,53-,54-,55-,56-,57-,62-,63-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071421
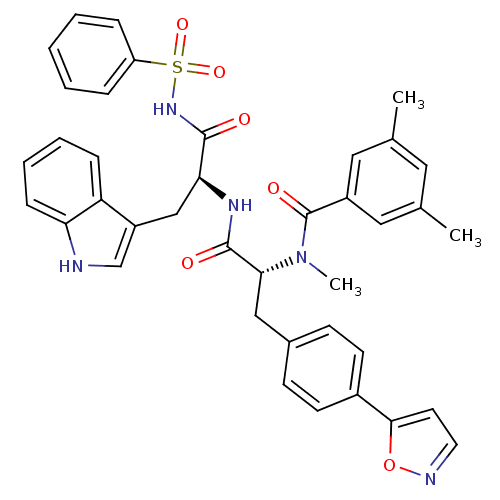
(CHEMBL310920 | N-[(R)-1-[(S)-2-Benzenesulfonylamin...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccno1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NS(=O)(=O)c1ccccc1)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C39H37N5O6S/c1-25-19-26(2)21-29(20-25)39(47)44(3)35(22-27-13-15-28(16-14-27)36-17-18-41-50-36)38(46)42-34(23-30-24-40-33-12-8-7-11-32(30)33)37(45)43-51(48,49)31-9-5-4-6-10-31/h4-21,24,34-35,40H,22-23H2,1-3H3,(H,42,46)(H,43,45)/t34-,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071439
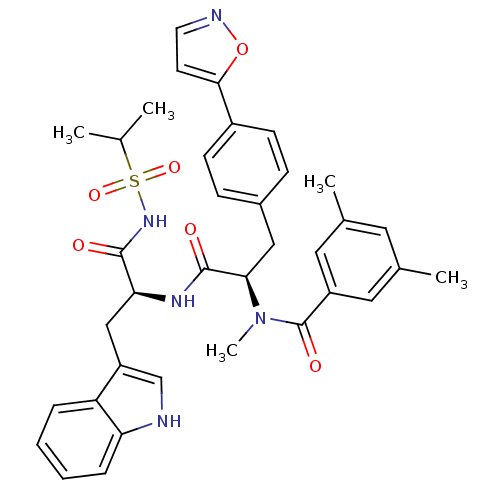
(CHEMBL73000 | N-[(R)-1-[(S)-1-(1H-Indol-3-ylmethyl...)Show SMILES CC(C)S(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H39N5O6S/c1-22(2)48(45,46)40-34(42)31(20-28-21-37-30-9-7-6-8-29(28)30)39-35(43)32(41(5)36(44)27-17-23(3)16-24(4)18-27)19-25-10-12-26(13-11-25)33-14-15-38-47-33/h6-18,21-22,31-32,37H,19-20H2,1-5H3,(H,39,43)(H,40,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071430
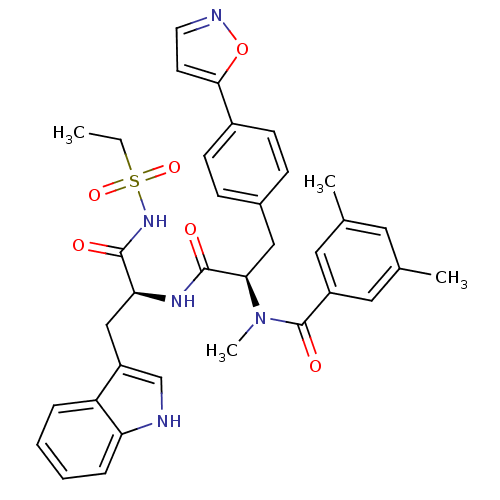
(CHEMBL73373 | N-[(R)-1-[(S)-2-Ethanesulfonylamino-...)Show SMILES CCS(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C35H37N5O6S/c1-5-47(44,45)39-33(41)30(20-27-21-36-29-9-7-6-8-28(27)29)38-34(42)31(40(4)35(43)26-17-22(2)16-23(3)18-26)19-24-10-12-25(13-11-24)32-14-15-37-46-32/h6-18,21,30-31,36H,5,19-20H2,1-4H3,(H,38,42)(H,39,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071428
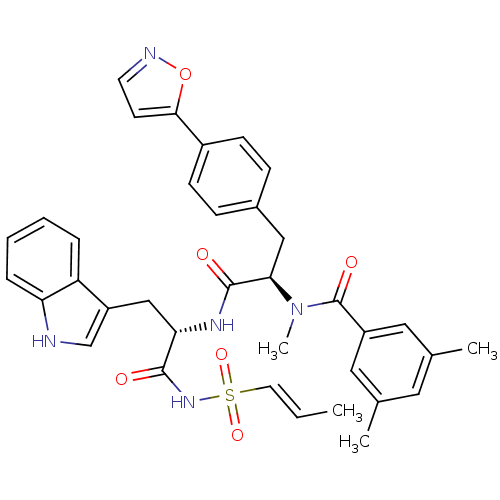
(CHEMBL307471 | N-[(R)-1-{(S)-1-(1H-Indol-3-ylmethy...)Show SMILES C\C=C\S(=O)(=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H37N5O6S/c1-5-16-48(45,46)40-34(42)31(21-28-22-37-30-9-7-6-8-29(28)30)39-35(43)32(41(4)36(44)27-18-23(2)17-24(3)19-27)20-25-10-12-26(13-11-25)33-14-15-38-47-33/h5-19,22,31-32,37H,20-21H2,1-4H3,(H,39,43)(H,40,42)/b16-5+/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071444

(CHEMBL307045 | N-[(R)-1-[1-(Butane-1-sulfonylamino...)Show SMILES CCCCS(=O)(=O)NC(=O)C(CCSC)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C31H40N4O6S2/c1-6-7-16-43(39,40)34-29(36)26(13-15-42-5)33-30(37)27(35(4)31(38)25-18-21(2)17-22(3)19-25)20-23-8-10-24(11-9-23)28-12-14-32-41-28/h8-12,14,17-19,26-27H,6-7,13,15-16,20H2,1-5H3,(H,33,37)(H,34,36)/t26?,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071443
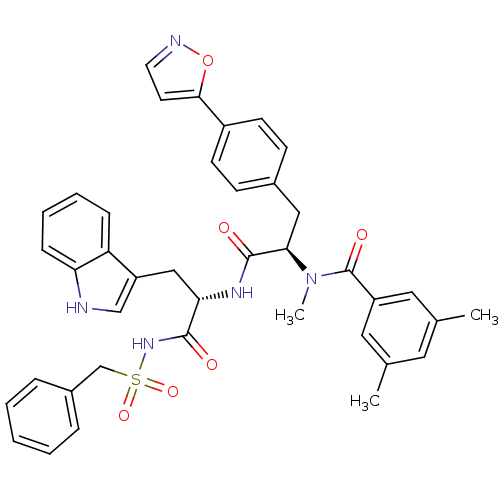
(CHEMBL308269 | N-[(R)-1-[(S)-1-(1H-Indol-3-ylmethy...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccno1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NS(=O)(=O)Cc1ccccc1)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C40H39N5O6S/c1-26-19-27(2)21-31(20-26)40(48)45(3)36(22-28-13-15-30(16-14-28)37-17-18-42-51-37)39(47)43-35(23-32-24-41-34-12-8-7-11-33(32)34)38(46)44-52(49,50)25-29-9-5-4-6-10-29/h4-21,24,35-36,41H,22-23,25H2,1-3H3,(H,43,47)(H,44,46)/t35-,36+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
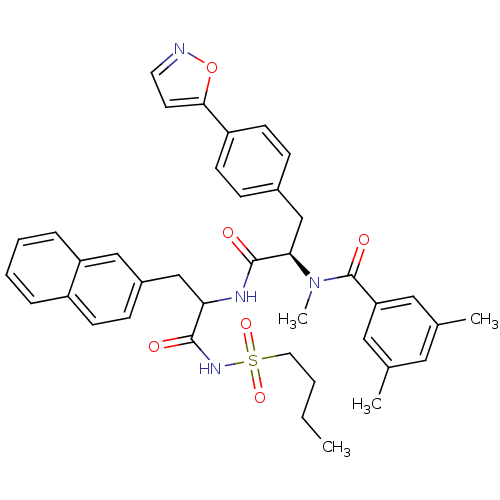
(Homo sapiens (Human)) | BDBM50071440
(CHEMBL307384 | N-[(R)-1-[2-(Butane-1-sulfonylamino...)Show SMILES CCCCS(=O)(=O)NC(=O)C(Cc1ccc2ccccc2c1)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C39H42N4O6S/c1-5-6-19-50(47,48)42-37(44)34(24-29-13-14-30-9-7-8-10-32(30)23-29)41-38(45)35(43(4)39(46)33-21-26(2)20-27(3)22-33)25-28-11-15-31(16-12-28)36-17-18-40-49-36/h7-18,20-23,34-35H,5-6,19,24-25H2,1-4H3,(H,41,45)(H,42,44)/t34?,35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
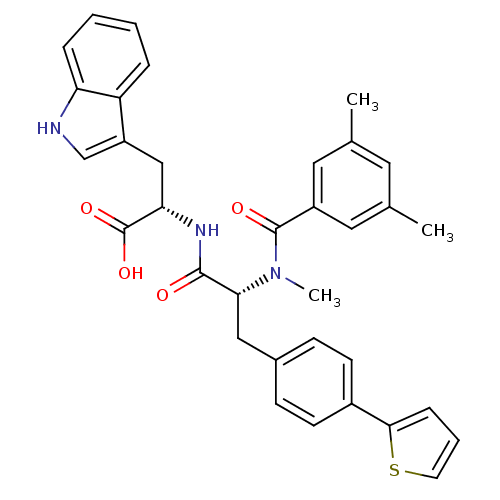
(Homo sapiens (Human)) | BDBM50071432
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1cccs1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C34H33N3O4S/c1-21-15-22(2)17-25(16-21)33(39)37(3)30(18-23-10-12-24(13-11-23)31-9-6-14-42-31)32(38)36-29(34(40)41)19-26-20-35-28-8-5-4-7-27(26)28/h4-17,20,29-30,35H,18-19H2,1-3H3,(H,36,38)(H,40,41)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
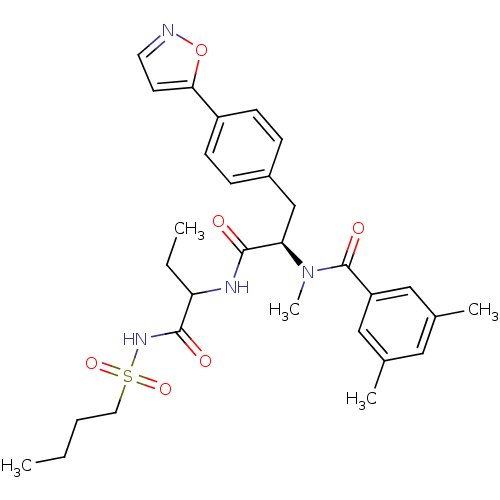
(Homo sapiens (Human)) | BDBM50071426
(CHEMBL73835 | N-[(R)-1-[1-(Butane-1-sulfonylaminoc...)Show SMILES CCCCS(=O)(=O)NC(=O)C(CC)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C30H38N4O6S/c1-6-8-15-41(38,39)33-28(35)25(7-2)32-29(36)26(34(5)30(37)24-17-20(3)16-21(4)18-24)19-22-9-11-23(12-10-22)27-13-14-31-40-27/h9-14,16-18,25-26H,6-8,15,19H2,1-5H3,(H,32,36)(H,33,35)/t25?,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071441
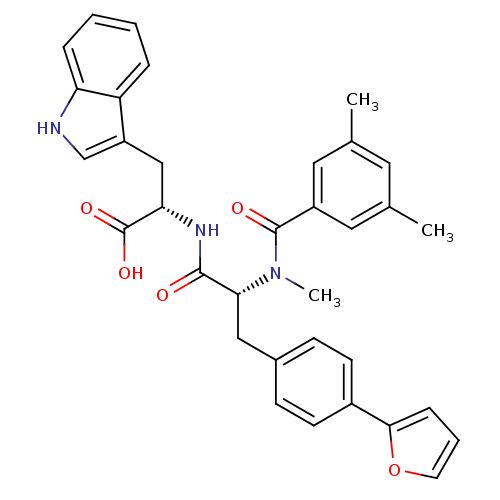
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccco1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C34H33N3O5/c1-21-15-22(2)17-25(16-21)33(39)37(3)30(18-23-10-12-24(13-11-23)31-9-6-14-42-31)32(38)36-29(34(40)41)19-26-20-35-28-8-5-4-7-27(26)28/h4-17,20,29-30,35H,18-19H2,1-3H3,(H,36,38)(H,40,41)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071427
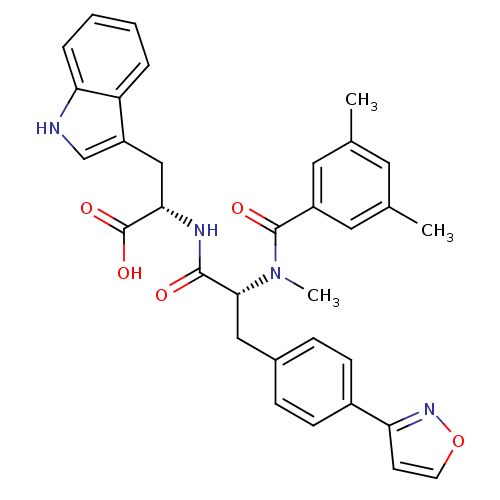
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccon1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C33H32N4O5/c1-20-14-21(2)16-24(15-20)32(39)37(3)30(17-22-8-10-23(11-9-22)27-12-13-42-36-27)31(38)35-29(33(40)41)18-25-19-34-28-7-5-4-6-26(25)28/h4-16,19,29-30,34H,17-18H2,1-3H3,(H,35,38)(H,40,41)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287883
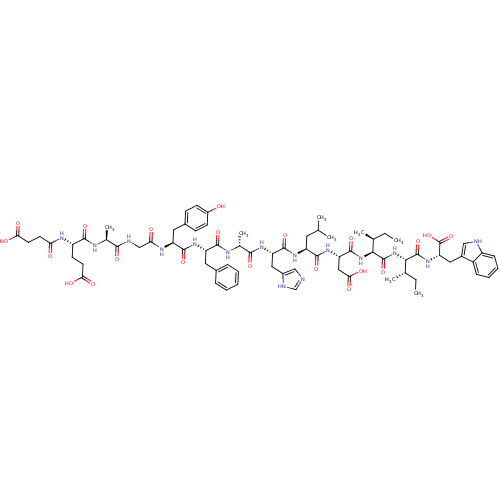
(CHEMBL412003 | Suc-Glu-Ala-Gly-Tyr-Phe-Ala-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C74H99N15O21/c1-9-39(5)62(72(107)87-56(74(109)110)31-45-34-76-49-19-15-14-18-48(45)49)89-73(108)63(40(6)10-2)88-71(106)55(33-61(97)98)86-68(103)51(28-38(3)4)84-70(105)54(32-46-35-75-37-78-46)83-65(100)42(8)80-67(102)53(29-43-16-12-11-13-17-43)85-69(104)52(30-44-20-22-47(90)23-21-44)82-58(92)36-77-64(99)41(7)79-66(101)50(24-26-59(93)94)81-57(91)25-27-60(95)96/h11-23,34-35,37-42,50-56,62-63,76,90H,9-10,24-33,36H2,1-8H3,(H,75,78)(H,77,99)(H,79,101)(H,80,102)(H,81,91)(H,82,92)(H,83,100)(H,84,105)(H,85,104)(H,86,103)(H,87,107)(H,88,106)(H,89,108)(H,93,94)(H,95,96)(H,97,98)(H,109,110)/t39-,40-,41-,42+,50-,51-,52-,53-,54-,55-,56-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50071433
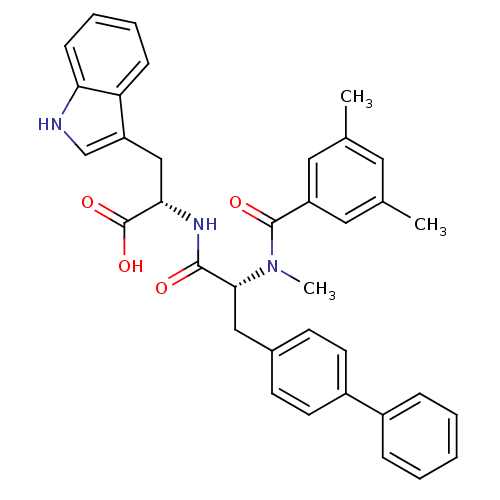
((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H35N3O4/c1-23-17-24(2)19-28(18-23)35(41)39(3)33(20-25-13-15-27(16-14-25)26-9-5-4-6-10-26)34(40)38-32(36(42)43)21-29-22-37-31-12-8-7-11-30(29)31/h4-19,22,32-33,37H,20-21H2,1-3H3,(H,38,40)(H,42,43)/t32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin receptor type B
(Sus scrofa) | BDBM50287882
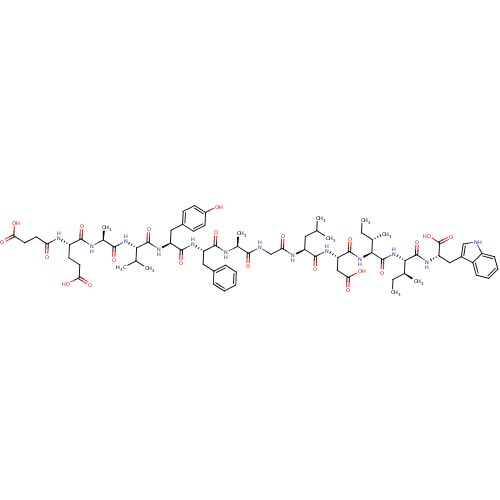
(CHEMBL412065 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-Gly-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C73H101N13O21/c1-11-39(7)61(71(104)83-54(73(106)107)33-45-35-74-48-21-17-16-20-47(45)48)86-72(105)62(40(8)12-2)85-69(102)53(34-59(94)95)81-67(100)50(30-37(3)4)79-56(89)36-75-63(96)41(9)76-66(99)51(31-43-18-14-13-15-19-43)80-68(101)52(32-44-22-24-46(87)25-23-44)82-70(103)60(38(5)6)84-64(97)42(10)77-65(98)49(26-28-57(90)91)78-55(88)27-29-58(92)93/h13-25,35,37-42,49-54,60-62,74,87H,11-12,26-34,36H2,1-10H3,(H,75,96)(H,76,99)(H,77,98)(H,78,88)(H,79,89)(H,80,101)(H,81,100)(H,82,103)(H,83,104)(H,84,97)(H,85,102)(H,86,105)(H,90,91)(H,92,93)(H,94,95)(H,106,107)/t39-,40-,41-,42-,49-,50-,51-,52-,53-,54-,60-,61-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071433

((S)-2-{(R)-3-Biphenyl-4-yl-2-[(3,5-dimethyl-benzoy...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C36H35N3O4/c1-23-17-24(2)19-28(18-23)35(41)39(3)33(20-25-13-15-27(16-14-25)26-9-5-4-6-10-26)34(40)38-32(36(42)43)21-29-22-37-31-12-8-7-11-30(29)31/h4-19,22,32-33,37H,20-21H2,1-3H3,(H,38,40)(H,42,43)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071425
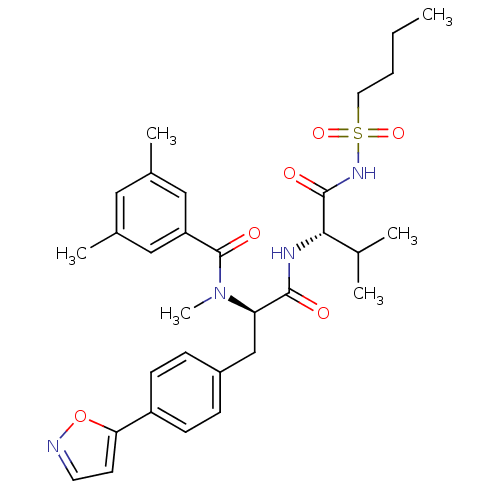
(CHEMBL311854 | N-[(R)-1-[(S)-1-(Butane-1-sulfonyla...)Show SMILES CCCCS(=O)(=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C(C)C Show InChI InChI=1S/C31H40N4O6S/c1-7-8-15-42(39,40)34-30(37)28(20(2)3)33-29(36)26(35(6)31(38)25-17-21(4)16-22(5)18-25)19-23-9-11-24(12-10-23)27-13-14-32-41-27/h9-14,16-18,20,26,28H,7-8,15,19H2,1-6H3,(H,33,36)(H,34,37)/t26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity against Endothelin B receptor |
Bioorg Med Chem Lett 8: 2247-52 (1999)
BindingDB Entry DOI: 10.7270/Q26M3609 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071425

(CHEMBL311854 | N-[(R)-1-[(S)-1-(Butane-1-sulfonyla...)Show SMILES CCCCS(=O)(=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C(C)C Show InChI InChI=1S/C31H40N4O6S/c1-7-8-15-42(39,40)34-30(37)28(20(2)3)33-29(36)26(35(6)31(38)25-17-21(4)16-22(5)18-25)19-23-9-11-24(12-10-23)27-13-14-32-41-27/h9-14,16-18,20,26,28H,7-8,15,19H2,1-6H3,(H,33,36)(H,34,37)/t26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Mus musculus) | BDBM50304497

(2-(5-{[4-(Benzo[1,3]dioxol-5-yl)-2-hydroxy-2-(4-me...)Show SMILES COc1ccc(cc1)C1(O)OC(=O)C(=C1Cc1cc(OC)c(OC)c(OCCOc2ccc(cc2)S(C)(=O)=O)c1)c1ccc2OCOc2c1 |c:14| Show InChI InChI=1S/C36H34O12S/c1-41-25-8-6-24(7-9-25)36(38)28(33(35(37)48-36)23-5-14-29-30(20-23)47-21-46-29)17-22-18-31(42-2)34(43-3)32(19-22)45-16-15-44-26-10-12-27(13-11-26)49(4,39)40/h5-14,18-20,38H,15-17,21H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital M£nster
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET1 from endothelin B receptor in DBA mouse microsomes |
Bioorg Med Chem 17: 7197-208 (2009)
Article DOI: 10.1016/j.bmc.2009.08.058
BindingDB Entry DOI: 10.7270/Q27S7NVF |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071434

(CHEMBL72217 | N-[(R)-1-[1-(Butane-1-sulfonylaminoc...)Show SMILES CCCCS(=O)(=O)NC(=O)C(CC(C)C)NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C32H42N4O6S/c1-7-8-15-43(40,41)35-30(37)27(16-21(2)3)34-31(38)28(36(6)32(39)26-18-22(4)17-23(5)19-26)20-24-9-11-25(12-10-24)29-13-14-33-42-29/h9-14,17-19,21,27-28H,7-8,15-16,20H2,1-6H3,(H,34,38)(H,35,37)/t27?,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071442
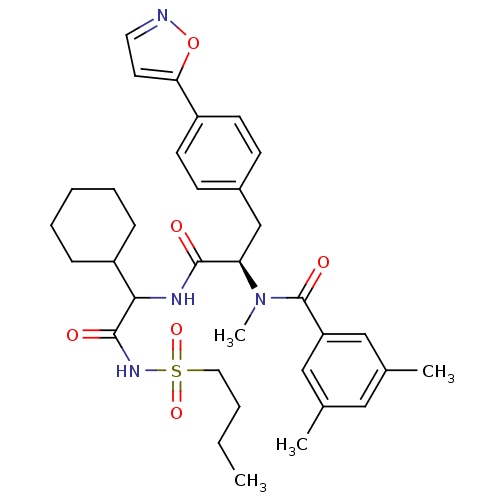
(CHEMBL72358 | N-[(R)-1-[2-(Butane-1-sulfonylamino)...)Show SMILES CCCCS(=O)(=O)NC(=O)C(NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C1CCCCC1 Show InChI InChI=1S/C34H44N4O6S/c1-5-6-18-45(42,43)37-33(40)31(27-10-8-7-9-11-27)36-32(39)29(38(4)34(41)28-20-23(2)19-24(3)21-28)22-25-12-14-26(15-13-25)30-16-17-35-44-30/h12-17,19-21,27,29,31H,5-11,18,22H2,1-4H3,(H,36,39)(H,37,40)/t29-,31?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071424

(CHEMBL263301 | N-[(R)-1-[1-(Butane-1-sulfonylamino...)Show SMILES CCCCS(=O)(=O)NC(=O)C(NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C(C)O Show InChI InChI=1S/C30H38N4O7S/c1-6-7-14-42(39,40)33-29(37)27(21(4)35)32-28(36)25(34(5)30(38)24-16-19(2)15-20(3)17-24)18-22-8-10-23(11-9-22)26-12-13-31-41-26/h8-13,15-17,21,25,27,35H,6-7,14,18H2,1-5H3,(H,32,36)(H,33,37)/t21?,25-,27?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50079426
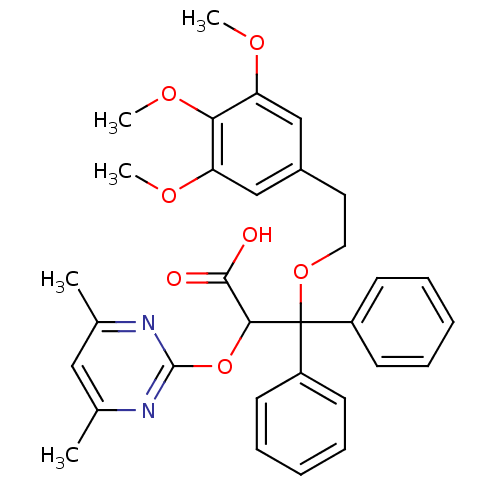
(2-(4,6-Dimethyl-pyrimidin-2-yloxy)-3,3-diphenyl-3-...)Show SMILES COc1cc(CCOC(C(Oc2nc(C)cc(C)n2)C(O)=O)(c2ccccc2)c2ccccc2)cc(OC)c1OC Show InChI InChI=1S/C32H34N2O7/c1-21-18-22(2)34-31(33-21)41-29(30(35)36)32(24-12-8-6-9-13-24,25-14-10-7-11-15-25)40-17-16-23-19-26(37-3)28(39-5)27(20-23)38-4/h6-15,18-20,29H,16-17H2,1-5H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-3 (ETB assay) binding to human cloned Endothelin B receptor in CHO cells |
J Med Chem 42: 3026-32 (1999)
Article DOI: 10.1021/jm9910425
BindingDB Entry DOI: 10.7270/Q2HD7TV6 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071422
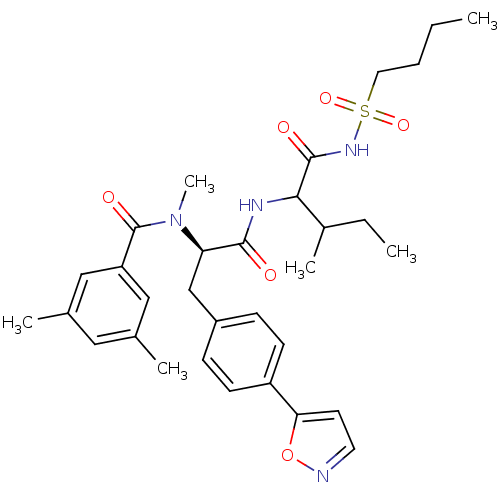
(CHEMBL73042 | N-[(R)-1-[1-(Butane-1-sulfonylaminoc...)Show SMILES CCCCS(=O)(=O)NC(=O)C(NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C(C)CC Show InChI InChI=1S/C32H42N4O6S/c1-7-9-16-43(40,41)35-31(38)29(23(5)8-2)34-30(37)27(36(6)32(39)26-18-21(3)17-22(4)19-26)20-24-10-12-25(13-11-24)28-14-15-33-42-28/h10-15,17-19,23,27,29H,7-9,16,20H2,1-6H3,(H,34,37)(H,35,38)/t23?,27-,29?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50071429
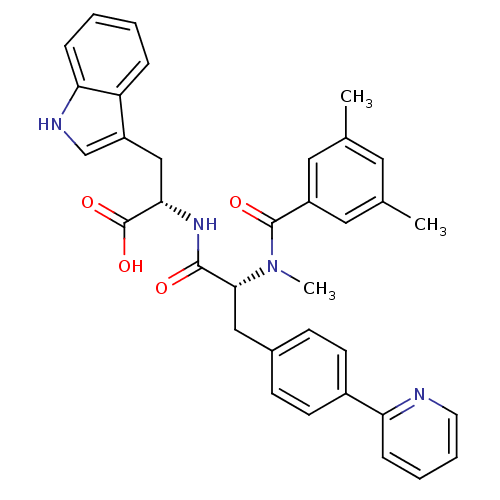
((S)-2-[(R)-2-[(3,5-Dimethyl-benzoyl)-methyl-amino]...)Show SMILES CN([C@H](Cc1ccc(cc1)-c1ccccn1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C35H34N4O4/c1-22-16-23(2)18-26(17-22)34(41)39(3)32(19-24-11-13-25(14-12-24)29-9-6-7-15-36-29)33(40)38-31(35(42)43)20-27-21-37-30-10-5-4-8-28(27)30/h4-18,21,31-32,37H,19-20H2,1-3H3,(H,38,40)(H,42,43)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to Endothelin B receptor |
Bioorg Med Chem Lett 8: 2241-6 (1999)
BindingDB Entry DOI: 10.7270/Q2BC3XQ7 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50079424
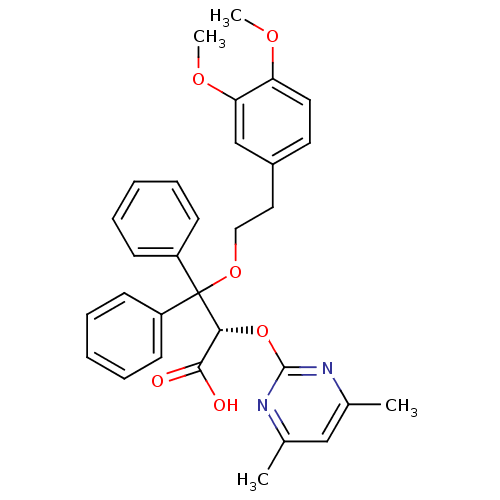
((S)-3-[2-(3,4-Dimethoxy-phenyl)-ethoxy]-2-(4,6-dim...)Show SMILES COc1ccc(CCOC([C@H](Oc2nc(C)cc(C)n2)C(O)=O)(c2ccccc2)c2ccccc2)cc1OC Show InChI InChI=1S/C31H32N2O6/c1-21-19-22(2)33-30(32-21)39-28(29(34)35)31(24-11-7-5-8-12-24,25-13-9-6-10-14-25)38-18-17-23-15-16-26(36-3)27(20-23)37-4/h5-16,19-20,28H,17-18H2,1-4H3,(H,34,35)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-3 (ETB assay) binding to human cloned Endothelin B receptor in CHO cells |
J Med Chem 42: 3026-32 (1999)
Article DOI: 10.1021/jm9910425
BindingDB Entry DOI: 10.7270/Q2HD7TV6 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50079424

((S)-3-[2-(3,4-Dimethoxy-phenyl)-ethoxy]-2-(4,6-dim...)Show SMILES COc1ccc(CCOC([C@H](Oc2nc(C)cc(C)n2)C(O)=O)(c2ccccc2)c2ccccc2)cc1OC Show InChI InChI=1S/C31H32N2O6/c1-21-19-22(2)33-30(32-21)39-28(29(34)35)31(24-11-7-5-8-12-24,25-13-9-6-10-14-25)38-18-17-23-15-16-26(36-3)27(20-23)37-4/h5-16,19-20,28H,17-18H2,1-4H3,(H,34,35)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Displacement of [125I]ET-3 from human ET-B receptor expressed in CHO cell membrane incubated for 30 mins by liquid scintillation counting method |
J Med Chem 59: 8168-88 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01781
BindingDB Entry DOI: 10.7270/Q22N55RM |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287878
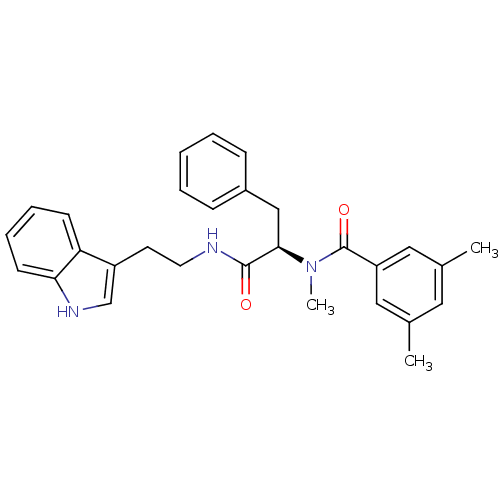
(CGP-49941 | CHEMBL305615 | N-{(R)-1-[2-(1H-Indol-3...)Show SMILES CN([C@H](Cc1ccccc1)C(=O)NCCc1c[nH]c2ccccc12)C(=O)c1cc(C)cc(C)c1 Show InChI InChI=1S/C29H31N3O2/c1-20-15-21(2)17-24(16-20)29(34)32(3)27(18-22-9-5-4-6-10-22)28(33)30-14-13-23-19-31-26-12-8-7-11-25(23)26/h4-12,15-17,19,27,31H,13-14,18H2,1-3H3,(H,30,33)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287888
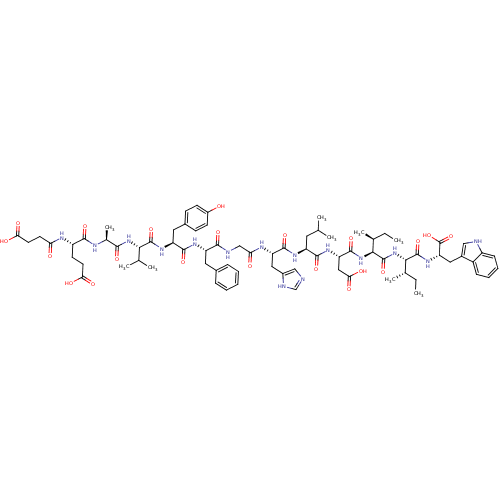
(CHEMBL407559 | Suc-Glu-Ala-Val-Tyr-Phe-Gly-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C76H103N15O21/c1-10-41(7)64(74(109)88-57(76(111)112)32-46-35-78-50-20-16-15-19-49(46)50)91-75(110)65(42(8)11-2)90-72(107)56(34-62(99)100)86-69(104)52(29-39(3)4)84-71(106)55(33-47-36-77-38-80-47)83-59(94)37-79-67(102)53(30-44-17-13-12-14-18-44)85-70(105)54(31-45-21-23-48(92)24-22-45)87-73(108)63(40(5)6)89-66(101)43(9)81-68(103)51(25-27-60(95)96)82-58(93)26-28-61(97)98/h12-24,35-36,38-43,51-57,63-65,78,92H,10-11,25-34,37H2,1-9H3,(H,77,80)(H,79,102)(H,81,103)(H,82,93)(H,83,94)(H,84,106)(H,85,105)(H,86,104)(H,87,108)(H,88,109)(H,89,101)(H,90,107)(H,91,110)(H,95,96)(H,97,98)(H,99,100)(H,111,112)/t41-,42-,43-,51-,52-,53-,54-,55-,56-,57-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50050964
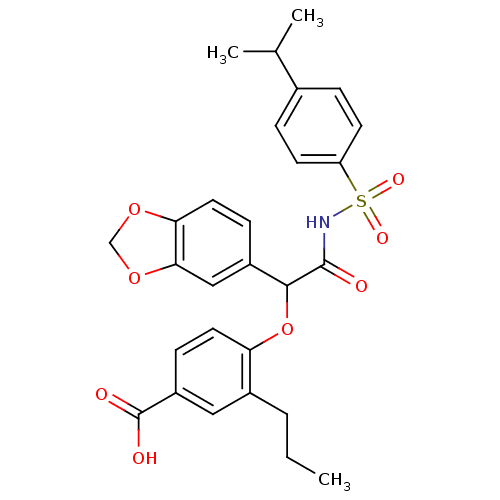
(4-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...)Show SMILES CCCc1cc(ccc1OC(C(=O)NS(=O)(=O)c1ccc(cc1)C(C)C)c1ccc2OCOc2c1)C(O)=O Show InChI InChI=1S/C28H29NO8S/c1-4-5-19-14-21(28(31)32)9-12-23(19)37-26(20-8-13-24-25(15-20)36-16-35-24)27(30)29-38(33,34)22-10-6-18(7-11-22)17(2)3/h6-15,17,26H,4-5,16H2,1-3H3,(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human Endothelin B receptor in chinese hamster ovary cells |
J Med Chem 39: 1039-48 (1996)
Article DOI: 10.1021/jm9505369
BindingDB Entry DOI: 10.7270/Q29G5KWT |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287886
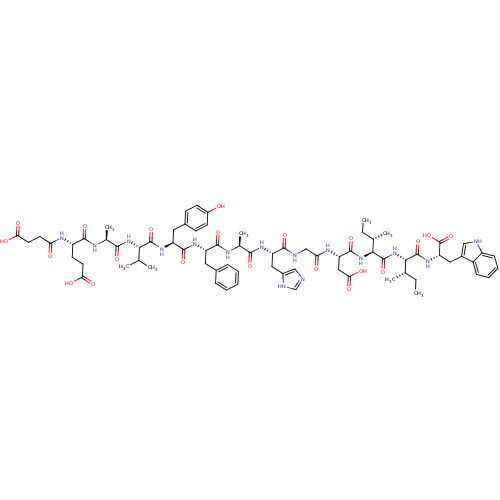
(CHEMBL405796 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Gly...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C73H97N15O21/c1-9-38(5)61(71(106)85-54(73(108)109)30-44-33-75-48-19-15-14-18-47(44)48)88-72(107)62(39(6)10-2)87-69(104)53(32-59(96)97)81-56(91)35-76-65(100)52(31-45-34-74-36-77-45)82-63(98)40(7)79-67(102)50(28-42-16-12-11-13-17-42)83-68(103)51(29-43-20-22-46(89)23-21-43)84-70(105)60(37(3)4)86-64(99)41(8)78-66(101)49(24-26-57(92)93)80-55(90)25-27-58(94)95/h11-23,33-34,36-41,49-54,60-62,75,89H,9-10,24-32,35H2,1-8H3,(H,74,77)(H,76,100)(H,78,101)(H,79,102)(H,80,90)(H,81,91)(H,82,98)(H,83,103)(H,84,105)(H,85,106)(H,86,99)(H,87,104)(H,88,107)(H,92,93)(H,94,95)(H,96,97)(H,108,109)/t38-,39-,40-,41-,49-,50-,51-,52-,53-,54-,60-,61-,62-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Homo sapiens (Human)) | BDBM50079417
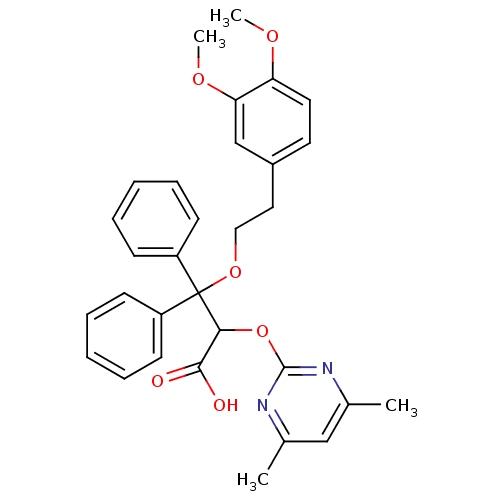
(3-[2-(3,4-Dimethoxy-phenyl)-ethoxy]-2-(4,6-dimethy...)Show SMILES COc1ccc(CCOC(C(Oc2nc(C)cc(C)n2)C(O)=O)(c2ccccc2)c2ccccc2)cc1OC Show InChI InChI=1S/C31H32N2O6/c1-21-19-22(2)33-30(32-21)39-28(29(34)35)31(24-11-7-5-8-12-24,25-13-9-6-10-14-25)38-18-17-23-15-16-26(36-3)27(20-23)37-4/h5-16,19-20,28H,17-18H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BASF AG
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-ET-3 (ETB assay) binding to human cloned Endothelin B receptor in CHO cells |
J Med Chem 42: 3026-32 (1999)
Article DOI: 10.1021/jm9910425
BindingDB Entry DOI: 10.7270/Q2HD7TV6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































