 Found 38 hits Enz. Inhib. hit(s) with Target = 'C-C motif chemokine 5'
Found 38 hits Enz. Inhib. hit(s) with Target = 'C-C motif chemokine 5' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329229

((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-phenyl...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)(F)F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38F3N5O/c1-20-27(21(2)34-19-33-20)28(38)37-16-24-14-36(15-25(24)17-37)13-10-26(22-6-4-3-5-7-22)23-8-11-35(12-9-23)18-29(30,31)32/h3-7,19,23-26H,8-18H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329246
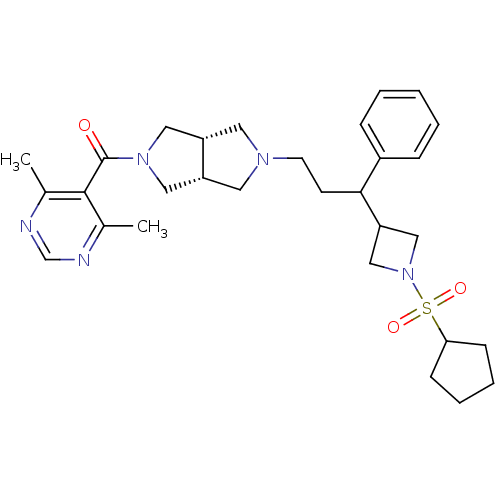
(((3aR,6aS)-5-(3-(1-(cyclopentylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H41N5O3S/c1-21-29(22(2)32-20-31-21)30(36)34-16-24-14-33(15-25(24)17-34)13-12-28(23-8-4-3-5-9-23)26-18-35(19-26)39(37,38)27-10-6-7-11-27/h3-5,8-9,20,24-28H,6-7,10-19H2,1-2H3/t24-,25+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329241
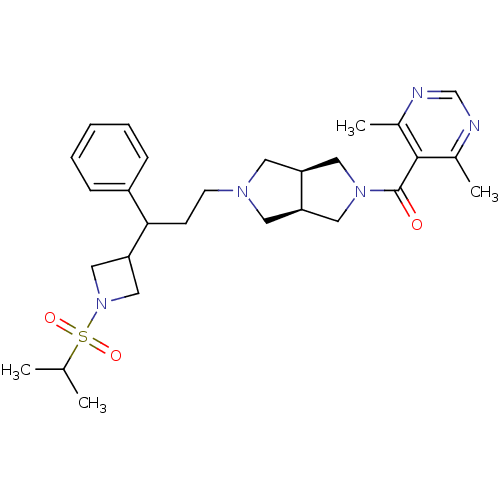
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(is...)Show SMILES CC(C)S(=O)(=O)N1CC(C1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C28H39N5O3S/c1-19(2)37(35,36)33-16-25(17-33)26(22-8-6-5-7-9-22)10-11-31-12-23-14-32(15-24(23)13-31)28(34)27-20(3)29-18-30-21(27)4/h5-9,18-19,23-26H,10-17H2,1-4H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329250

(((3aR,6aS)-5-(3-(1-(cyclopentylsulfonyl)piperidin-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C32H45N5O3S/c1-23-31(24(2)34-22-33-23)32(38)36-20-27-18-35(19-28(27)21-36)15-14-30(25-8-4-3-5-9-25)26-12-16-37(17-13-26)41(39,40)29-10-6-7-11-29/h3-5,8-9,22,26-30H,6-7,10-21H2,1-2H3/t27-,28+,30? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329232

(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3cc(F)cc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H37F4N5O/c1-18-28(19(2)35-17-34-18)29(39)38-14-22-12-37(13-23(22)15-38)8-5-26(21-9-24(30)11-25(31)10-21)20-3-6-36(7-4-20)16-27(32)33/h9-11,17,20,22-23,26-27H,3-8,12-16H2,1-2H3/t22-,23+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329249
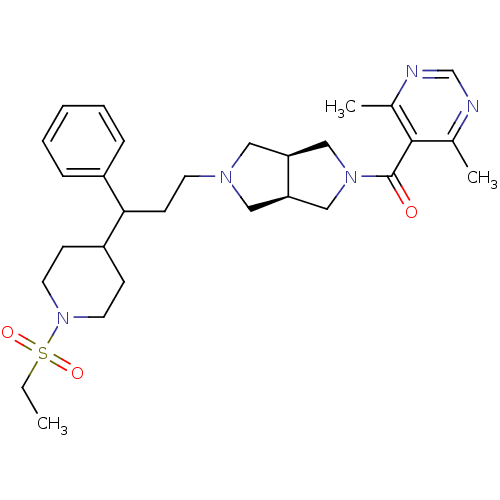
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(et...)Show SMILES CCS(=O)(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C29H41N5O3S/c1-4-38(36,37)34-14-10-24(11-15-34)27(23-8-6-5-7-9-23)12-13-32-16-25-18-33(19-26(25)17-32)29(35)28-21(2)30-20-31-22(28)3/h5-9,20,24-27H,4,10-19H2,1-3H3/t25-,26+,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329255
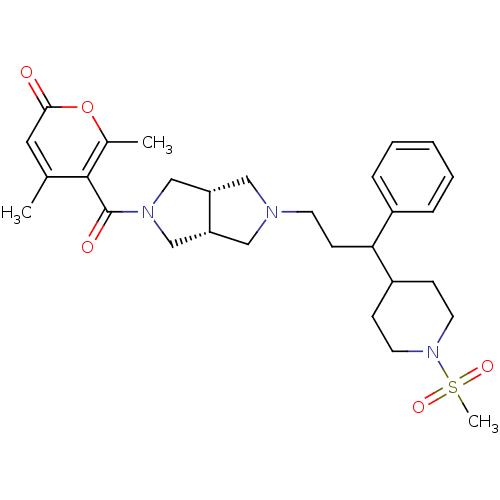
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(=O)oc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39N3O5S/c1-20-15-27(33)37-21(2)28(20)29(34)31-18-24-16-30(17-25(24)19-31)12-11-26(22-7-5-4-6-8-22)23-9-13-32(14-10-23)38(3,35)36/h4-8,15,23-26H,9-14,16-19H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329248
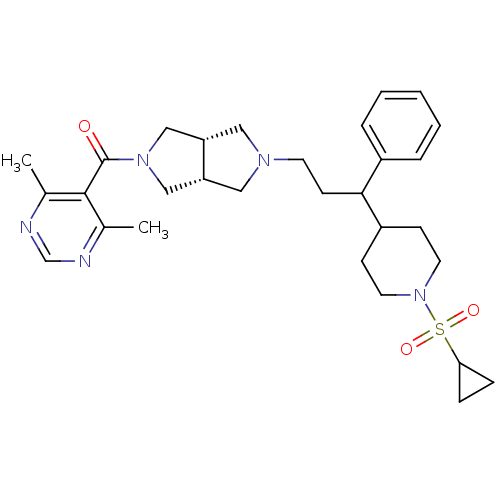
(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)piperidin-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)C3CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H41N5O3S/c1-21-29(22(2)32-20-31-21)30(36)34-18-25-16-33(17-26(25)19-34)13-12-28(23-6-4-3-5-7-23)24-10-14-35(15-11-24)39(37,38)27-8-9-27/h3-7,20,24-28H,8-19H2,1-2H3/t25-,26+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329230
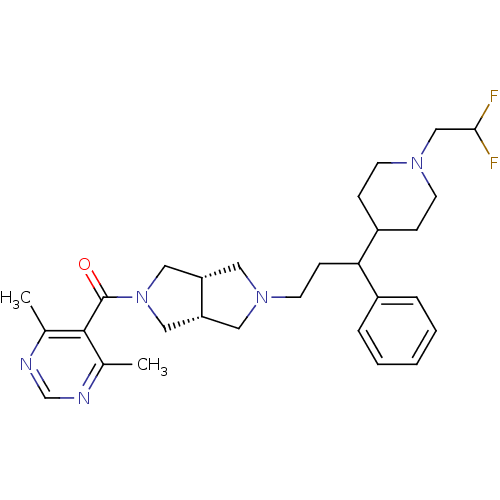
(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H39F2N5O/c1-20-28(21(2)33-19-32-20)29(37)36-16-24-14-35(15-25(24)17-36)13-10-26(22-6-4-3-5-7-22)23-8-11-34(12-9-23)18-27(30)31/h3-7,19,23-27H,8-18H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329256
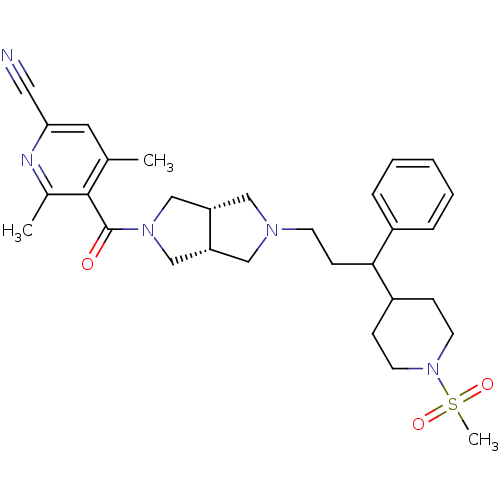
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C30H39N5O3S/c1-21-15-27(16-31)32-22(2)29(21)30(36)34-19-25-17-33(18-26(25)20-34)12-11-28(23-7-5-4-6-8-23)24-9-13-35(14-10-24)39(3,37)38/h4-8,15,24-26,28H,9-14,17-20H2,1-3H3/t25-,26+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329235

(1-(4-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES CCC(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C30H41N5O2/c1-4-28(36)34-14-10-24(11-15-34)27(23-8-6-5-7-9-23)12-13-33-16-25-18-35(19-26(25)17-33)30(37)29-21(2)31-20-32-22(29)3/h5-9,20,24-27H,4,10-19H2,1-3H3/t25-,26+,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329251
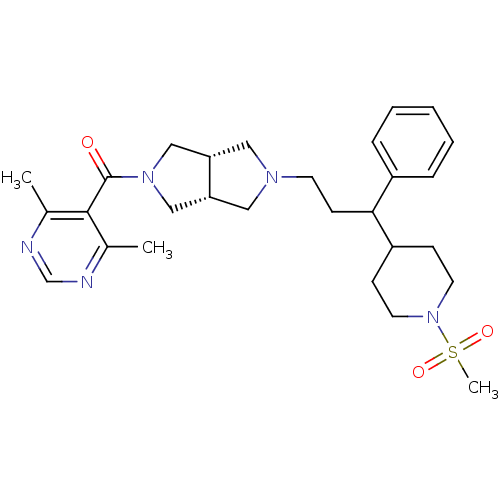
((4,6-dimethyl pyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(m...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H39N5O3S/c1-20-27(21(2)30-19-29-20)28(34)32-17-24-15-31(16-25(24)18-32)12-11-26(22-7-5-4-6-8-22)23-9-13-33(14-10-23)37(3,35)36/h4-8,19,23-26H,9-18H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329251

((4,6-dimethyl pyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(m...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H39N5O3S/c1-20-27(21(2)30-19-29-20)28(34)32-17-24-15-31(16-25(24)18-32)12-11-26(22-7-5-4-6-8-22)23-9-13-33(14-10-23)37(3,35)36/h4-8,19,23-26H,9-18H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329231
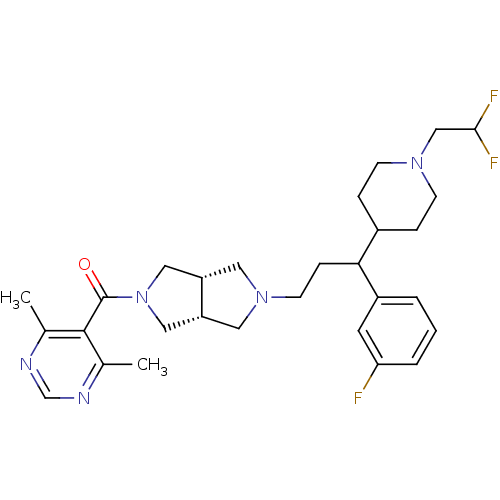
(((3aR,6aS)-5-(3-(1-(2,2-difluoroethyl)piperidin-4-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC(F)F)CC3)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38F3N5O/c1-19-28(20(2)34-18-33-19)29(38)37-15-23-13-36(14-24(23)16-37)11-8-26(22-4-3-5-25(30)12-22)21-6-9-35(10-7-21)17-27(31)32/h3-5,12,18,21,23-24,26-27H,6-11,13-17H2,1-2H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329244
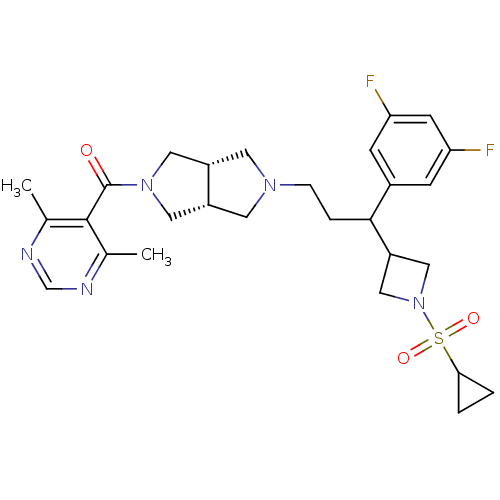
(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CC3)c3cc(F)cc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H35F2N5O3S/c1-17-27(18(2)32-16-31-17)28(36)34-12-20-10-33(11-21(20)13-34)6-5-26(19-7-23(29)9-24(30)8-19)22-14-35(15-22)39(37,38)25-3-4-25/h7-9,16,20-22,25-26H,3-6,10-15H2,1-2H3/t20-,21+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329252
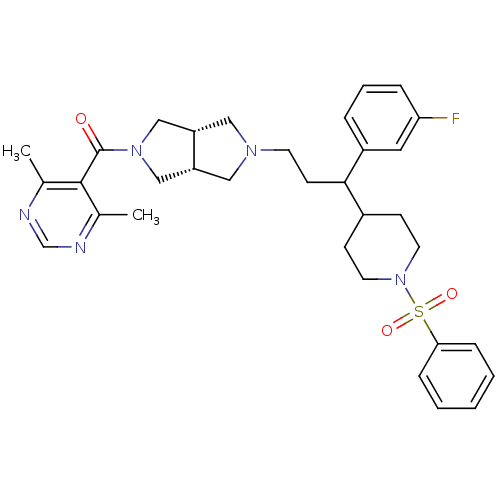
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(3-flu...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)c3ccccc3)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C33H40FN5O3S/c1-23-32(24(2)36-22-35-23)33(40)38-20-27-18-37(19-28(27)21-38)14-13-31(26-7-6-8-29(34)17-26)25-11-15-39(16-12-25)43(41,42)30-9-4-3-5-10-30/h3-10,17,22,25,27-28,31H,11-16,18-21H2,1-2H3/t27-,28+,31? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329243

(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CC3)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H36FN5O3S/c1-18-27(19(2)31-17-30-18)28(35)33-13-21-11-32(12-22(21)14-33)9-8-26(20-4-3-5-24(29)10-20)23-15-34(16-23)38(36,37)25-6-7-25/h3-5,10,17,21-23,25-26H,6-9,11-16H2,1-2H3/t21-,22+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329242
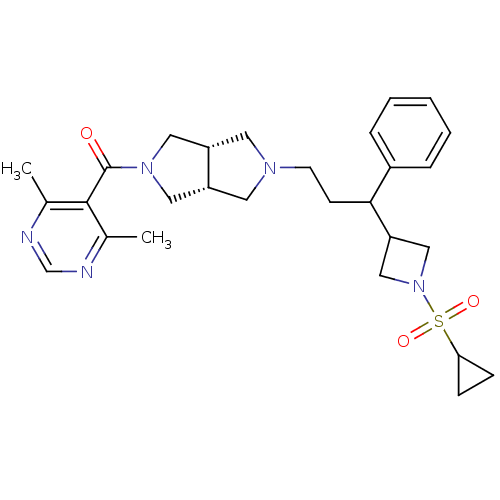
(((3aR,6aS)-5-(3-(1-(cyclopropylsulfonyl)azetidin-3...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(=O)(=O)C3CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H37N5O3S/c1-19-27(20(2)30-18-29-19)28(34)32-14-22-12-31(13-23(22)15-32)11-10-26(21-6-4-3-5-7-21)24-16-33(17-24)37(35,36)25-8-9-25/h3-7,18,22-26H,8-17H2,1-2H3/t22-,23+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329227
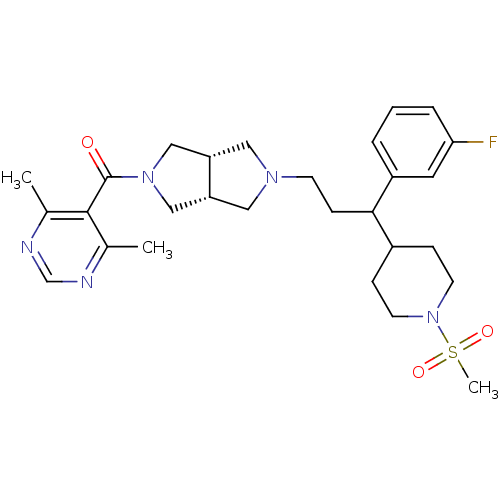
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(3-flu...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H38FN5O3S/c1-19-27(20(2)31-18-30-19)28(35)33-16-23-14-32(15-24(23)17-33)10-9-26(22-5-4-6-25(29)13-22)21-7-11-34(12-8-21)38(3,36)37/h4-6,13,18,21,23-24,26H,7-12,14-17H2,1-3H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329227

((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(3-flu...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H38FN5O3S/c1-19-27(20(2)31-18-30-19)28(35)33-16-23-14-32(15-24(23)17-33)10-9-26(22-5-4-6-25(29)13-22)21-7-11-34(12-8-21)38(3,36)37/h4-6,13,18,21,23-24,26H,7-12,14-17H2,1-3H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329234
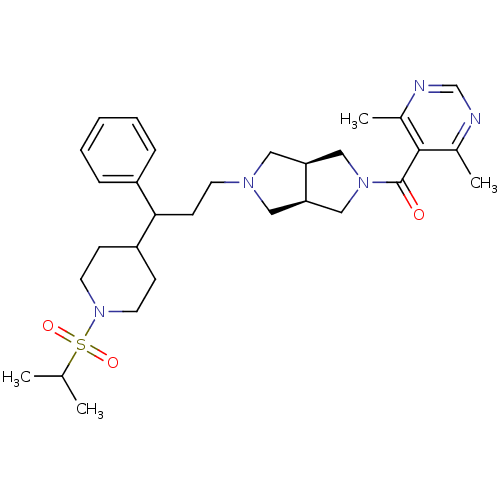
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(is...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C30H43N5O3S/c1-21(2)39(37,38)35-14-10-25(11-15-35)28(24-8-6-5-7-9-24)12-13-33-16-26-18-34(19-27(26)17-33)30(36)29-22(3)31-20-32-23(29)4/h5-9,20-21,25-28H,10-19H2,1-4H3/t26-,27+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329247
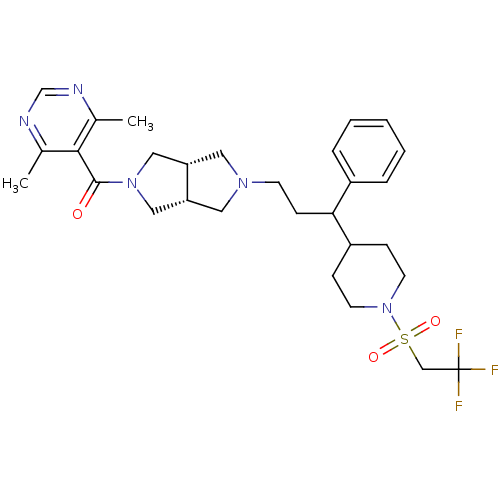
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-phenyl...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(=O)(=O)CC(F)(F)F)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38F3N5O3S/c1-20-27(21(2)34-19-33-20)28(38)36-16-24-14-35(15-25(24)17-36)11-10-26(22-6-4-3-5-7-22)23-8-12-37(13-9-23)41(39,40)18-29(30,31)32/h3-7,19,23-26H,8-18H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329245

((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(me...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C26H35N5O3S/c1-18-25(19(2)28-17-27-18)26(32)30-13-21-11-29(12-22(21)14-30)10-9-24(20-7-5-4-6-8-20)23-15-31(16-23)35(3,33)34/h4-8,17,21-24H,9-16H2,1-3H3/t21-,22+,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329237

(1-(4-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES CC(C)CC(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C32H45N5O2/c1-22(2)16-30(38)36-14-10-26(11-15-36)29(25-8-6-5-7-9-25)12-13-35-17-27-19-37(20-28(27)18-35)32(39)31-23(3)33-21-34-24(31)4/h5-9,21-22,26-29H,10-20H2,1-4H3/t27-,28+,29? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329236
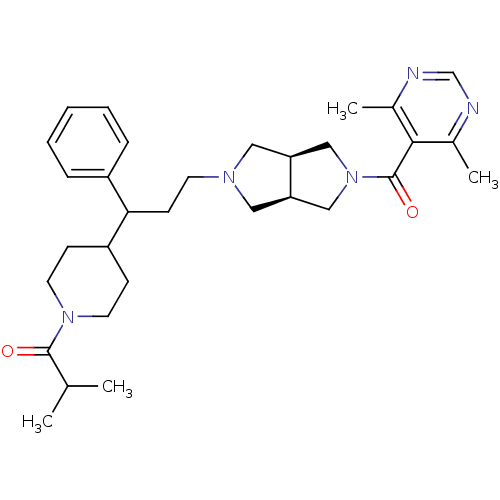
(1-(4-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES CC(C)C(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C31H43N5O2/c1-21(2)30(37)35-14-10-25(11-15-35)28(24-8-6-5-7-9-24)12-13-34-16-26-18-36(19-27(26)17-34)31(38)29-22(3)32-20-33-23(29)4/h5-9,20-21,25-28H,10-19H2,1-4H3/t26-,27+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329233
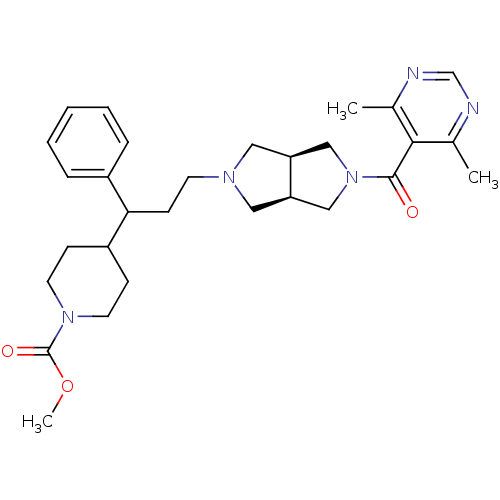
(CHEMBL1270900 | methyl 4-(3-((3aR,6aS)-5-(4,6-dime...)Show SMILES COC(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C29H39N5O3/c1-20-27(21(2)31-19-30-20)28(35)34-17-24-15-32(16-25(24)18-34)12-11-26(22-7-5-4-6-8-22)23-9-13-33(14-10-23)29(36)37-3/h4-8,19,23-26H,9-18H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329253
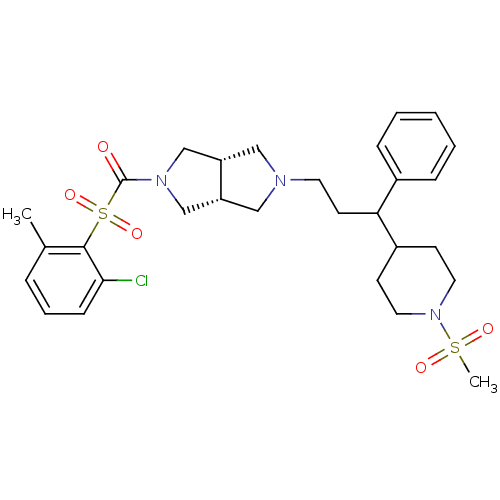
((2-chloro-6-methylphenylsulfonyl)((3aR,6aS)-5-(3-(...)Show SMILES Cc1cccc(Cl)c1S(=O)(=O)C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38ClN3O5S2/c1-21-7-6-10-27(30)28(21)40(37,38)29(34)32-19-24-17-31(18-25(24)20-32)14-13-26(22-8-4-3-5-9-22)23-11-15-33(16-12-23)39(2,35)36/h3-10,23-26H,11-20H2,1-2H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329239
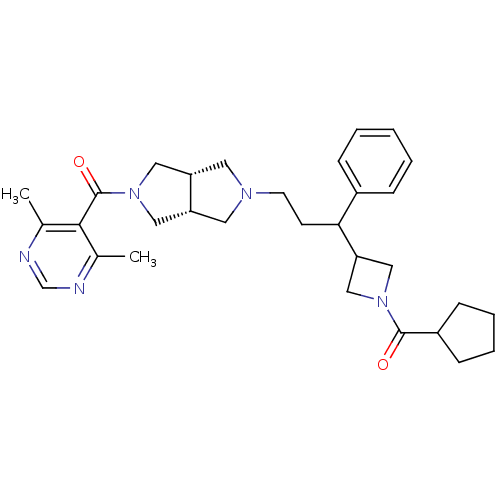
(((3aR,6aS)-5-(3-(1-(cyclopentanecarbonyl)azetidin-...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(C3)C(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H41N5O2/c1-21-29(22(2)33-20-32-21)31(38)36-16-25-14-34(15-26(25)17-36)13-12-28(23-8-4-3-5-9-23)27-18-35(19-27)30(37)24-10-6-7-11-24/h3-5,8-9,20,24-28H,6-7,10-19H2,1-2H3/t25-,26+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329254
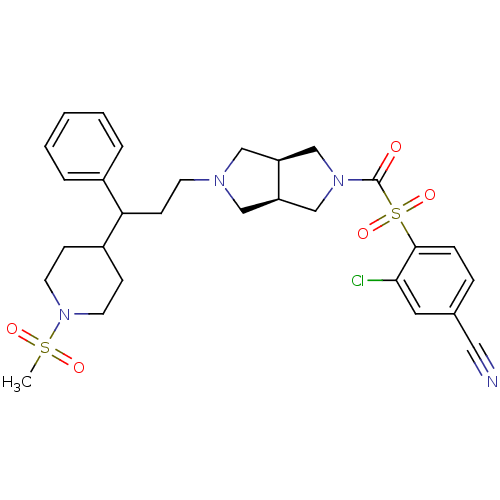
(3-chloro-4-((3aR,6aS)-5-(3-(1-(methylsulfonyl)pipe...)Show SMILES CS(=O)(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)S(=O)(=O)c1ccc(cc1Cl)C#N)c1ccccc1 |r| Show InChI InChI=1S/C29H35ClN4O5S2/c1-40(36,37)34-13-9-23(10-14-34)26(22-5-3-2-4-6-22)11-12-32-17-24-19-33(20-25(24)18-32)29(35)41(38,39)28-8-7-21(16-31)15-27(28)30/h2-8,15,23-26H,9-14,17-20H2,1H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329251

((4,6-dimethyl pyrimidin-5-yl)((3aR,6aS)-5-(3-(1-(m...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H39N5O3S/c1-20-27(21(2)30-19-29-20)28(34)32-17-24-15-31(16-25(24)18-32)12-11-26(22-7-5-4-6-8-22)23-9-13-33(14-10-23)37(3,35)36/h4-8,19,23-26H,9-18H2,1-3H3/t24-,25+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329228

(4-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-carbon...)Show SMILES CC(C)NC(=O)N1CCC(CC1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C31H44N6O2/c1-21(2)34-31(39)36-14-10-25(11-15-36)28(24-8-6-5-7-9-24)12-13-35-16-26-18-37(19-27(26)17-35)30(38)29-22(3)32-20-33-23(29)4/h5-9,20-21,25-28H,10-19H2,1-4H3,(H,34,39)/t26-,27+,28? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329238
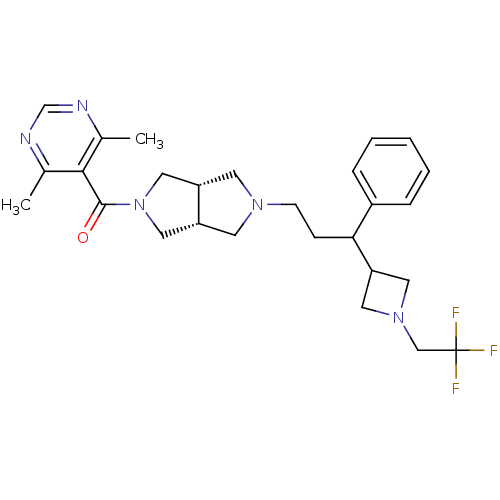
((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-phenyl...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CN(CC(F)(F)F)C3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C27H34F3N5O/c1-18-25(19(2)32-17-31-18)26(36)35-14-21-10-33(11-22(21)15-35)9-8-24(20-6-4-3-5-7-20)23-12-34(13-23)16-27(28,29)30/h3-7,17,21-24H,8-16H2,1-2H3/t21-,22+,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329227

((4,6-dimethylpyrimidin-5-yl)((3aR,6aS)-5-(3-(3-flu...)Show SMILES Cc1ncnc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3cccc(F)c3)C[C@@H]2C1 |r| Show InChI InChI=1S/C28H38FN5O3S/c1-19-27(20(2)31-18-30-19)28(35)33-16-23-14-32(15-24(23)17-33)10-9-26(22-5-4-6-25(29)13-22)21-7-11-34(12-8-21)38(3,36)37/h4-6,13,18,21,23-24,26H,7-12,14-17H2,1-3H3/t23-,24+,26? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50329240
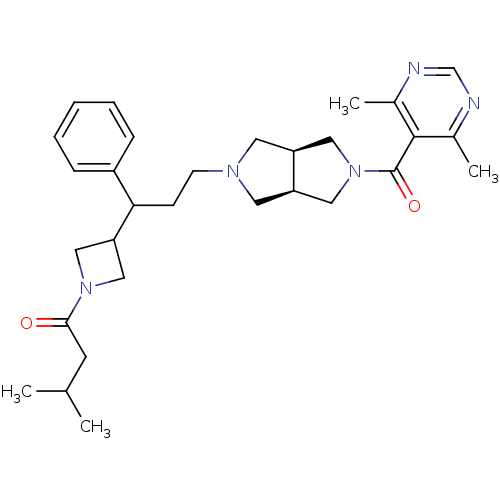
(1-(3-(3-((3aR,6aS)-5-(4,6-dimethylpyrimidine-5-car...)Show SMILES CC(C)CC(=O)N1CC(C1)C(CCN1C[C@H]2CN(C[C@H]2C1)C(=O)c1c(C)ncnc1C)c1ccccc1 |r| Show InChI InChI=1S/C30H41N5O2/c1-20(2)12-28(36)34-17-26(18-34)27(23-8-6-5-7-9-23)10-11-33-13-24-15-35(16-25(24)14-33)30(37)29-21(3)31-19-32-22(29)4/h5-9,19-20,24-27H,10-18H2,1-4H3/t24-,25+,27? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of RANTES |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50017345
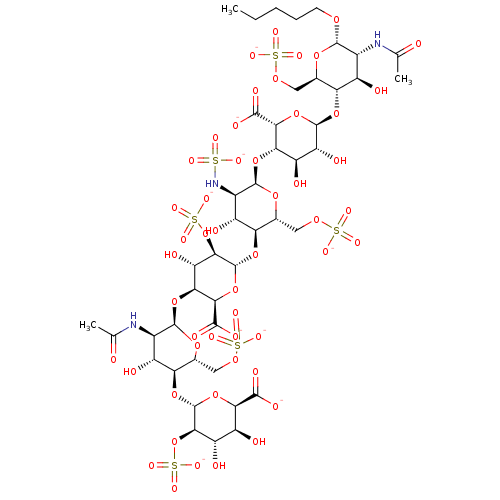
(CHEMBL3288260)Show SMILES [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[H][C@]1(O[C@@H]2O[C@@H](C([O-])=O)[C@@]([H])(O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@]([H])(O[C@@H]4O[C@@H](C([O-])=O)[C@@]([H])(O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@]([H])(O[C@@H]6O[C@H]([C@@H](O)[C@H](O)[C@H]6OS([O-])(=O)=O)C([O-])=O)[C@H](O)[C@H]5NC(C)=O)[C@H](O)[C@H]4OS([O-])(=O)=O)[C@H](O)[C@H]3NS([O-])(=O)=O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](NC(C)=O)[C@@H](OCCCCC)O[C@@H]1COS([O-])(=O)=O |r| Show InChI InChI=1S/C45H73N3O51S6.9Na/c1-4-5-6-7-83-40-16(46-11(2)49)19(51)27(13(87-40)8-84-101(68,69)70)90-43-25(57)24(56)30(35(96-43)38(61)62)93-42-18(48-100(65,66)67)21(53)29(15(89-42)10-86-103(74,75)76)92-45-34(99-105(80,81)82)26(58)31(36(97-45)39(63)64)94-41-17(47-12(3)50)20(52)28(14(88-41)9-85-102(71,72)73)91-44-33(98-104(77,78)79)23(55)22(54)32(95-44)37(59)60;;;;;;;;;/h13-36,40-45,48,51-58H,4-10H2,1-3H3,(H,46,49)(H,47,50)(H,59,60)(H,61,62)(H,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82);;;;;;;;;/q;9*+1/p-9/t13-,14-,15-,16-,17-,18-,19-,20-,21-,22+,23+,24-,25-,26+,27-,28-,29-,30+,31+,32-,33-,34-,35-,36-,40+,41-,42-,43-,44-,45-;;;;;;;;;/m1........./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Momenta Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant CCL5 by surface plasmon resonance assay |
J Med Chem 57: 4511-20 (2014)
Article DOI: 10.1021/jm4016069
BindingDB Entry DOI: 10.7270/Q2ZG6TSN |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50017347
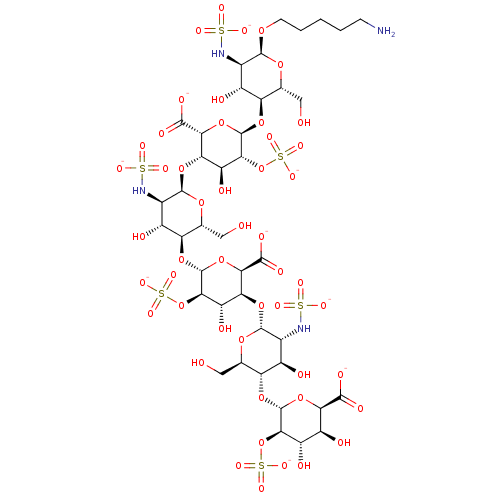
(CHEMBL3288259)Show SMILES [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[H][C@]1(O[C@@H]2O[C@H]([C@@H](O)[C@H](O)[C@H]2OS([O-])(=O)=O)C([O-])=O)[C@H](O)[C@@H](NS([O-])(=O)=O)[C@@H](O[C@@]2([H])[C@H](O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@@]3([H])[C@H](O)[C@@H](NS([O-])(=O)=O)[C@@H](O[C@@]4([H])[C@H](O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@@]5([H])[C@H](O)[C@@H](NS([O-])(=O)=O)[C@@H](OCCCCCN)O[C@@H]5CO)O[C@H]4C([O-])=O)O[C@@H]3CO)O[C@H]2C([O-])=O)O[C@@H]1CO |r| Show InChI InChI=1S/C41H70N4O49S6.9Na/c42-4-2-1-3-5-80-36-12(43-95(62,63)64)15(49)22(9(6-46)81-36)85-40-29(93-99(74,75)76)20(54)25(31(90-40)34(58)59)88-38-14(45-97(68,69)70)17(51)24(11(8-48)83-38)86-41-30(94-100(77,78)79)21(55)26(32(91-41)35(60)61)87-37-13(44-96(65,66)67)16(50)23(10(7-47)82-37)84-39-28(92-98(71,72)73)19(53)18(52)27(89-39)33(56)57;;;;;;;;;/h9-32,36-41,43-55H,1-8,42H2,(H,56,57)(H,58,59)(H,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79);;;;;;;;;/q;9*+1/p-9/t9-,10-,11-,12-,13-,14-,15-,16-,17-,18+,19+,20+,21+,22-,23-,24-,25+,26+,27-,28-,29-,30-,31-,32-,36+,37-,38-,39-,40-,41-;;;;;;;;;/m1........./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Momenta Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant CCL5 by surface plasmon resonance assay |
J Med Chem 57: 4511-20 (2014)
Article DOI: 10.1021/jm4016069
BindingDB Entry DOI: 10.7270/Q2ZG6TSN |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50017346
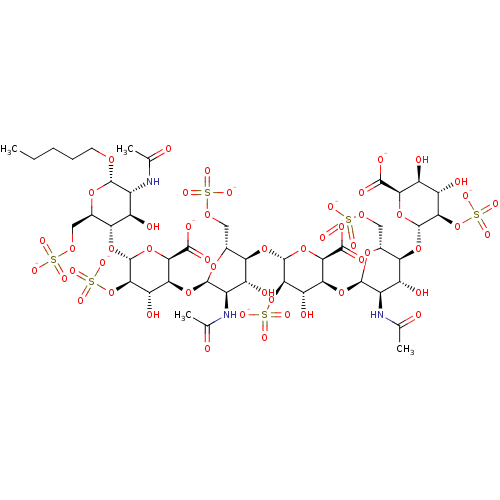
(CHEMBL3288258)Show SMILES [Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[H][C@]1(O[C@@H]2O[C@@H](C([O-])=O)[C@@]([H])(O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@]([H])(O[C@@H]4O[C@@H](C([O-])=O)[C@@]([H])(O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@]([H])(O[C@@H]6O[C@H]([C@@H](O)[C@H](O)[C@H]6OS([O-])(=O)=O)C([O-])=O)[C@H](O)[C@H]5NC(C)=O)[C@H](O)[C@H]4OS([O-])(=O)=O)[C@H](O)[C@H]3NC(C)=O)[C@H](O)[C@H]2OS([O-])(=O)=O)[C@H](O)[C@@H](NC(C)=O)[C@@H](OCCCCC)O[C@@H]1COS([O-])(=O)=O |r| Show InChI InChI=1S/C47H75N3O52S6.9Na/c1-5-6-7-8-85-42-18(48-12(2)51)21(54)28(15(89-42)9-86-103(67,68)69)93-46-35(101-107(79,80)81)26(59)31(37(98-46)40(63)64)96-44-20(50-14(4)53)23(56)30(17(91-44)11-88-105(73,74)75)94-47-36(102-108(82,83)84)27(60)32(38(99-47)41(65)66)95-43-19(49-13(3)52)22(55)29(16(90-43)10-87-104(70,71)72)92-45-34(100-106(76,77)78)25(58)24(57)33(97-45)39(61)62;;;;;;;;;/h15-38,42-47,54-60H,5-11H2,1-4H3,(H,48,51)(H,49,52)(H,50,53)(H,61,62)(H,63,64)(H,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84);;;;;;;;;/q;9*+1/p-9/t15-,16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28-,29-,30-,31+,32+,33-,34-,35-,36-,37-,38-,42+,43-,44-,45-,46-,47-;;;;;;;;;/m1........./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Momenta Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant CCL5 by surface plasmon resonance assay |
J Med Chem 57: 4511-20 (2014)
Article DOI: 10.1021/jm4016069
BindingDB Entry DOI: 10.7270/Q2ZG6TSN |
More data for this
Ligand-Target Pair | |
C-C motif chemokine 5
(Homo sapiens (Human)) | BDBM50585055

(CHEMBL5081237)Show SMILES [H][C@@]1(O[C@H]2[C@H](O)[C@@H](OS(O)(=O)=O)[C@H](OCCOCCN)O[C@H]2C(O)=O)O[C@H]([C@@H](O[C@]2([H])O[C@H]([C@@H](O[C@]3([H])O[C@H]([C@@H](OS(O)(=O)=O)[C@H](O)[C@H]3OS(O)(=O)=O)C(O)=O)[C@H](O)[C@H]2OS(O)(=O)=O)C(O)=O)[C@H](O)[C@H]1OS(O)(=O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.72E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human CCL5 assessed as dissociation constant incubated for 3 mins by surface plasmon resonance analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01800
BindingDB Entry DOI: 10.7270/Q2WH2TW6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data