

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50205472 ((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of EphB3 | Proc Natl Acad Sci USA 104: 20523-8 (2007) Checked by Author Article DOI: 10.1073/pnas.0708800104 BindingDB Entry DOI: 10.7270/Q2DB82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50205477 ((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of EPHB3 | Bioorg Med Chem Lett 17: 2134-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.081 BindingDB Entry DOI: 10.7270/Q2BV7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50205472 ((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of EPHB3 | Bioorg Med Chem Lett 17: 2134-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.081 BindingDB Entry DOI: 10.7270/Q2BV7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50205468 ((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of EPHB3 | Bioorg Med Chem Lett 17: 2134-8 (2007) Article DOI: 10.1016/j.bmcl.2007.01.081 BindingDB Entry DOI: 10.7270/Q2BV7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50598820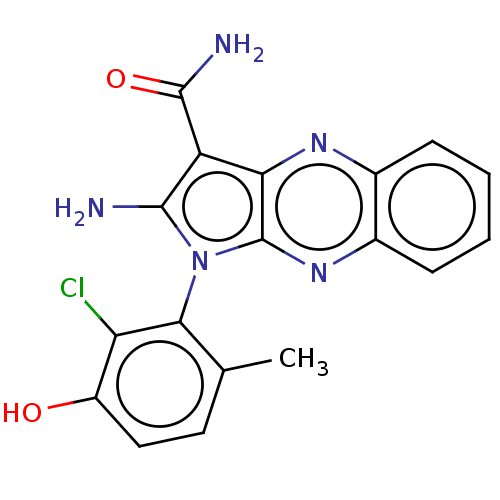 (CHEMBL5191665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00552 BindingDB Entry DOI: 10.7270/Q2028WKQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50598829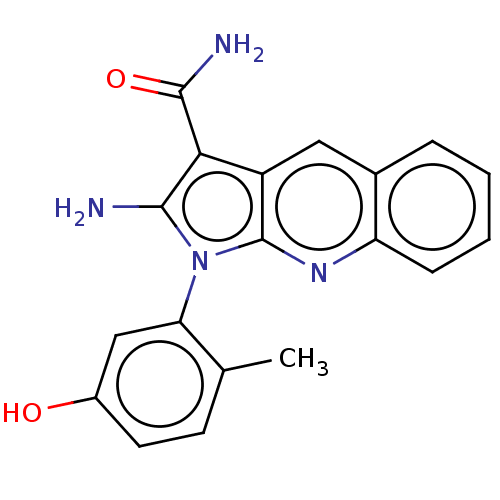 (CHEMBL5203261) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00552 BindingDB Entry DOI: 10.7270/Q2028WKQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50100316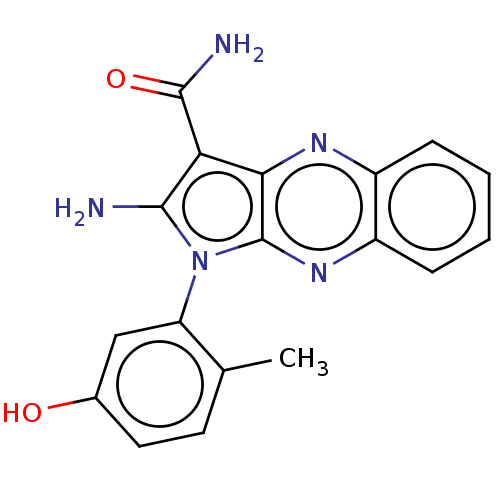 (CHEMBL3321809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00552 BindingDB Entry DOI: 10.7270/Q2028WKQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50299218 (8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Inhibition of EphB3 by [gamma33-P]ATP based assay | J Med Chem 52: 6433-46 (2009) Article DOI: 10.1021/jm9009444 BindingDB Entry DOI: 10.7270/Q2BZ663G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50598810 (CHEMBL5202826) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00552 BindingDB Entry DOI: 10.7270/Q2028WKQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50538442 (CHEMBL4638981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of EPHB3 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based ... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50538438 (CHEMBL4640297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of EPHB3 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based ... | ACS Med Chem Lett 11: 491-496 (2020) Article DOI: 10.1021/acsmedchemlett.9b00612 BindingDB Entry DOI: 10.7270/Q2377D68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311312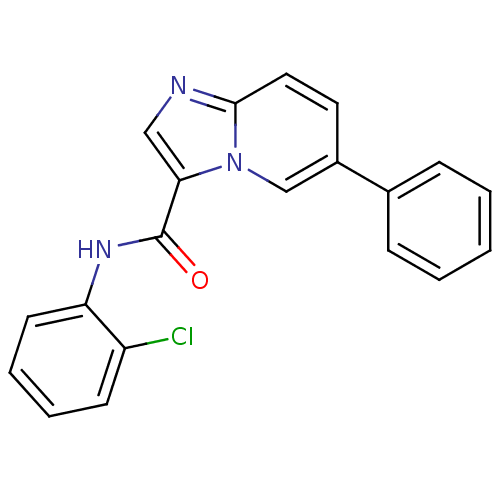 (CHEMBL1078739 | N-(2-chlorophenyl)-6-phenylimidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311306 (CHEMBL1078214 | N-(2-chlorophenyl)-5-(piperidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311302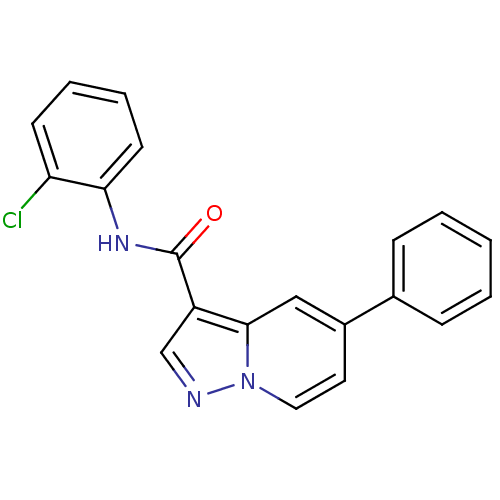 (CHEMBL1078107 | N-(2-chlorophenyl)-5-phenylpyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311303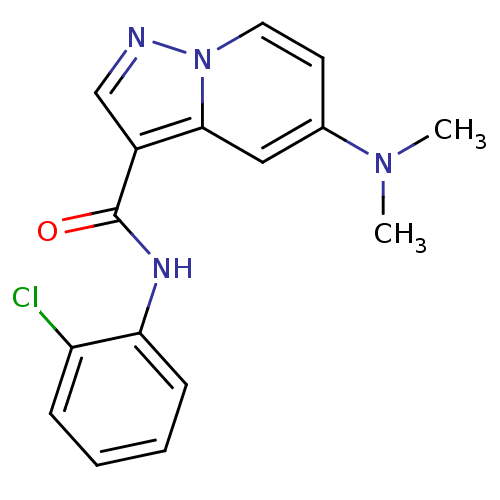 (CHEMBL1077571 | N-(2-chlorophenyl)-5-(dimethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311316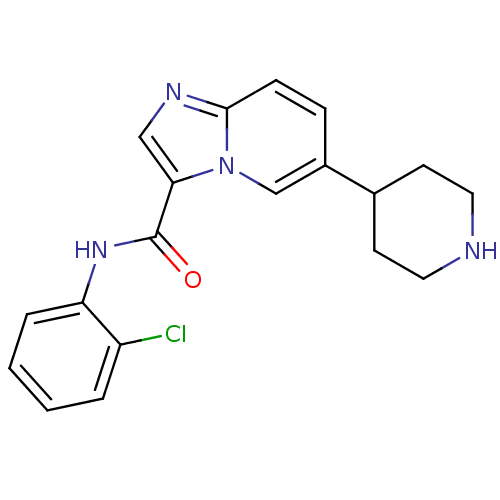 (CHEMBL1077739 | LDN-211904 | N-(2-chlorophenyl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311313 (CHEMBL1078840 | N-(2-chlorophenyl)-6-(piperidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311318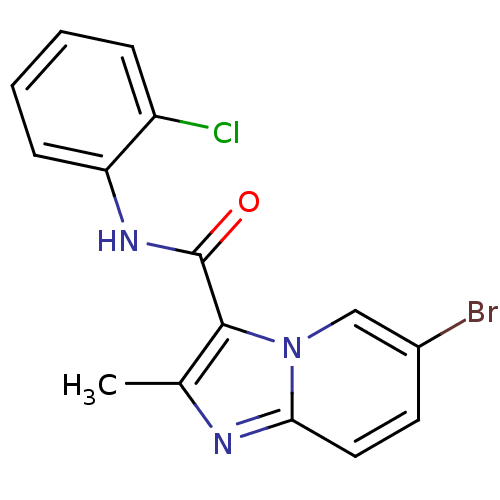 (6-bromo-N-(2-chlorophenyl)-2-methylimidazo[1,2-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311301 (CHEMBL1080959 | N-(2-chlorophenyl)-5-methoxypyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311304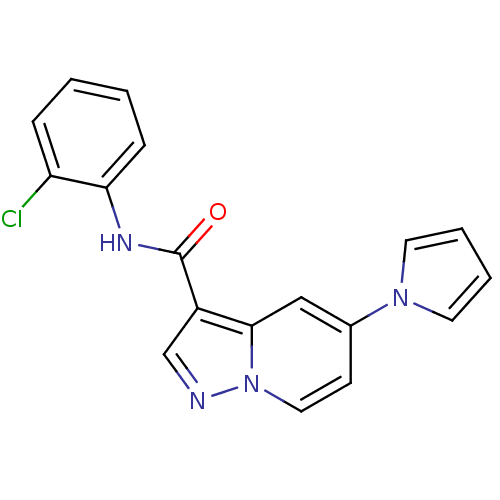 (CHEMBL1078108 | N-(2-chlorophenyl)-5-(1H-pyrrol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311317 (CHEMBL1077740 | N-(2-chlorophenyl)-6-(4-hydroxypip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311305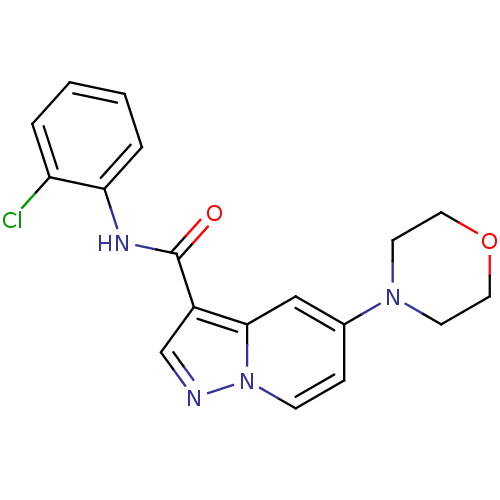 (CHEMBL1078109 | N-(2-chlorophenyl)-5-morpholinopyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311296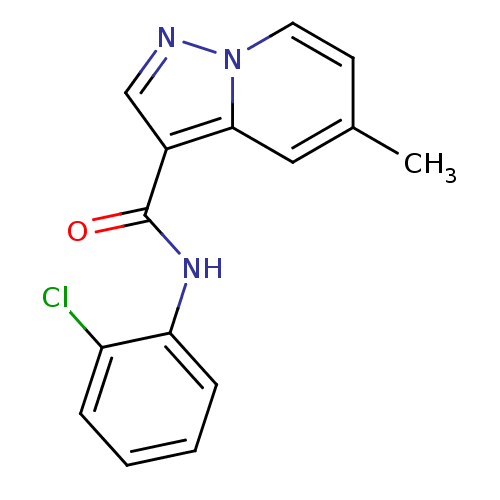 (CHEMBL1081498 | N-(2-chlorophenyl)-5-methylpyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50357333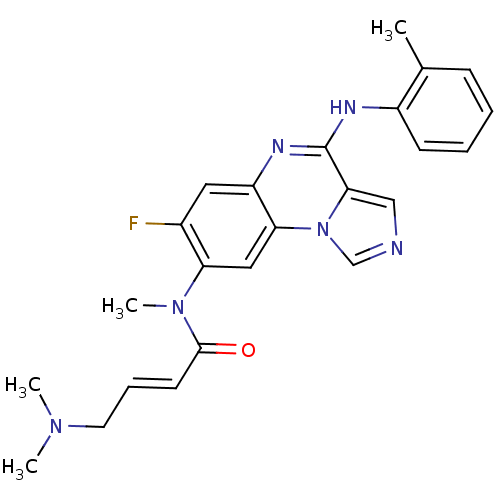 (CHEMBL1916891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of EPHB3 relative to control | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311311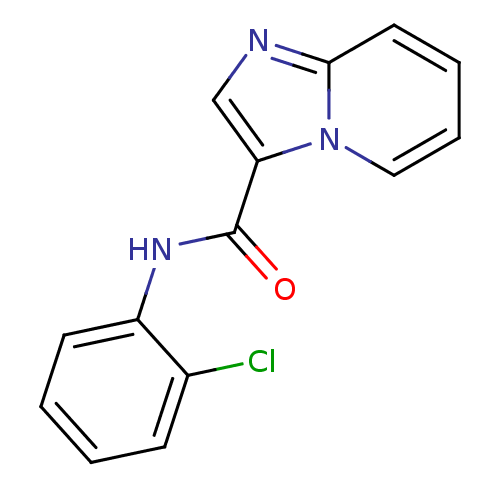 (CHEMBL1078738 | N-(2-chlorophenyl)imidazo[1,2-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EPHB3 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311288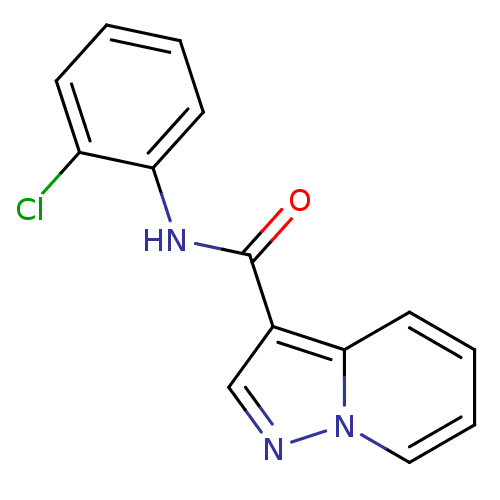 (CHEMBL1078785 | N-(2-chlorophenyl)pyrazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50363957 (CHEMBL1952210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of EphB3 | Bioorg Med Chem 13: 4835-41 (2005) Article DOI: 10.1016/j.bmc.2005.05.012 BindingDB Entry DOI: 10.7270/Q2H41RWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50182212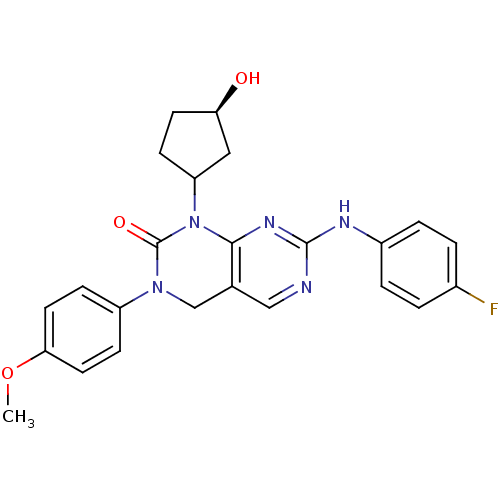 (7-(4-fluorophenylamino)-1-((1S,3R)-3-hydroxycyclop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibitory activity against EphB3 | Bioorg Med Chem Lett 16: 1950-3 (2006) Article DOI: 10.1016/j.bmcl.2005.12.092 BindingDB Entry DOI: 10.7270/Q2S46SRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human EPHB3 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311299 (5-chloro-N-(2-chlorophenyl)pyrazolo[1,5-a]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50134791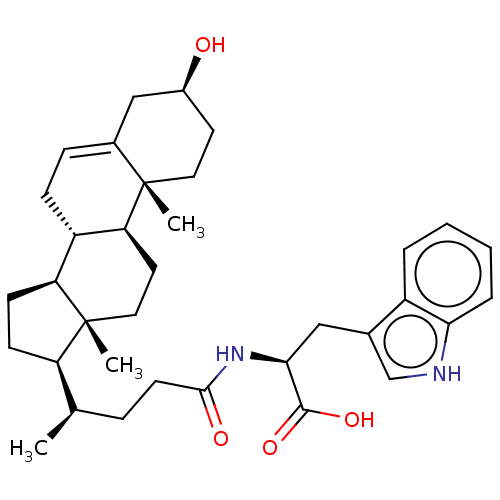 (CHEMBL3734863) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Displacement of biotinylated ephrin-B1-Fc from EphB3 (unknown origin) preincubated for 1 hr followed by biotinylated-ephrin-B1-Fc addition measured a... | Eur J Med Chem 103: 312-24 (2015) Article DOI: 10.1016/j.ejmech.2015.08.048 BindingDB Entry DOI: 10.7270/Q2KW5HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50428059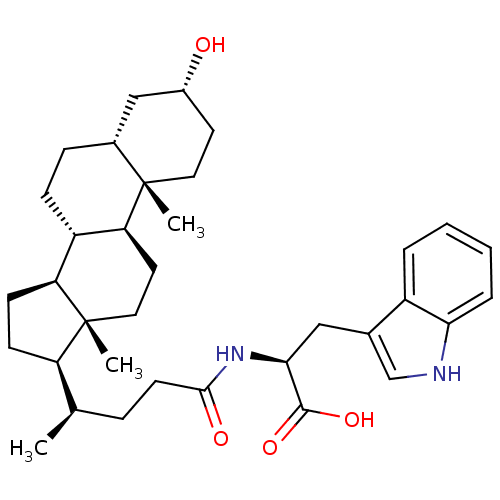 (CHEMBL2322989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Displacement of ephrin-B1-Fc from EphB3 receptor Fc ectodomain (unknown origin) after 1 hr by ELISA | J Med Chem 56: 2936-47 (2013) Article DOI: 10.1021/jm301890k BindingDB Entry DOI: 10.7270/Q2JW8G7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris Curated by ChEMBL | Assay Description Inhibition of EPHB3 | Bioorg Med Chem Lett 21: 7155-65 (2011) Article DOI: 10.1016/j.bmcl.2011.09.078 BindingDB Entry DOI: 10.7270/Q2NC61NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50135286 (CHEMBL3745885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EPHB3 using poly[Glu:Tyr] (4:1) as substrate | Bioorg Med Chem 24: 521-44 (2016) Article DOI: 10.1016/j.bmc.2015.11.045 BindingDB Entry DOI: 10.7270/Q24Q7WT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50519662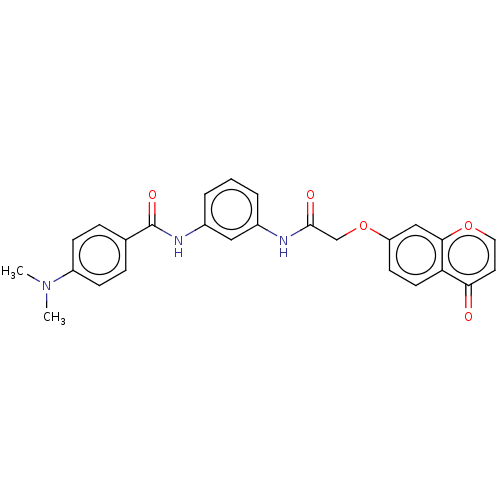 (CHEMBL4438748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human EphB3 (599 to 920 residues) using poly(Glu, Tyr) 4:1 as substrate after 40 mins in presence of [gamma-33ATP] by radio... | J Med Chem 62: 10691-10710 (2019) Article DOI: 10.1021/acs.jmedchem.9b01143 BindingDB Entry DOI: 10.7270/Q2MC93FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Mus musculus) | BDBM50551201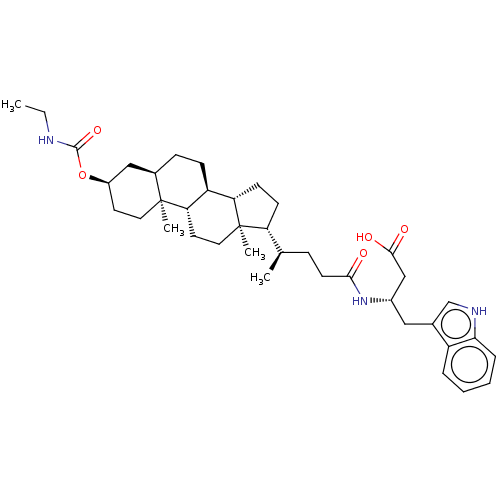 (CHEMBL4761556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of biotinylated ephrin-B1-Fc from mouse EphB3 Fc preincubated for 1 hr followed by ephrin-B1-FC addition and measured after 4 hrs by ELI... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112083 BindingDB Entry DOI: 10.7270/Q2X35233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311310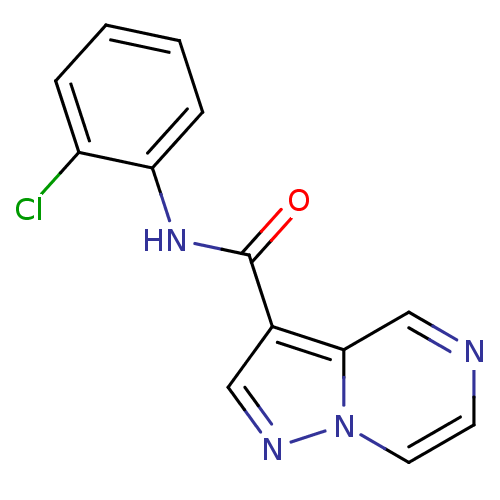 (CHEMBL1077850 | N-(2-chlorophenyl)pyrazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311295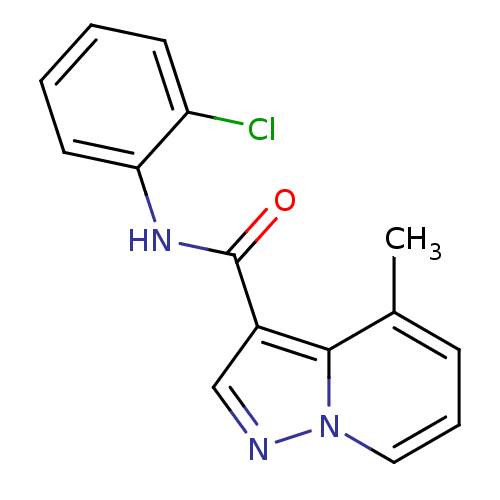 (CHEMBL1080223 | N-(2-chlorophenyl)-4-methylpyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311297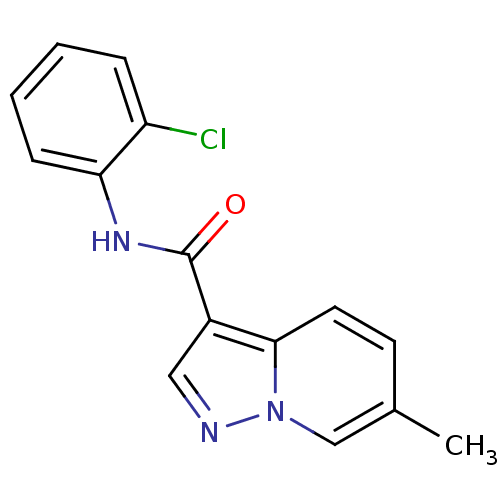 (CHEMBL1081499 | N-(2-chlorophenyl)-6-methylpyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311293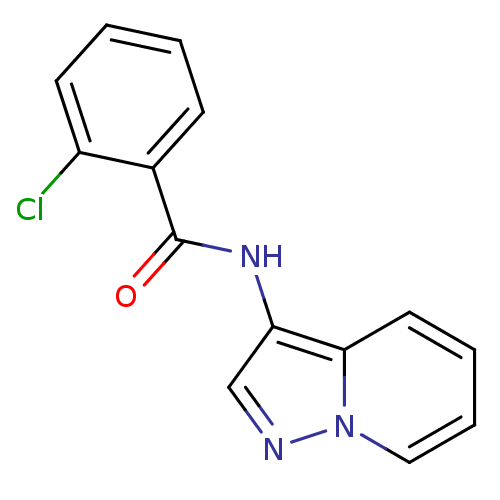 (2-chloro-N-(pyrazolo[1,5-a]pyridin-3-yl)benzamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311294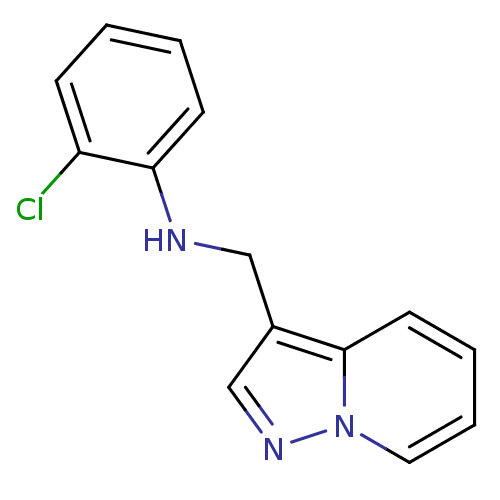 (2-chloro-N-(pyrazolo[1,5-a]pyridin-3-ylmethyl)anil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311298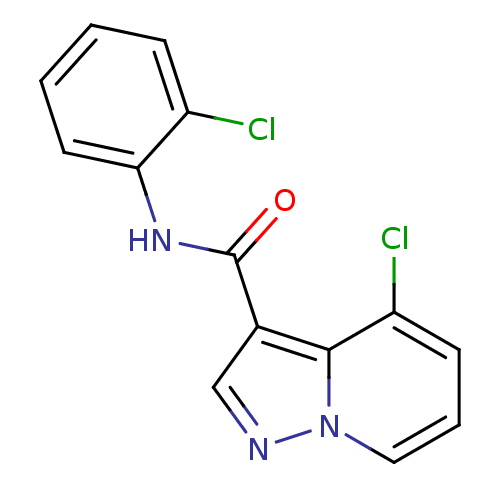 (4-chloro-N-(2-chlorophenyl)pyrazolo[1,5-a]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311300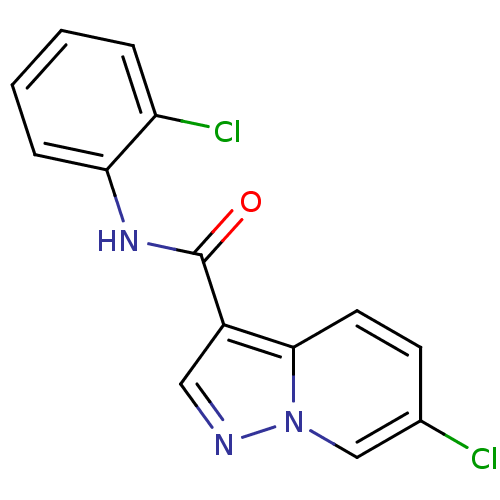 (6-chloro-N-(2-chlorophenyl)pyrazolo[1,5-a]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311307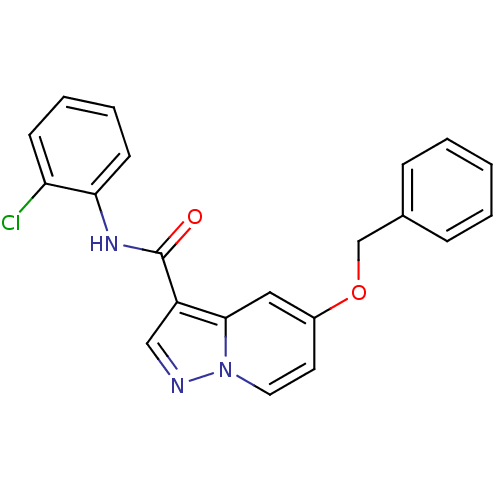 (5-(benzyloxy)-N-(2-chlorophenyl)pyrazolo[1,5-a]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311308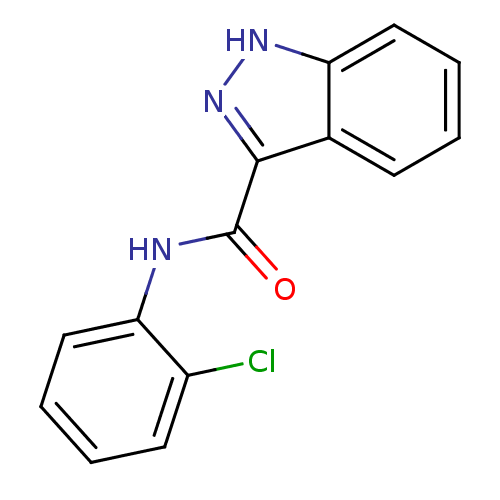 (CHEMBL1078238 | N-(2-chlorophenyl)-1H-indazole-3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311315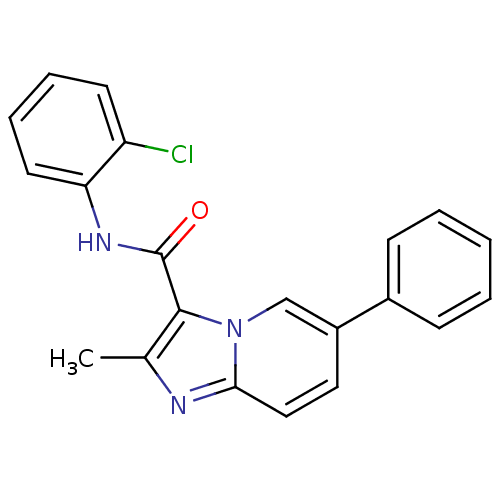 (CHEMBL1077738 | N-(2-chlorophenyl)-2-methyl-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311309 (CHEMBL1078239 | N-(2-chlorophenyl)-[1,2,3]triazolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311314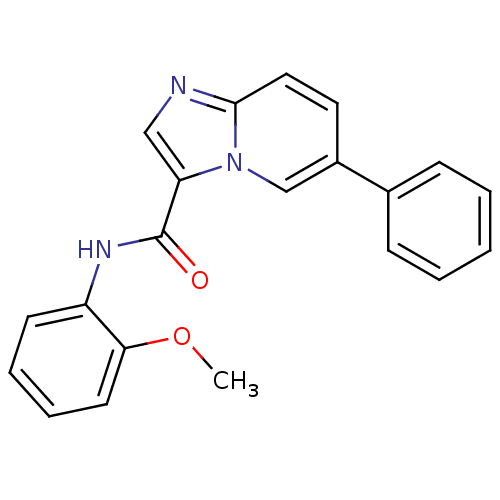 (CHEMBL1078542 | N-(2-methoxyphenyl)-6-phenylimidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-B receptor 3 (Homo sapiens (Human)) | BDBM50311289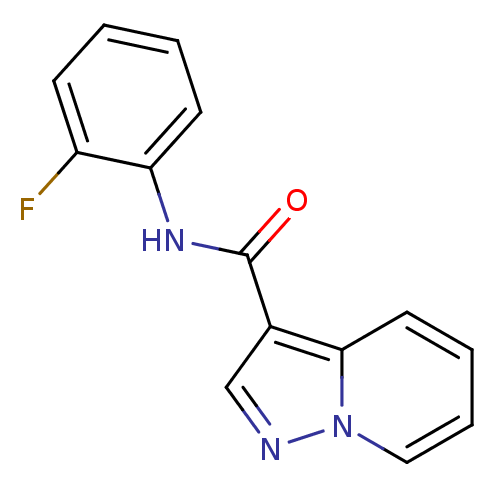 (CHEMBL1078965 | N-(2-fluorophenyl)pyrazolo[1,5-a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant EphB3 kinase-mediated BTK-peptide phosphorylation assessed as 33P incorporation after 30 mins by scintillation counti... | Bioorg Med Chem Lett 19: 6122-6 (2009) Article DOI: 10.1016/j.bmcl.2009.09.010 BindingDB Entry DOI: 10.7270/Q2T43T66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |