

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096105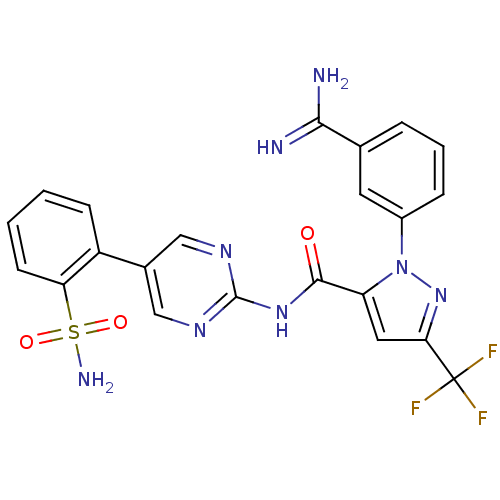 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17122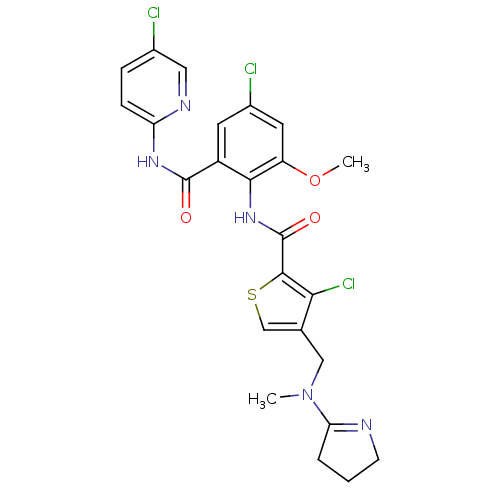 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17129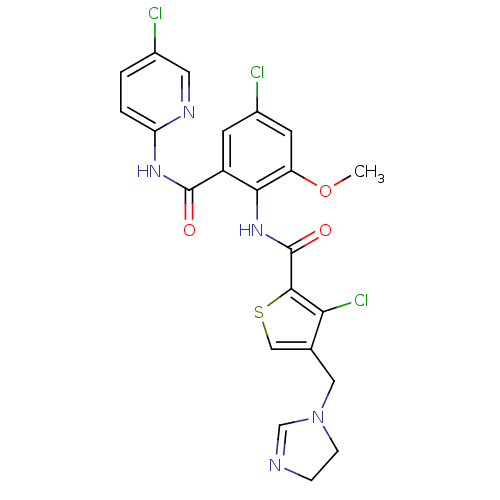 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17127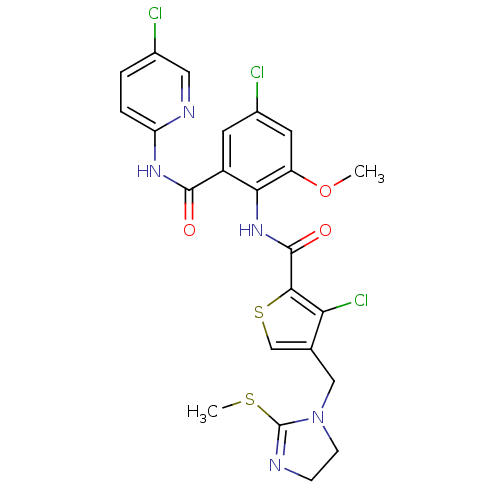 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096099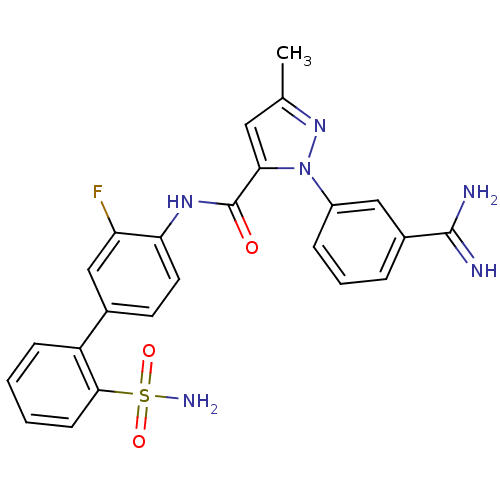 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135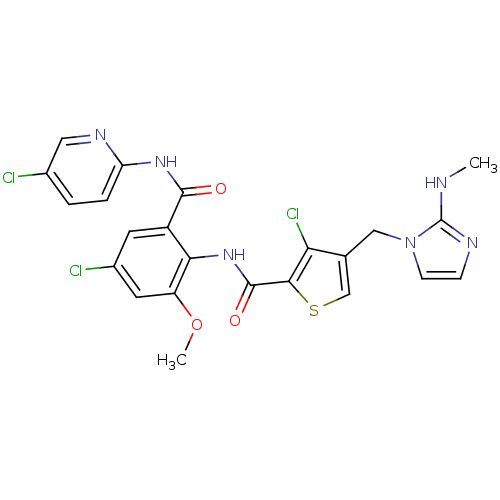 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377655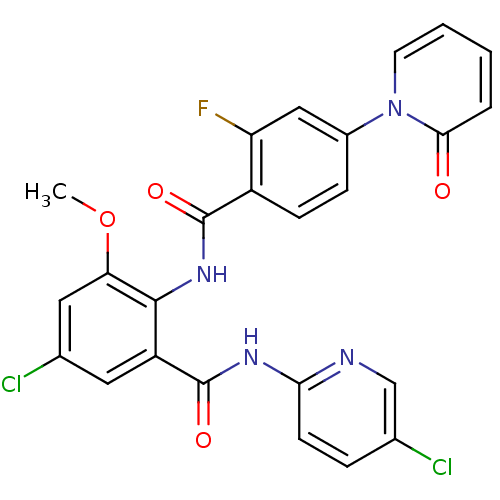 (CHEMBL260160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17136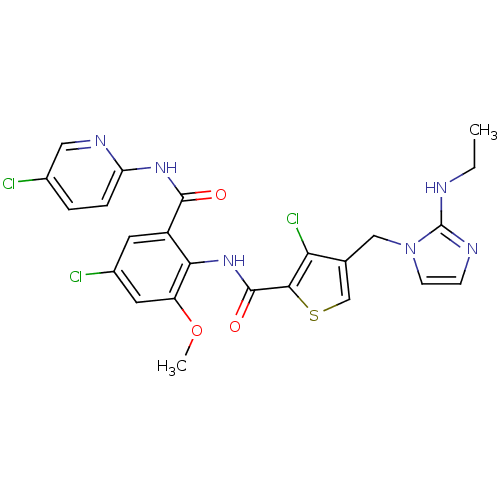 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17134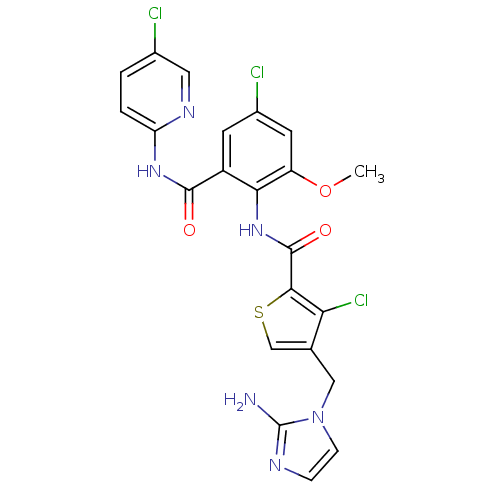 (4-[(2-amino-1H-imidazol-1-yl)methyl]-3-chloro-N-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096101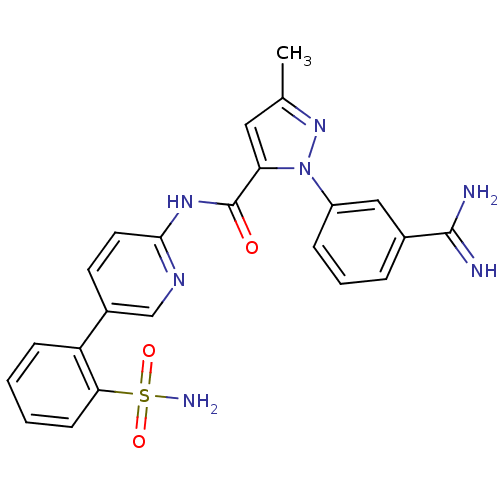 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039635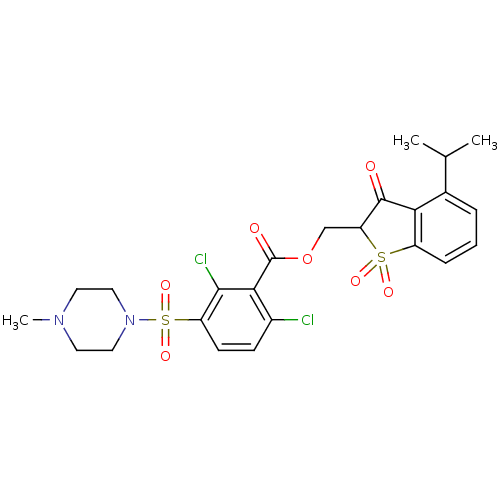 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17111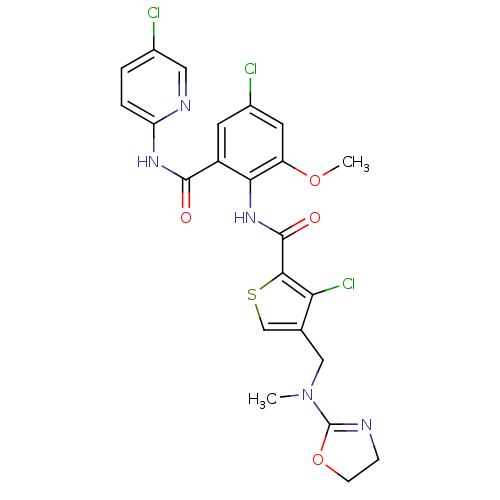 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096091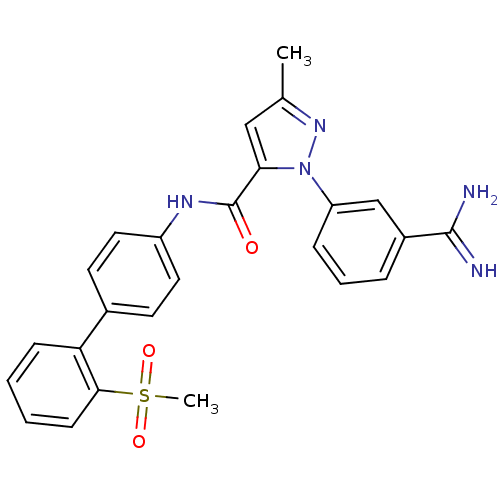 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096110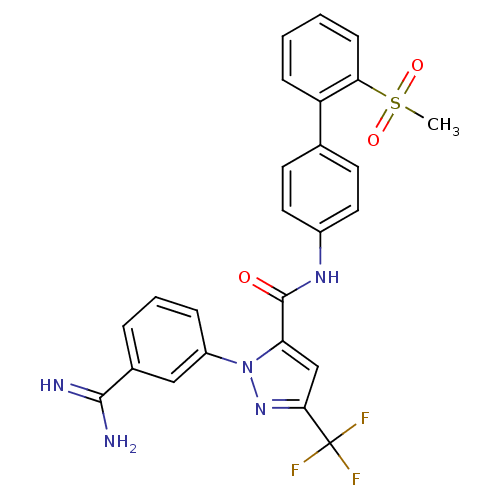 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17137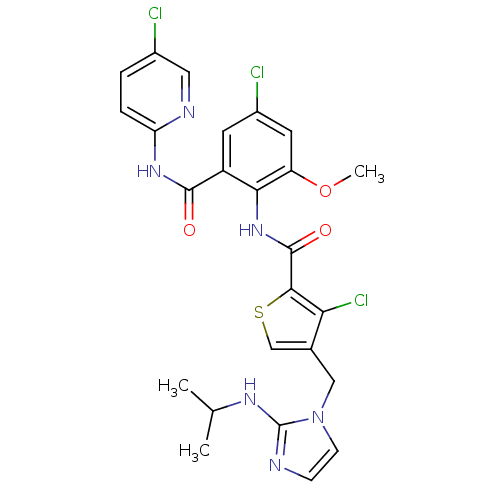 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036480 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039646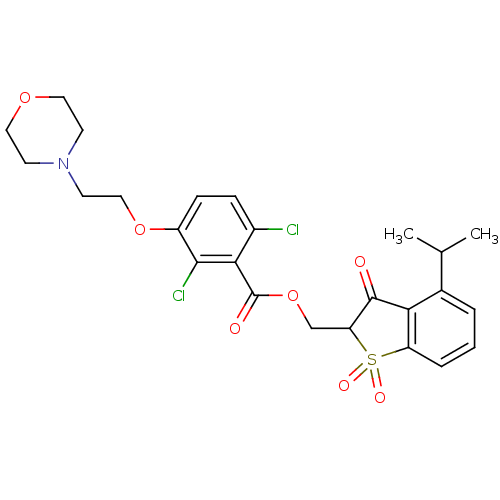 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096108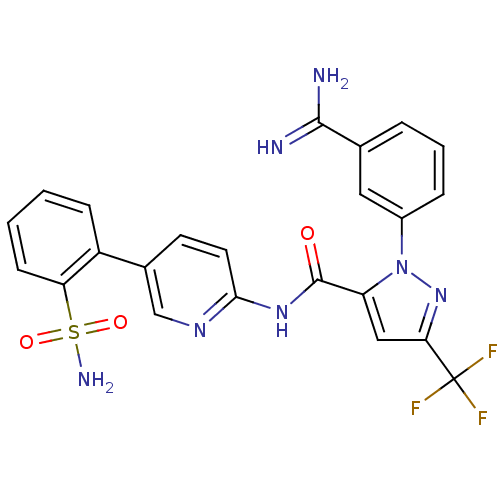 (2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096085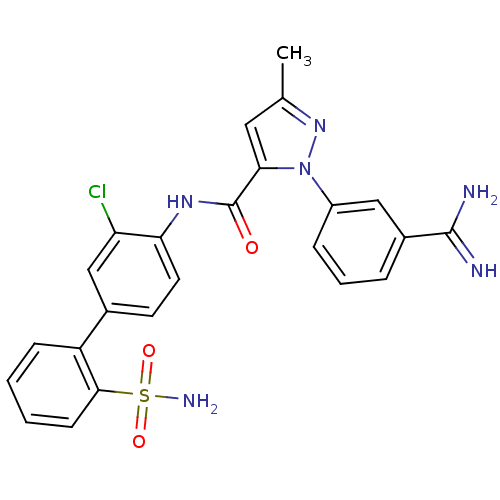 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17123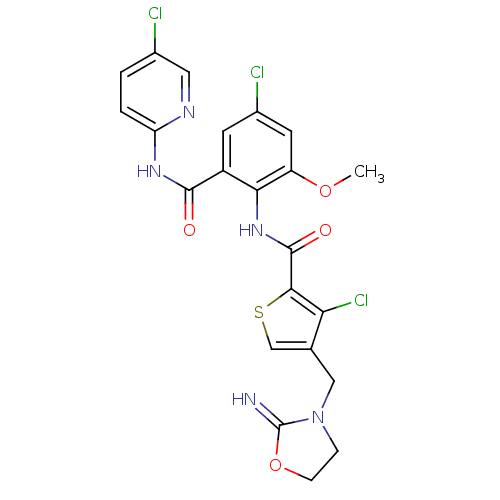 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039637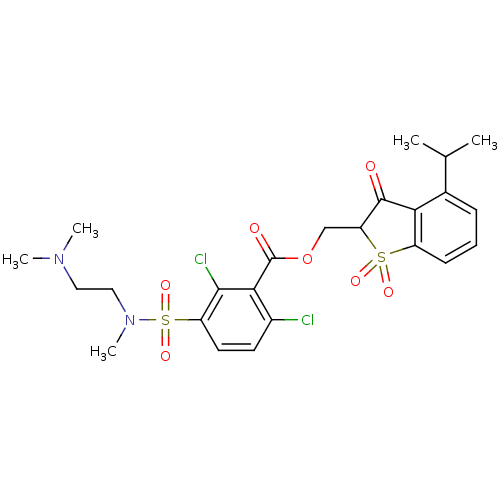 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceutical Research Division Curated by ChEMBL | Assay Description In vitro inhibition constant of human leukocyte elastase. | J Med Chem 37: 2623-6 (1994) BindingDB Entry DOI: 10.7270/Q2ST7QGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17124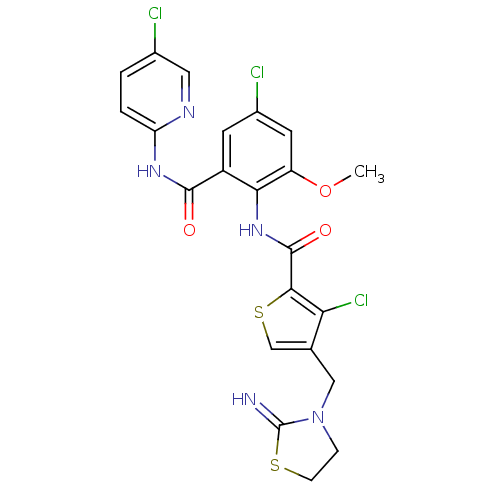 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17133 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17131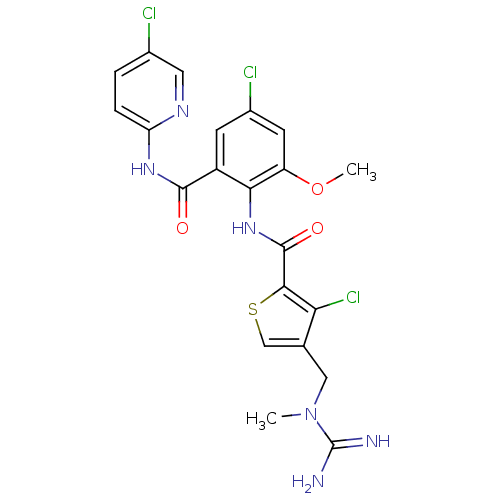 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17130 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17128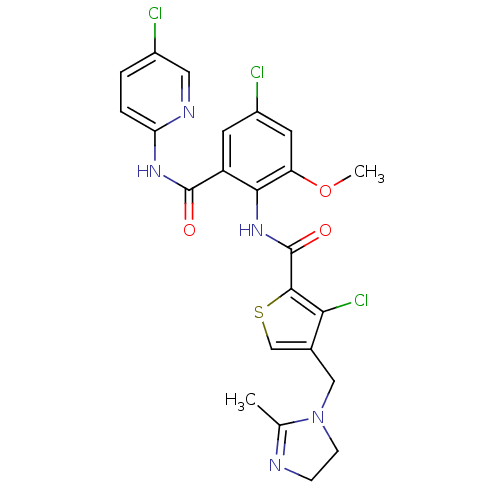 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50096098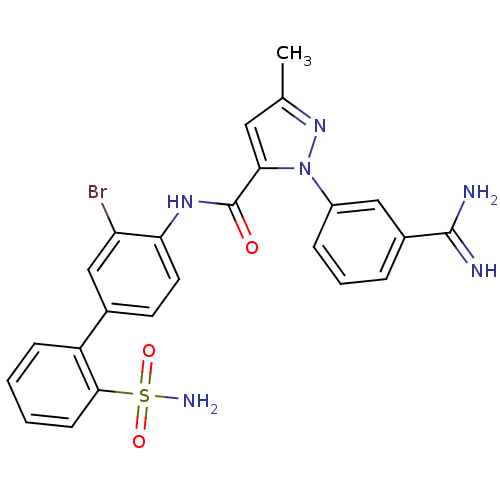 (2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17125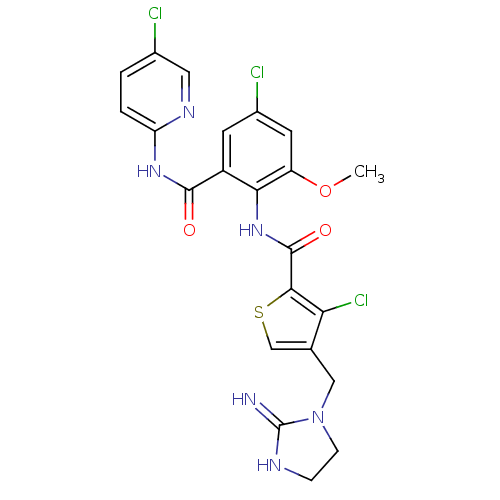 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036476 (2,6-Dichloro-3-(4-methyl-piperazine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036478 (3-Carboxymethoxy-2,6-dichloro-benzoic acid 4-isopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50142111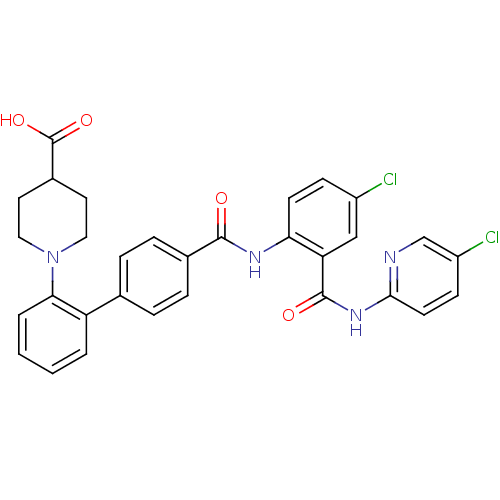 (1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against coagulation factor Xa. | Bioorg Med Chem Lett 14: 983-7 (2004) Article DOI: 10.1016/j.bmcl.2003.11.079 BindingDB Entry DOI: 10.7270/Q21V5DF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17112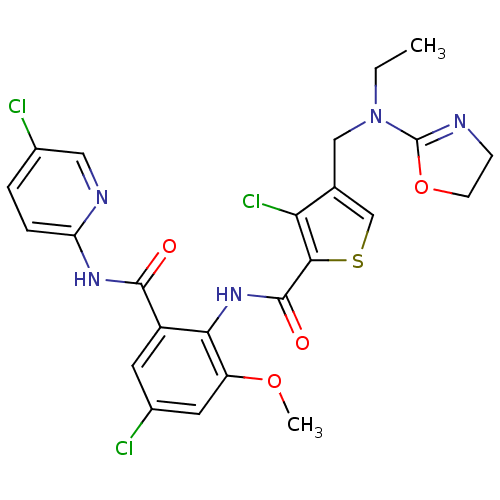 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17118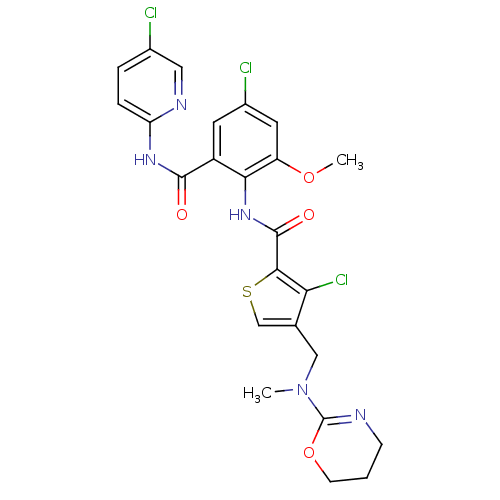 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined | Bioorg Med Chem Lett 12: 1511-5 (2002) BindingDB Entry DOI: 10.7270/Q2P84B6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036481 (2,6-Dichloro-3-[(2-dimethylamino-ethyl)-methyl-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036477 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Inc. Curated by ChEMBL | Assay Description Potency of inhibition against human leukocyte elastase (HLE) expressed as an apparent binding constant | J Med Chem 38: 739-44 (1995) BindingDB Entry DOI: 10.7270/Q2W66JTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology Curated by ChEMBL | Assay Description Inhibition of human factor 10a using S-2765 as substrate after 30 mins by spectrophotometric method | Eur J Med Chem 146: 299-317 (2018) Article DOI: 10.1016/j.ejmech.2018.01.067 BindingDB Entry DOI: 10.7270/Q2251MTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were determined ... | J Med Chem 46: 4405-18 (2003) Article DOI: 10.1021/jm020578e BindingDB Entry DOI: 10.7270/Q2TT4P78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124984 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374877 (CHEMBL270221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 566-78 (2001) BindingDB Entry DOI: 10.7270/Q2RF5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036477 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Binding affinity against Elastase. | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Tested for binding affinity against human Coagulation factor Xa (trypsin-like serine protease) | Bioorg Med Chem Lett 12: 1651-5 (2002) BindingDB Entry DOI: 10.7270/Q2VT1RFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377635 (CHEMBL402980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328697 ((R)-5-guanidino-N-(2-((S)-5-guanidino-1-oxo-1-(thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 21396 total ) | Next | Last >> |