 Found 33 hits Enz. Inhib. hit(s) with Target = 'Chondroitin sulfate N-acetylgalactosaminyltransferase 1'
Found 33 hits Enz. Inhib. hit(s) with Target = 'Chondroitin sulfate N-acetylgalactosaminyltransferase 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386129

(CHEMBL2042217)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(Cl)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20ClFN4O5/c1-11(2)19(21(30)31)27-20(29)18-10-17(28-33-18)12-3-6-14(7-4-12)25-22(32)26-16-8-5-13(23)9-15(16)24/h3-11,19H,1-2H3,(H,27,29)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386149

(CHEMBL2042351)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)-c1ccc(Nc2nc3ccc(F)cc3s2)cc1)C(O)=O |r| Show InChI InChI=1S/C25H22FN3O3S/c1-14(2)22(24(31)32)29-23(30)17-5-3-15(4-6-17)16-7-10-19(11-8-16)27-25-28-20-12-9-18(26)13-21(20)33-25/h3-14,22H,1-2H3,(H,27,28)(H,29,30)(H,31,32)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386126
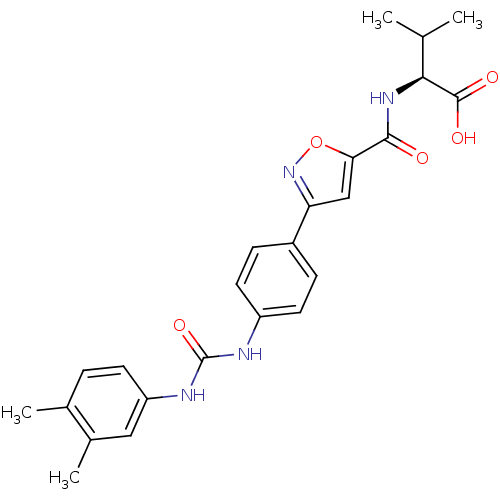
(CHEMBL2042213)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(C)c(C)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H26N4O5/c1-13(2)21(23(30)31)27-22(29)20-12-19(28-33-20)16-6-9-17(10-7-16)25-24(32)26-18-8-5-14(3)15(4)11-18/h5-13,21H,1-4H3,(H,27,29)(H,30,31)(H2,25,26,32)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386120
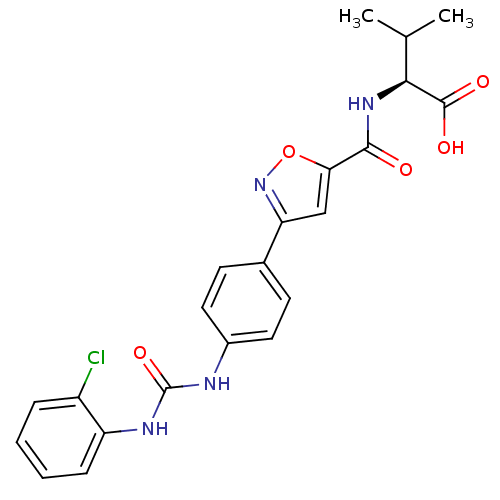
(CHEMBL2042201)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21ClN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-7-9-14(10-8-13)24-22(31)25-16-6-4-3-5-15(16)23/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386122

(CHEMBL2042204)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cccc(Cl)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21ClN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-6-8-15(9-7-13)24-22(31)25-16-5-3-4-14(23)10-16/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386145
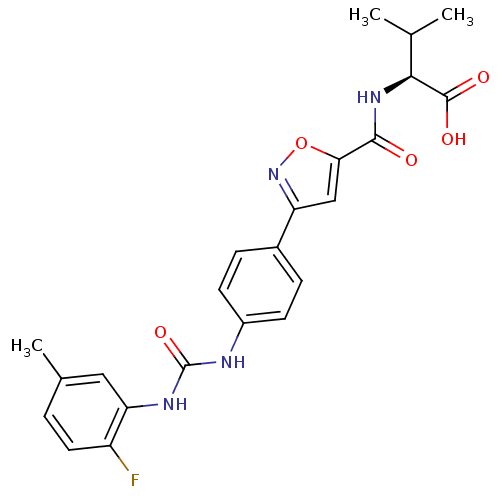
(CHEMBL2042347)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cc(C)ccc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C23H23FN4O5/c1-12(2)20(22(30)31)27-21(29)19-11-17(28-33-19)14-5-7-15(8-6-14)25-23(32)26-18-10-13(3)4-9-16(18)24/h4-12,20H,1-3H3,(H,27,29)(H,30,31)(H2,25,26,32)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386137

(CHEMBL2042339)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccccc2C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O5/c1-12(2)19(21(32)33)29-20(31)18-11-17(30-35-18)13-7-9-14(10-8-13)27-22(34)28-16-6-4-3-5-15(16)23(24,25)26/h3-12,19H,1-2H3,(H,29,31)(H,32,33)(H2,27,28,34)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386136
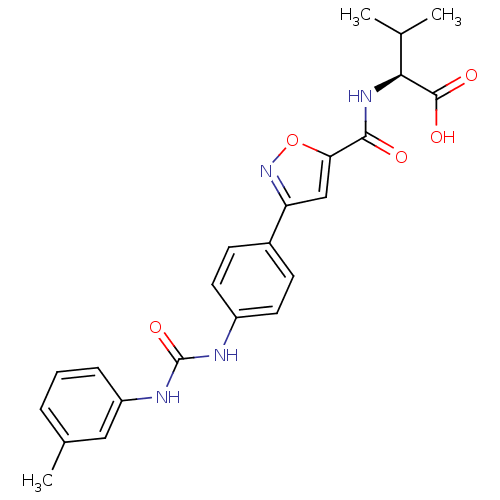
(CHEMBL2042338)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C23H24N4O5/c1-13(2)20(22(29)30)26-21(28)19-12-18(27-32-19)15-7-9-16(10-8-15)24-23(31)25-17-6-4-5-14(3)11-17/h4-13,20H,1-3H3,(H,26,28)(H,29,30)(H2,24,25,31)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386131
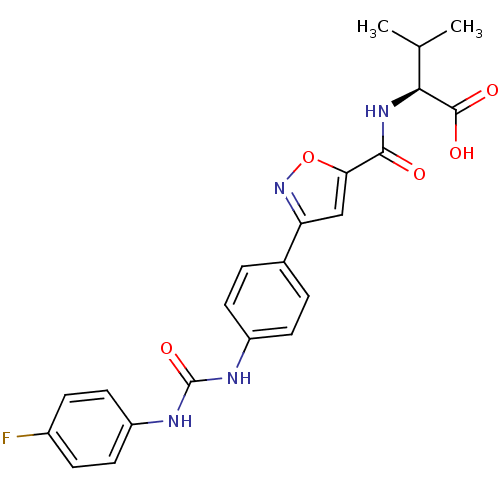
(CHEMBL2042221)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(F)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21FN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-3-7-15(8-4-13)24-22(31)25-16-9-5-14(23)6-10-16/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386132

(CHEMBL2042223)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(C)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C23H24N4O5/c1-13(2)20(22(29)30)26-21(28)19-12-18(27-32-19)15-6-10-17(11-7-15)25-23(31)24-16-8-4-14(3)5-9-16/h4-13,20H,1-3H3,(H,26,28)(H,29,30)(H2,24,25,31)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386130

(CHEMBL2042219)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20F2N4O5/c1-11(2)19(21(30)31)27-20(29)18-10-17(28-33-18)12-3-6-14(7-4-12)25-22(32)26-16-8-5-13(23)9-15(16)24/h3-11,19H,1-2H3,(H,27,29)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386133

(CHEMBL2042334)Show SMILES COc1ccc(NC(=O)Nc2ccc(cc2)-c2cc(on2)C(=O)N[C@@H](C(C)C)C(O)=O)cc1 |r| Show InChI InChI=1S/C23H24N4O6/c1-13(2)20(22(29)30)26-21(28)19-12-18(27-33-19)14-4-6-15(7-5-14)24-23(31)25-16-8-10-17(32-3)11-9-16/h4-13,20H,1-3H3,(H,26,28)(H,29,30)(H2,24,25,31)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386124
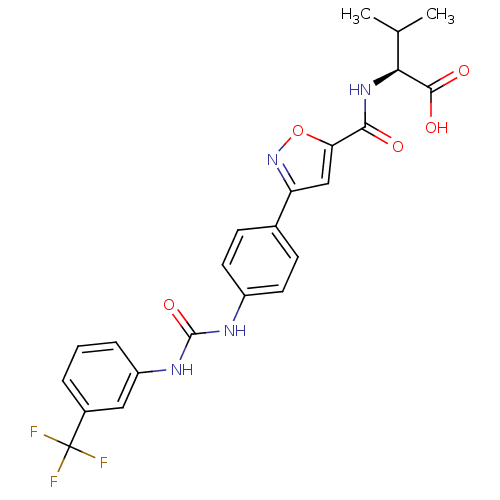
(CHEMBL2042210)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cccc(c2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O5/c1-12(2)19(21(32)33)29-20(31)18-11-17(30-35-18)13-6-8-15(9-7-13)27-22(34)28-16-5-3-4-14(10-16)23(24,25)26/h3-12,19H,1-2H3,(H,29,31)(H,32,33)(H2,27,28,34)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386148
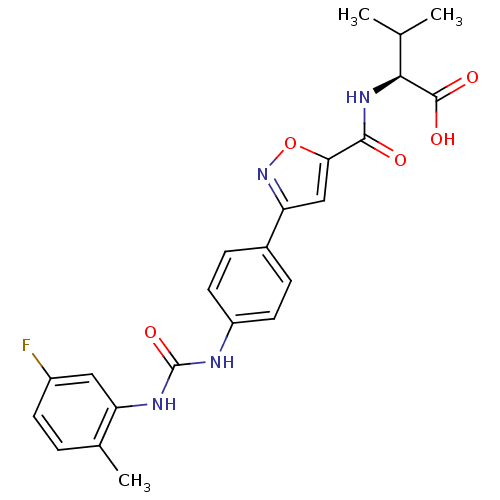
(CHEMBL2042350)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cc(F)ccc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C23H23FN4O5/c1-12(2)20(22(30)31)27-21(29)19-11-18(28-33-19)14-5-8-16(9-6-14)25-23(32)26-17-10-15(24)7-4-13(17)3/h4-12,20H,1-3H3,(H,27,29)(H,30,31)(H2,25,26,32)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386140

(CHEMBL2042342)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cc(F)cc(F)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20F2N4O5/c1-11(2)19(21(30)31)27-20(29)18-10-17(28-33-18)12-3-5-15(6-4-12)25-22(32)26-16-8-13(23)7-14(24)9-16/h3-11,19H,1-2H3,(H,27,29)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386139

(CHEMBL2042341)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(F)c(F)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20F2N4O5/c1-11(2)19(21(30)31)27-20(29)18-10-17(28-33-18)12-3-5-13(6-4-12)25-22(32)26-14-7-8-15(23)16(24)9-14/h3-11,19H,1-2H3,(H,27,29)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386138
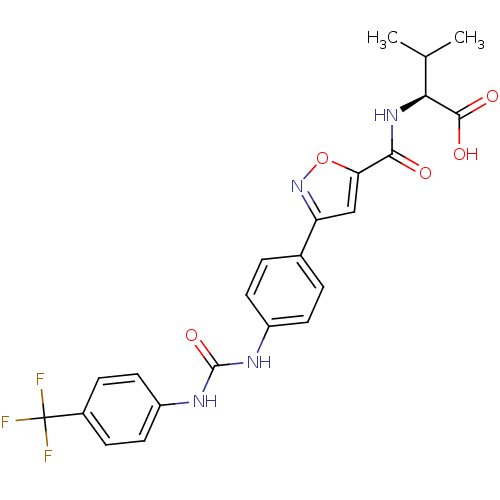
(CHEMBL2042340)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(cc2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O5/c1-12(2)19(21(32)33)29-20(31)18-11-17(30-35-18)13-3-7-15(8-4-13)27-22(34)28-16-9-5-14(6-10-16)23(24,25)26/h3-12,19H,1-2H3,(H,29,31)(H,32,33)(H2,27,28,34)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386135
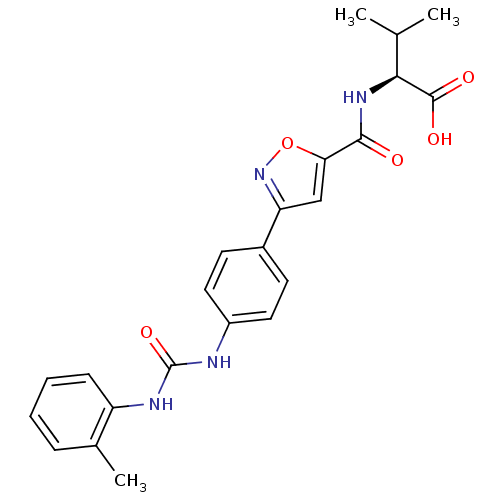
(CHEMBL2042337)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccccc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C23H24N4O5/c1-13(2)20(22(29)30)26-21(28)19-12-18(27-32-19)15-8-10-16(11-9-15)24-23(31)25-17-7-5-4-6-14(17)3/h4-13,20H,1-3H3,(H,26,28)(H,29,30)(H2,24,25,31)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386143
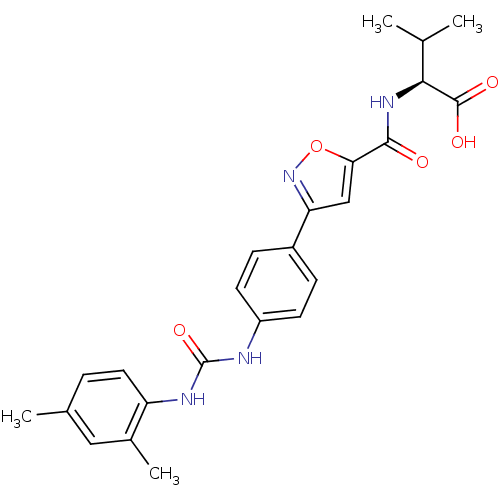
(CHEMBL2042345)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(C)cc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C24H26N4O5/c1-13(2)21(23(30)31)27-22(29)20-12-19(28-33-20)16-6-8-17(9-7-16)25-24(32)26-18-10-5-14(3)11-15(18)4/h5-13,21H,1-4H3,(H,27,29)(H,30,31)(H2,25,26,32)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386127
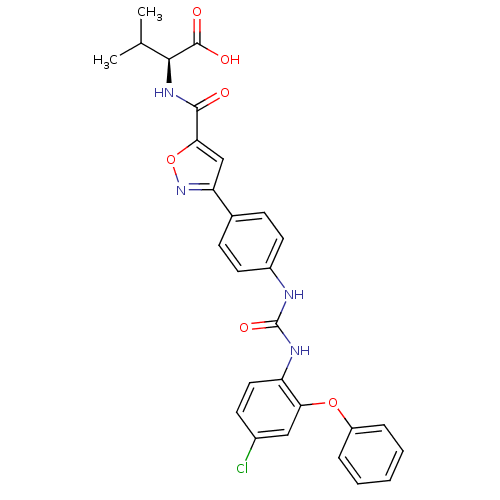
(CHEMBL2042215)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(Cl)cc2Oc2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H25ClN4O6/c1-16(2)25(27(35)36)32-26(34)24-15-22(33-39-24)17-8-11-19(12-9-17)30-28(37)31-21-13-10-18(29)14-23(21)38-20-6-4-3-5-7-20/h3-16,25H,1-2H3,(H,32,34)(H,35,36)(H2,30,31,37)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386147
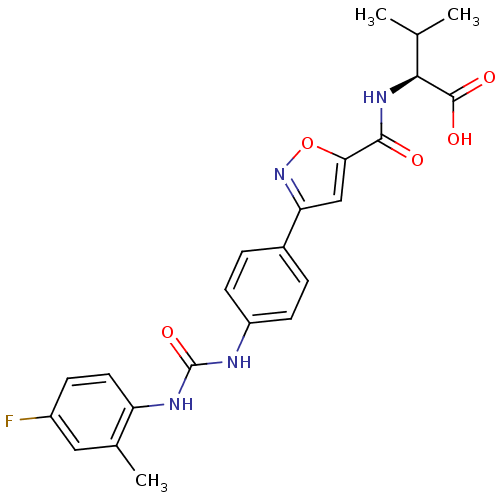
(CHEMBL2042349)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(F)cc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C23H23FN4O5/c1-12(2)20(22(30)31)27-21(29)19-11-18(28-33-19)14-4-7-16(8-5-14)25-23(32)26-17-9-6-15(24)10-13(17)3/h4-12,20H,1-3H3,(H,27,29)(H,30,31)(H2,25,26,32)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386134
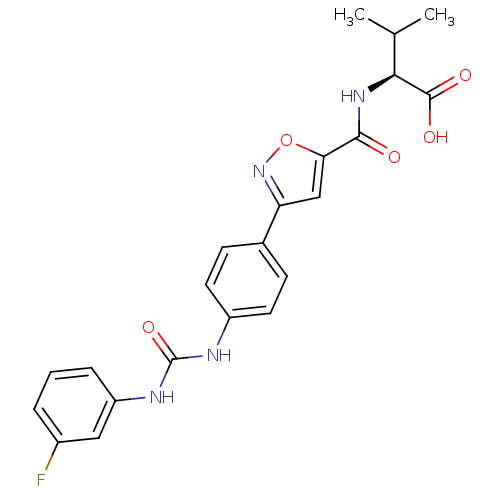
(CHEMBL2042336)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21FN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-6-8-15(9-7-13)24-22(31)25-16-5-3-4-14(23)10-16/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386144

(CHEMBL2042346)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cc(C)cc(C)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C24H26N4O5/c1-13(2)21(23(30)31)27-22(29)20-12-19(28-33-20)16-5-7-17(8-6-16)25-24(32)26-18-10-14(3)9-15(4)11-18/h5-13,21H,1-4H3,(H,27,29)(H,30,31)(H2,25,26,32)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386151
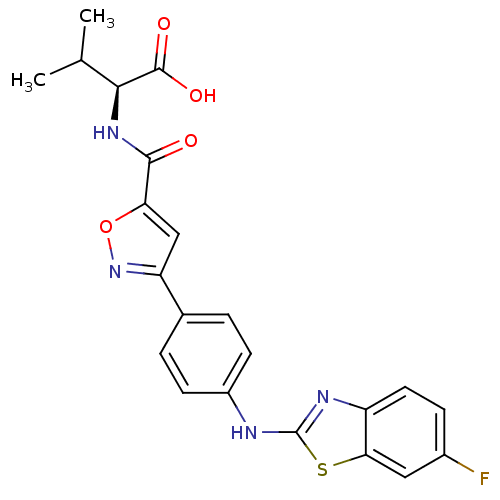
(CHEMBL2042352)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(Nc2nc3ccc(F)cc3s2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H19FN4O4S/c1-11(2)19(21(29)30)26-20(28)17-10-16(27-31-17)12-3-6-14(7-4-12)24-22-25-15-8-5-13(23)9-18(15)32-22/h3-11,19H,1-2H3,(H,24,25)(H,26,28)(H,29,30)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386123
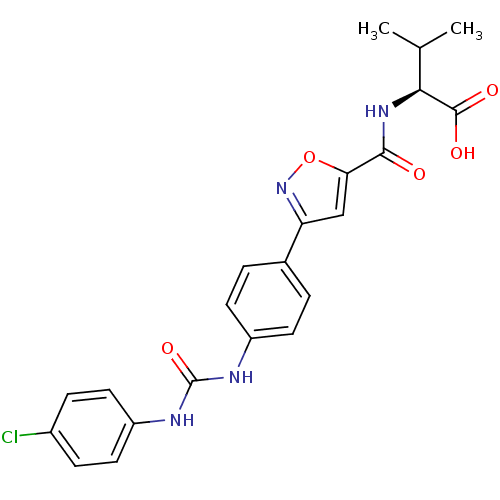
(CHEMBL2042207)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(Cl)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21ClN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-3-7-15(8-4-13)24-22(31)25-16-9-5-14(23)6-10-16/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386119

(CHEMBL2042335)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccccc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21FN4O5/c1-12(2)19(21(29)30)26-20(28)18-11-17(27-32-18)13-7-9-14(10-8-13)24-22(31)25-16-6-4-3-5-15(16)23/h3-12,19H,1-2H3,(H,26,28)(H,29,30)(H2,24,25,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386141
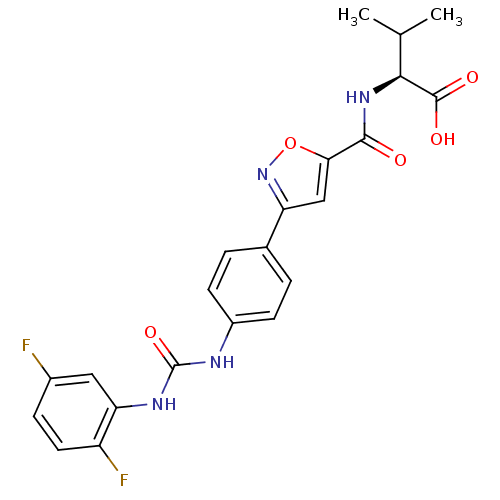
(CHEMBL2042343)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2cc(F)ccc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20F2N4O5/c1-11(2)19(21(30)31)27-20(29)18-10-16(28-33-18)12-3-6-14(7-4-12)25-22(32)26-17-9-13(23)5-8-15(17)24/h3-11,19H,1-2H3,(H,27,29)(H,30,31)(H2,25,26,32)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386146
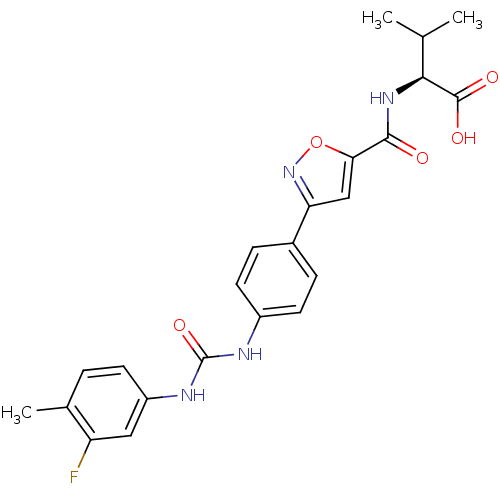
(CHEMBL2042348)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2ccc(C)c(F)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C23H23FN4O5/c1-12(2)20(22(30)31)27-21(29)19-11-18(28-33-19)14-5-8-15(9-6-14)25-23(32)26-16-7-4-13(3)17(24)10-16/h4-12,20H,1-3H3,(H,27,29)(H,30,31)(H2,25,26,32)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386142
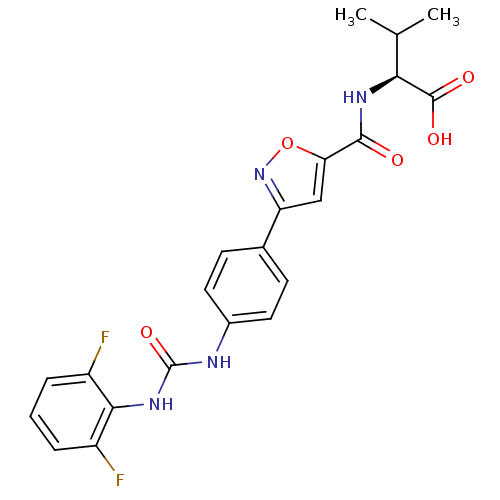
(CHEMBL2042344)Show SMILES CC(C)[C@H](NC(=O)c1cc(no1)-c1ccc(NC(=O)Nc2c(F)cccc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C22H20F2N4O5/c1-11(2)18(21(30)31)26-20(29)17-10-16(28-33-17)12-6-8-13(9-7-12)25-22(32)27-19-14(23)4-3-5-15(19)24/h3-11,18H,1-2H3,(H,26,29)(H,30,31)(H2,25,27,32)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386128
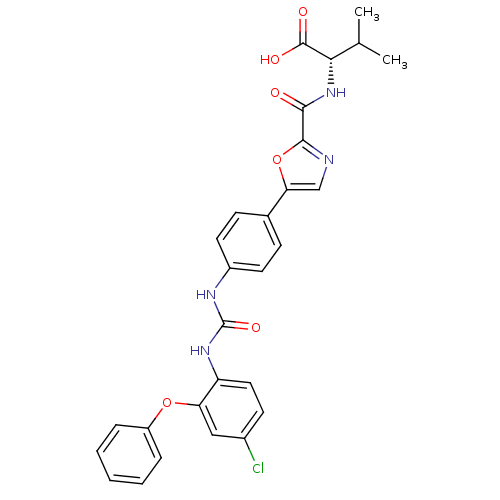
(CHEMBL2042216)Show SMILES CC(C)[C@H](NC(=O)c1ncc(o1)-c1ccc(NC(=O)Nc2ccc(Cl)cc2Oc2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C28H25ClN4O6/c1-16(2)24(27(35)36)33-25(34)26-30-15-23(39-26)17-8-11-19(12-9-17)31-28(37)32-21-13-10-18(29)14-22(21)38-20-6-4-3-5-7-20/h3-16,24H,1-2H3,(H,33,34)(H,35,36)(H2,31,32,37)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386150

(CHEMBL2042220)Show SMILES CC(C)[C@H](NC(=O)c1nc(no1)-c1ccc(NC(=O)Nc2ccc(F)cc2F)cc1)C(O)=O |r| Show InChI InChI=1S/C21H19F2N5O5/c1-10(2)16(20(30)31)26-18(29)19-27-17(28-33-19)11-3-6-13(7-4-11)24-21(32)25-15-8-5-12(22)9-14(15)23/h3-10,16H,1-2H3,(H,26,29)(H,30,31)(H2,24,25,32)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386121

(CHEMBL2042202)Show SMILES CC(C)[C@H](NC(=O)c1ncc(o1)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1)C(O)=O |r| Show InChI InChI=1S/C22H21ClN4O5/c1-12(2)18(21(29)30)27-19(28)20-24-11-17(32-20)13-7-9-14(10-8-13)25-22(31)26-16-6-4-3-5-15(16)23/h3-12,18H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |
Chondroitin sulfate N-acetylgalactosaminyltransferase 1
(Homo sapiens (Human)) | BDBM50386125
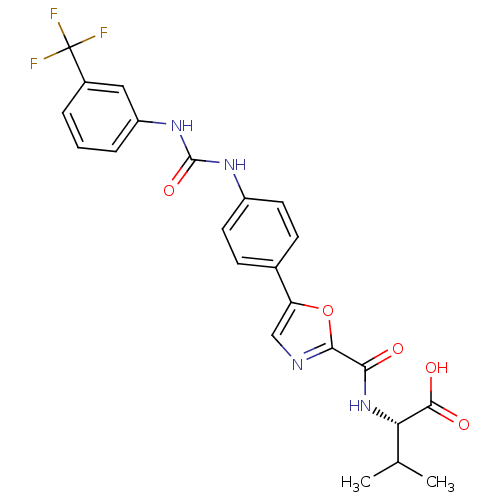
(CHEMBL2042211)Show SMILES CC(C)[C@H](NC(=O)c1ncc(o1)-c1ccc(NC(=O)Nc2cccc(c2)C(F)(F)F)cc1)C(O)=O |r| Show InChI InChI=1S/C23H21F3N4O5/c1-12(2)18(21(32)33)30-19(31)20-27-11-17(35-20)13-6-8-15(9-7-13)28-22(34)29-16-5-3-4-14(10-16)23(24,25)26/h3-12,18H,1-2H3,(H,30,31)(H,32,33)(H2,28,29,34)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 assessed as conversion of [14C]-oleoyl-CoA to [14C]-triglyceride after 10 mins by scintillation counting |
Eur J Med Chem 54: 324-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.016
BindingDB Entry DOI: 10.7270/Q25B03HK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data