

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85134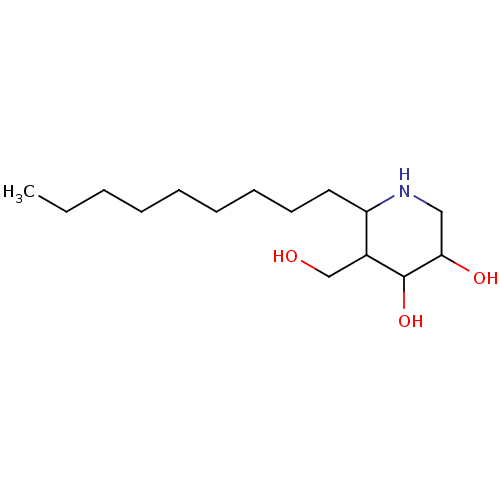 (Isofagomine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85134 (Isofagomine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383315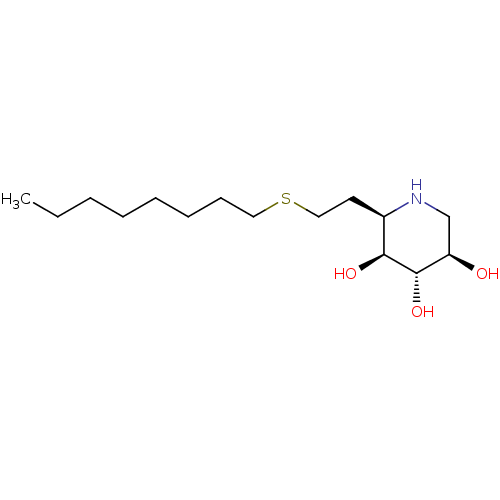 (CHEMBL2029773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108418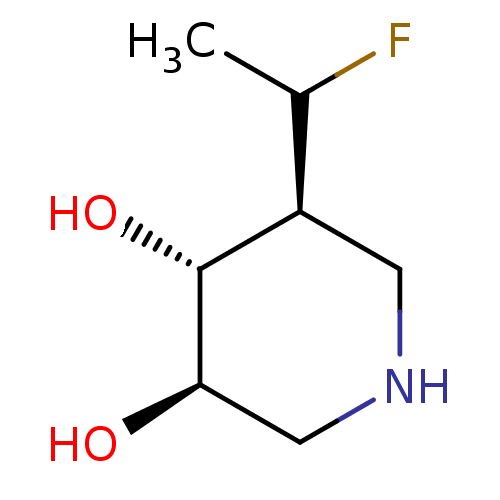 (US8604206, 13 | US8604206, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | 17.1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM162702 (US9056847, Fluorophore 1-cyclophellitol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.950 | n/a | 2 | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM US Patent | Assay Description Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... | US Patent US9056847 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50254115 (CHEMBL4080589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Competitive inhibition of recombinant beta-glucocerebrosidase (unknown origin) using 4-nitrophenyl beta-D-galactopyranoside as substrate pre-incubate... | Bioorg Med Chem Lett 27: 3431-3435 (2017) Article DOI: 10.1016/j.bmcl.2017.05.086 BindingDB Entry DOI: 10.7270/Q29889D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827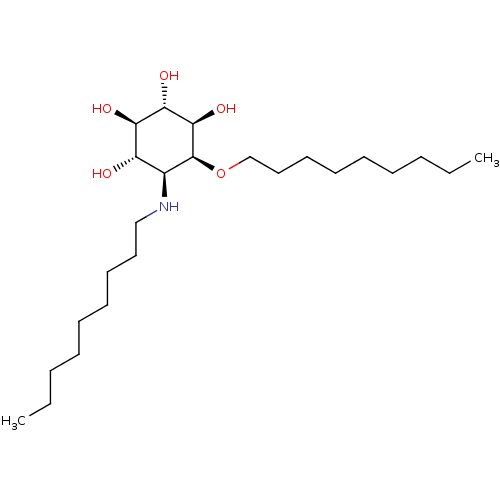 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50394827 (CHEMBL2164231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Qu£mica Avan£ada de Catalunya (IQAC-CSIC) Curated by ChEMBL | Assay Description Binding affinity to recombinant imiglucerase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 30 mins before substrate... | J Med Chem 55: 4479-88 (2012) Article DOI: 10.1021/jm300342q BindingDB Entry DOI: 10.7270/Q2GQ6ZV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018014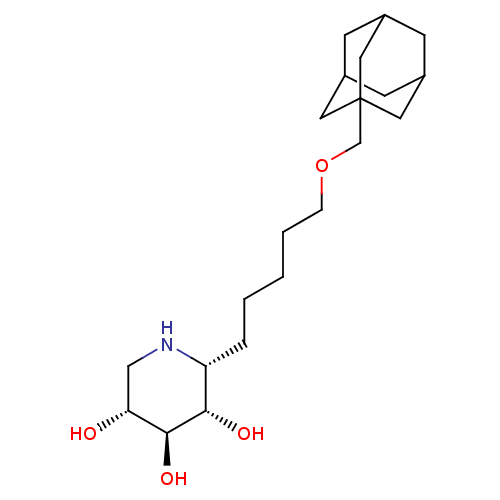 (CHEMBL3289676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220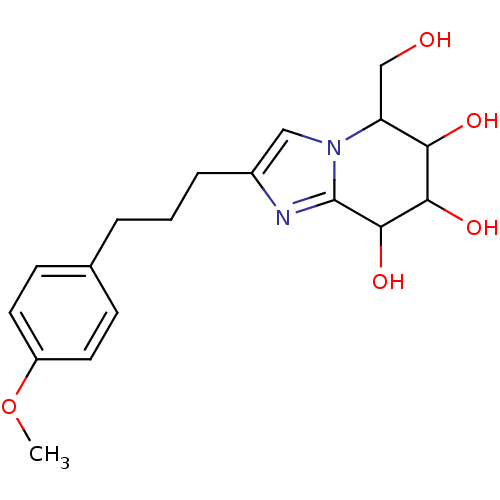 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50099006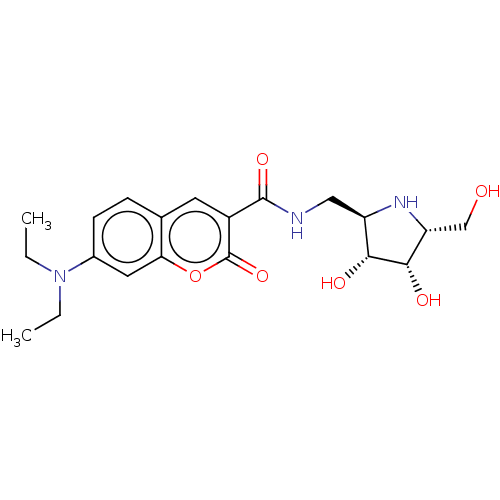 (7-Diethylamino-2-oxo-2H-chromene-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Tested for inhibitory activity of the compound against Beta-glucosidase from Agrobacterium species | Bioorg Med Chem Lett 11: 1063-4 (2001) BindingDB Entry DOI: 10.7270/Q2222T12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219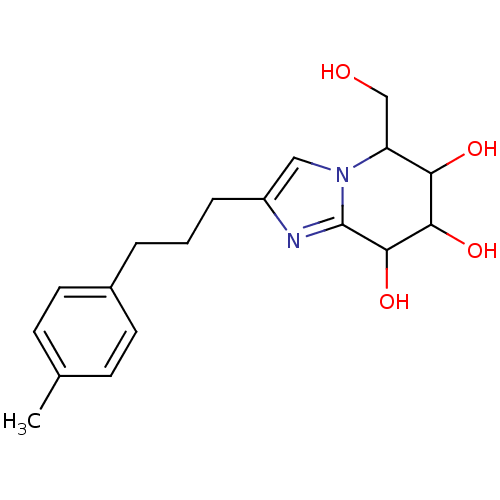 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM60418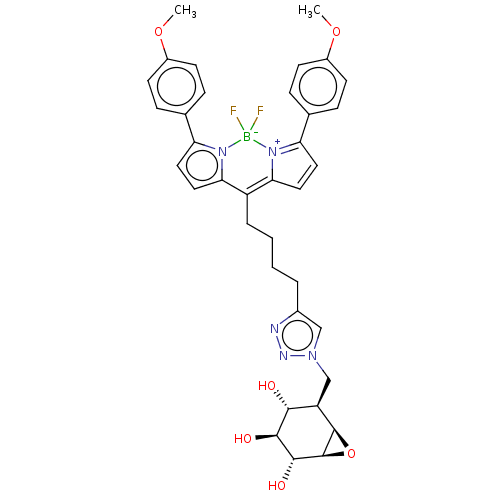 (US9056847, Fluorophore 2-cyclophellitol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
ACADEMISCH MEDISCH CENTRUM BIJ UNIVERSITEIT VAN AMSTERDAM US Patent | Assay Description Activity of GBA was measured at 37° C. with 4-methylumbelliferyl β-D-glucopyranoside as substrate as reported previously. To determine the IC50 ... | US Patent US9056847 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801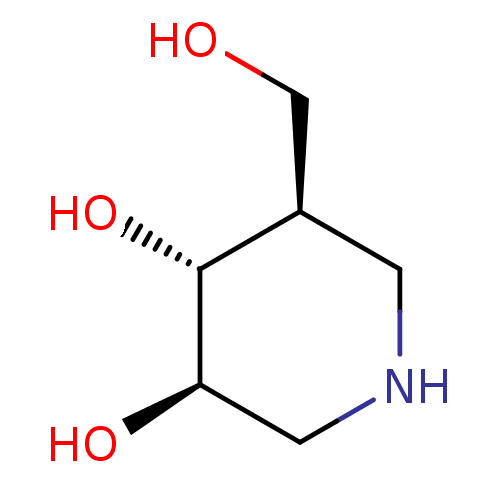 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383327 (CHEMBL2029772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108222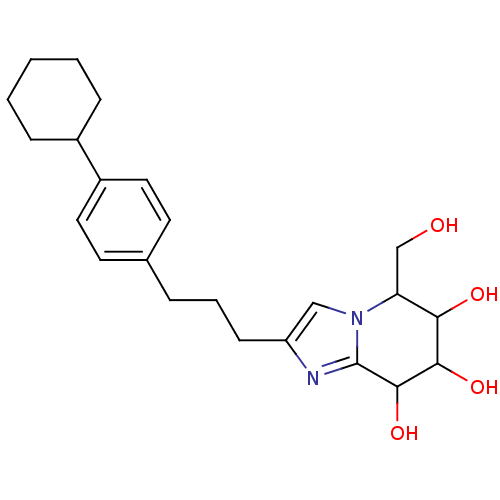 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50100428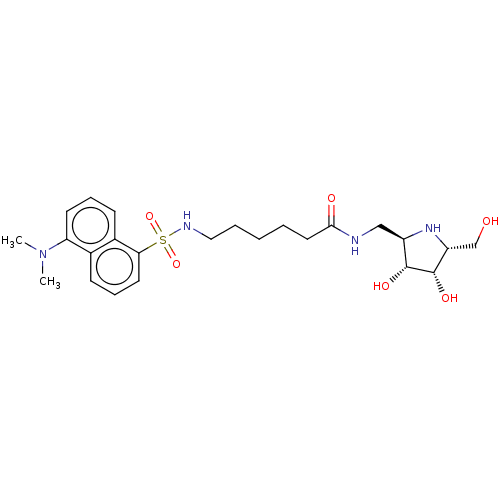 (6-(5-Dimethylamino-naphthalene-1-sulfonylamino)-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285821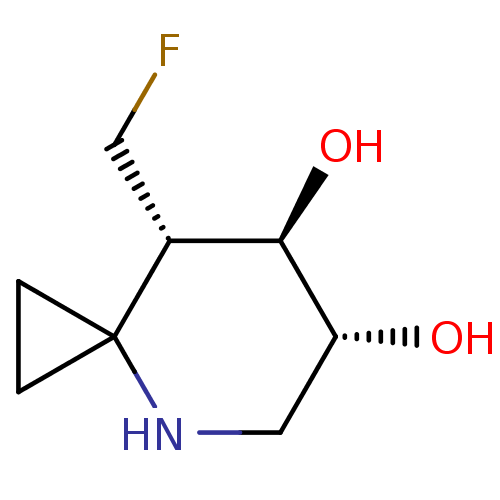 ((6R,7R,8S)-8-(fluoromethyl)-4-azaspiro[2.5]octane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285821 ((6R,7R,8S)-8-(fluoromethyl)-4-azaspiro[2.5]octane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250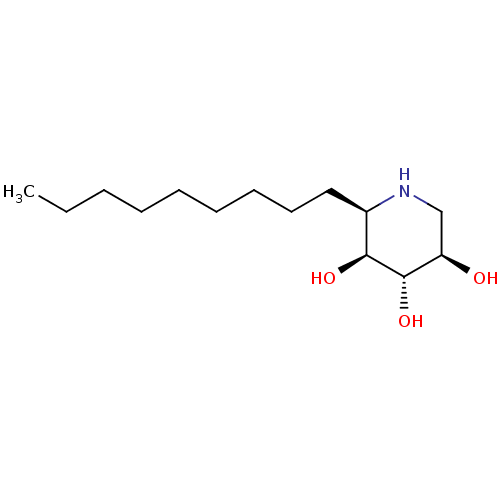 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant beta-glucocerebrosidase assessed as p-nitrophenolate accumulation preincubated for 10 mins before p-nitrophenyl-beta-... | Bioorg Med Chem 18: 2645-50 (2010) Article DOI: 10.1016/j.bmc.2010.02.027 BindingDB Entry DOI: 10.7270/Q24F1QVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50100426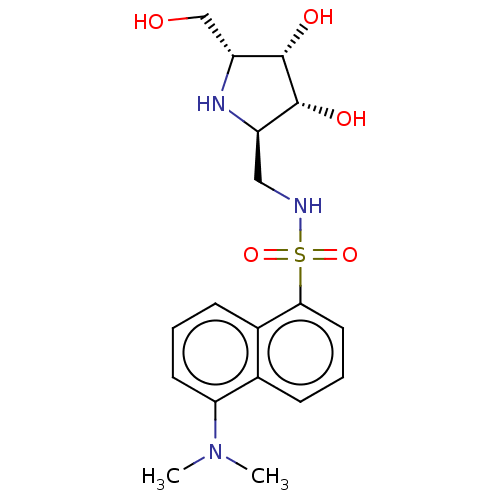 (5-Dimethylamino-naphthalene-1-sulfonic acid ((3S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie der Technischen Universität Graz Curated by ChEMBL | Assay Description Inhibitory activity against Agrobacterium sp. Beta-glucosidase employing fluorescence spectrometric method | Bioorg Med Chem Lett 11: 1339-42 (2001) BindingDB Entry DOI: 10.7270/Q2JQ1097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285819 ((6R,7R,8S)-8-methyl-4-azaspiro[2.5]octane-6,7-diol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285819 ((6R,7R,8S)-8-methyl-4-azaspiro[2.5]octane-6,7-diol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383321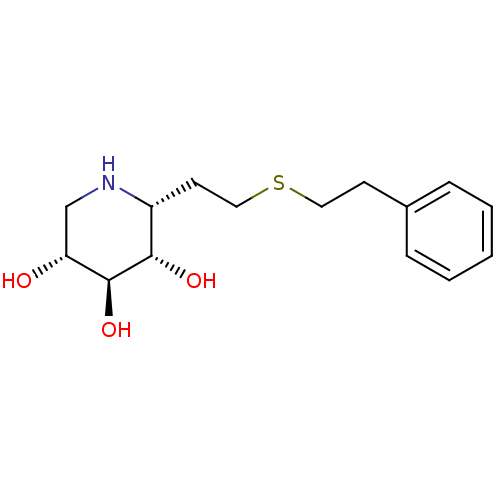 (CHEMBL2029780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108417 (US8604206, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.06 | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216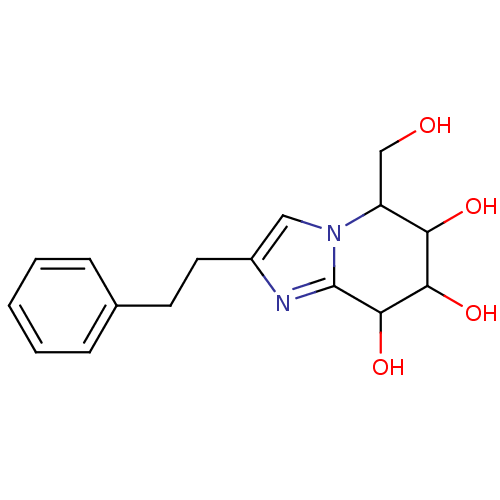 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223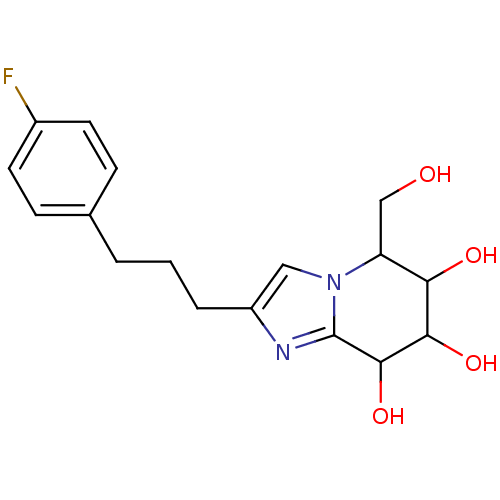 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221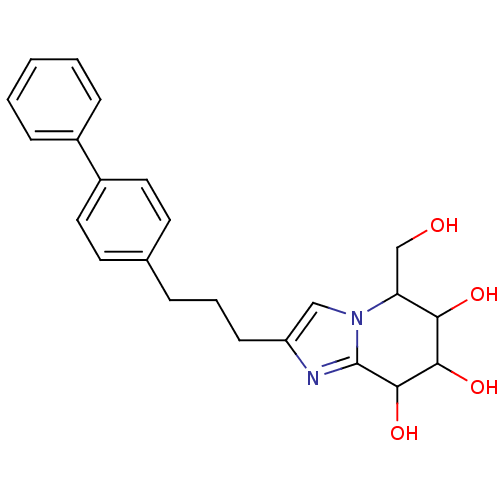 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.62 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108418 (US8604206, 13 | US8604206, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383323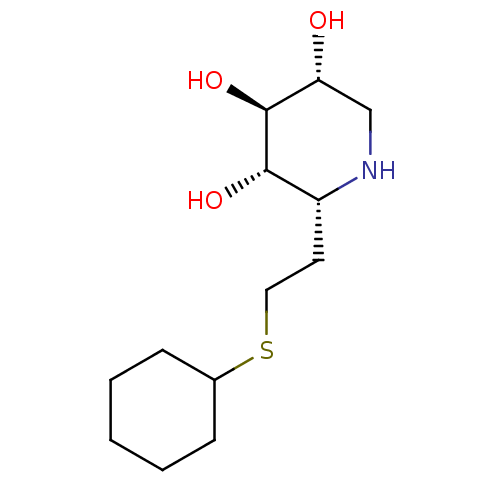 (CHEMBL2029778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108224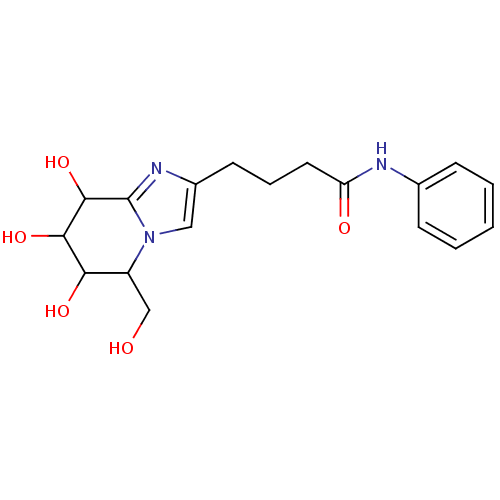 (N-phenyl-4-[6,7,8-trihydroxy-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383325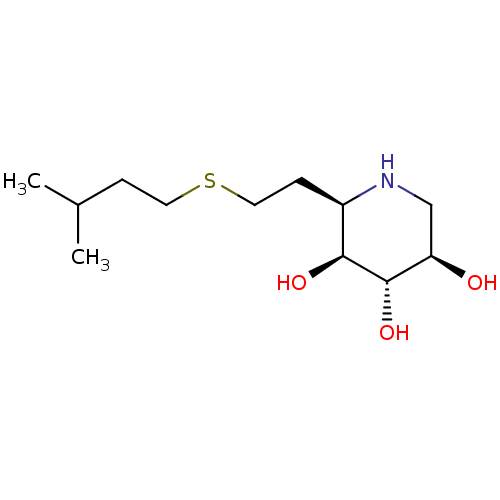 (CHEMBL2029776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM636738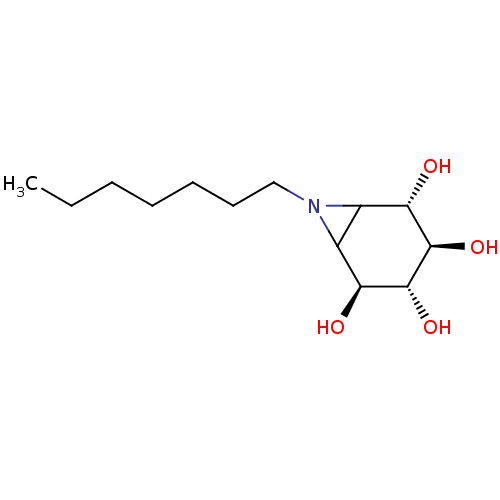 (US11826435, Compound 1.7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50075408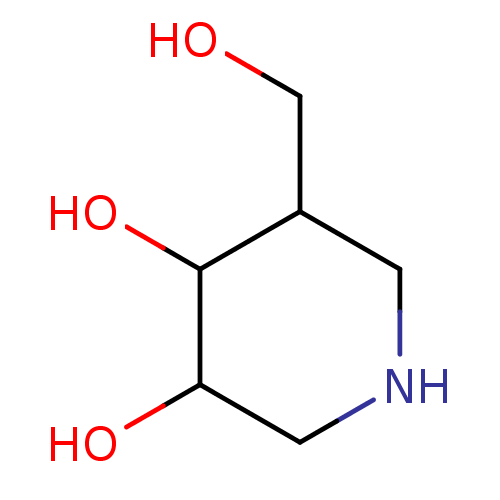 (5-(hydroxymethyl)piperidine-3,4-diol | CHEMBL34512...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108217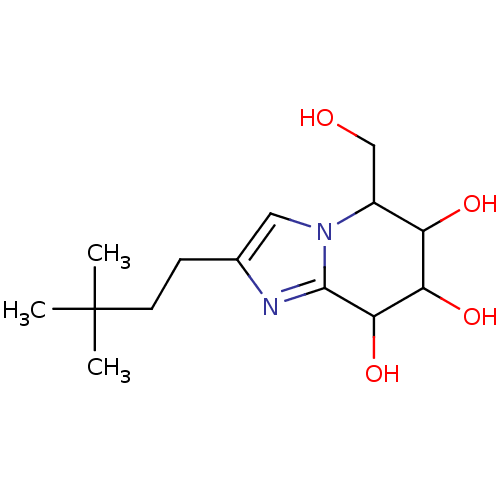 (2-(3,3-dimethylbutyl)-5-(hydroxymethyl)- 5H,6H,7H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.34 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108218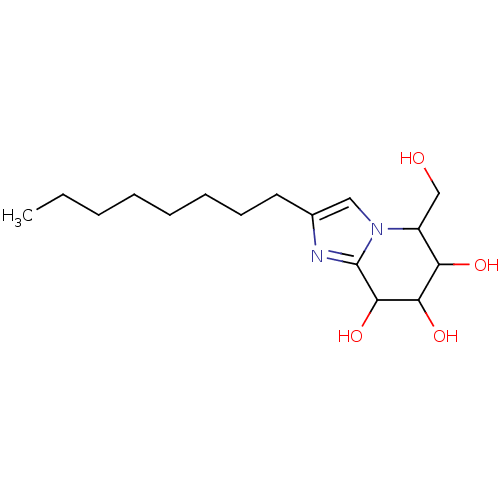 (5-(hydroxymethyl)-2-octyl-5H,6H,7H,8H-imidazo[1,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.38 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383324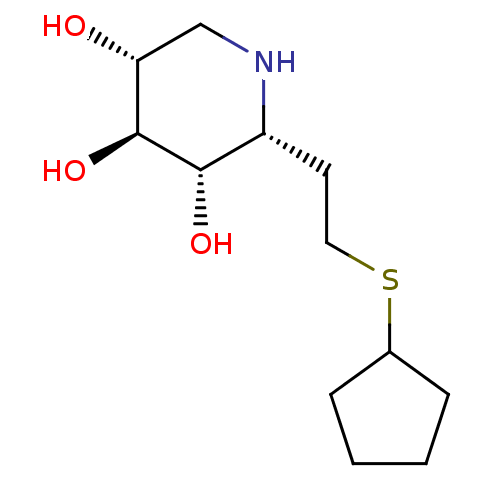 (CHEMBL2029777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285839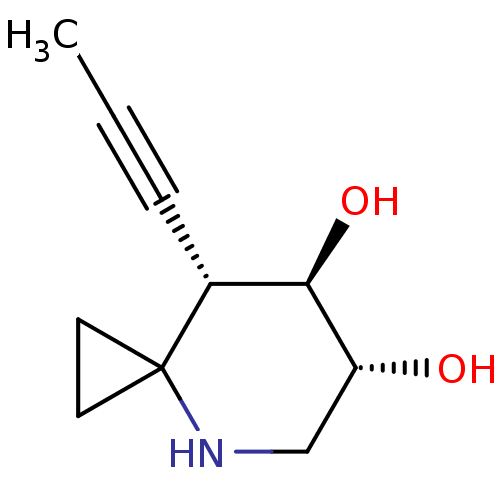 ((6R,7R,8S)-8-(prop-1-yn-1-yl)-4-azaspiro[2.5]octan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285839 ((6R,7R,8S)-8-(prop-1-yn-1-yl)-4-azaspiro[2.5]octan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36514 (CID46912122 | MDW933, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.4 | 1.24 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285820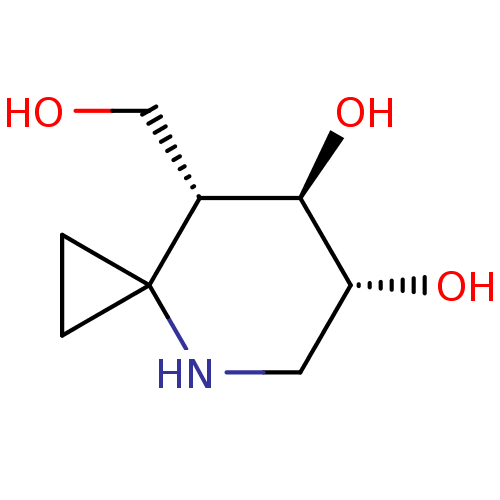 ((6R,7R,8R)-8-(hydroxymethyl)-4-azaspiro[2.5]octane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285820 ((6R,7R,8R)-8-(hydroxymethyl)-4-azaspiro[2.5]octane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018018 (CHEMBL3289679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285834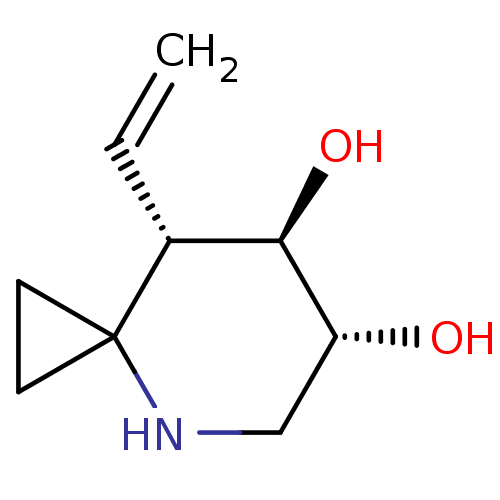 ((6R,7R,8S)-8-vinyl-4-azaspiro[2.5]octane-6,7-diol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285834 ((6R,7R,8S)-8-vinyl-4-azaspiro[2.5]octane-6,7-diol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285838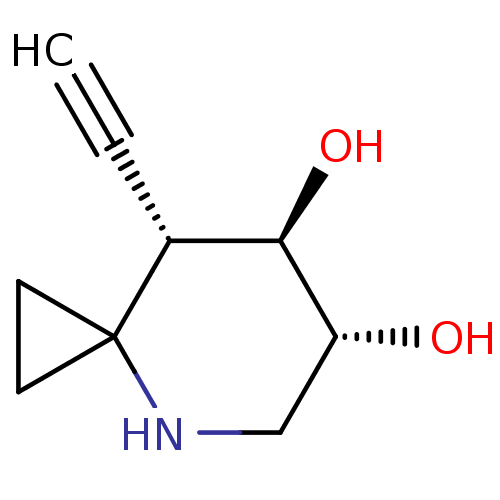 ((6R,7R,8S)-8-ethynyl-4-azaspiro[2.5]octane-6,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US9796680 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM285838 ((6R,7R,8S)-8-ethynyl-4-azaspiro[2.5]octane-6,7-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Alectos Therapeutics Inc. US Patent | Assay Description Various concentrations of test compounds were prepared in DMSO and then diluted into buffer consisting of 50 mM sodium phosphate 0.25% w/v sodium tau... | US Patent US10081601 (2018) BindingDB Entry DOI: 10.7270/Q2S184JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36515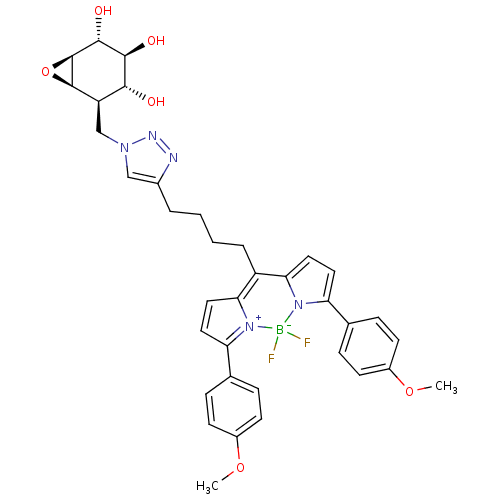 (CID46912120 | MDW941, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | 1.94 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1384 total ) | Next | Last >> |