 Found 33 hits Enz. Inhib. hit(s) with Target = 'Glutathione S-transferase A2'
Found 33 hits Enz. Inhib. hit(s) with Target = 'Glutathione S-transferase A2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50111440
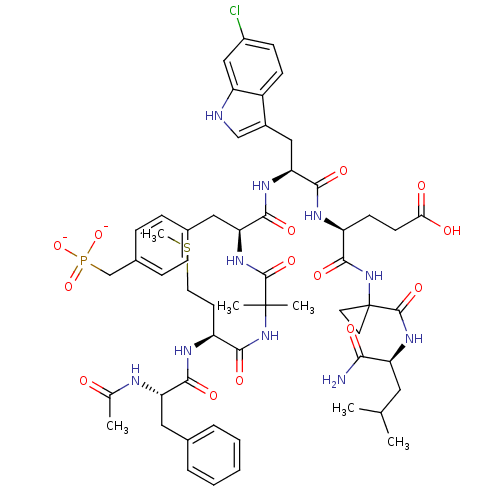
(4-(1-carbamoyl-4-methylpentanamide-2-yl-cyclopropy...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)NC(C)(C)C(=O)N[C@@H](Cc1ccc(CP([O-])([O-])=O)cc1)C(=O)N[C@@H](Cc1c[nH]c2cc(Cl)ccc12)C(=O)N[C@@H](CCC(O)=O)C(=O)NC1(CC1)C(=O)N[C@@H](CC(C)C)C(N)=O Show InChI InChI=1S/C56H74ClN10O14PS/c1-31(2)24-42(47(58)71)64-54(78)56(21-22-56)67-52(76)39(18-19-46(69)70)61-50(74)45(27-36-29-59-41-28-37(57)16-17-38(36)41)63-49(73)44(26-34-12-14-35(15-13-34)30-82(79,80)81)65-53(77)55(4,5)66-51(75)40(20-23-83-6)62-48(72)43(60-32(3)68)25-33-10-8-7-9-11-33/h7-17,28-29,31,39-40,42-45,59H,18-27,30H2,1-6H3,(H2,58,71)(H,60,68)(H,61,74)(H,62,72)(H,63,73)(H,64,78)(H,65,77)(H,66,75)(H,67,76)(H,69,70)(H2,79,80,81)/p-2/t39-,40-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of p53 binding to Glutathione S-transferase 2 (hdm2-GST) |
J Med Chem 45: 1543-58 (2002)
BindingDB Entry DOI: 10.7270/Q2CN74ND |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088969

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(1-ethyl-...)Show SMILES CCC(CC(C)=O)SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O Show InChI InChI=1S/C16H27N3O7S/c1-3-10(6-9(2)20)27-8-12(15(24)18-7-14(22)23)19-13(21)5-4-11(17)16(25)26/h10-12H,3-8,17H2,1-2H3,(H,18,24)(H,19,21)(H,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088974
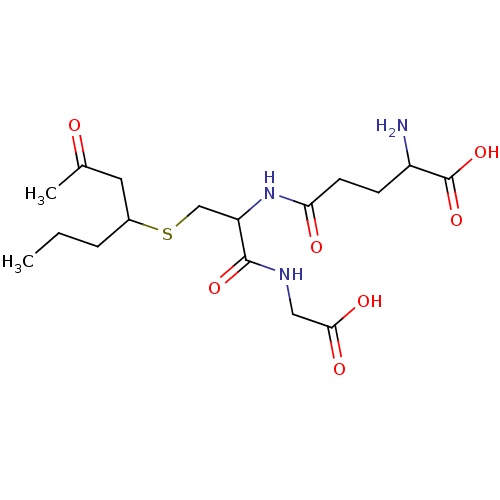
(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(3-oxo-1-...)Show SMILES CCCC(CC(C)=O)SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O Show InChI InChI=1S/C17H29N3O7S/c1-3-4-11(7-10(2)21)28-9-13(16(25)19-8-15(23)24)20-14(22)6-5-12(18)17(26)27/h11-13H,3-9,18H2,1-2H3,(H,19,25)(H,20,22)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088966
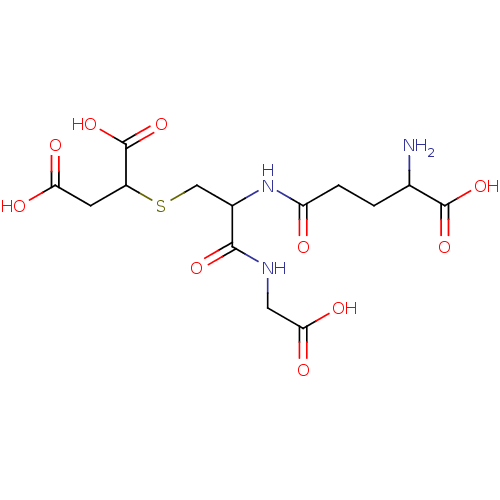
(2-[2-(4-Amino-4-carboxy-butyrylamino)-2-(carboxyme...)Show SMILES NC(CCC(=O)NC(CSC(CC(O)=O)C(O)=O)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C14H21N3O10S/c15-6(13(24)25)1-2-9(18)17-7(12(23)16-4-11(21)22)5-28-8(14(26)27)3-10(19)20/h6-8H,1-5,15H2,(H,16,23)(H,17,18)(H,19,20)(H,21,22)(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088967
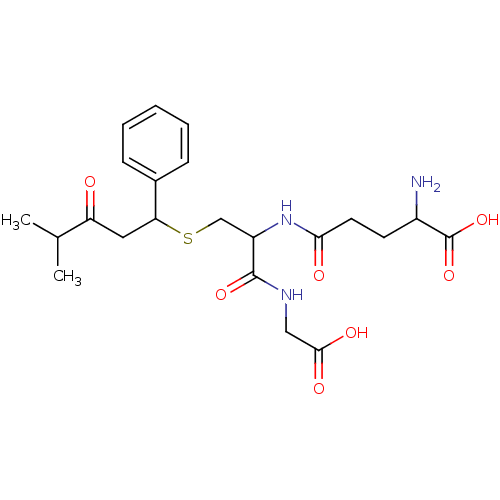
(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(4-methyl...)Show SMILES CC(C)C(=O)CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)c1ccccc1 Show InChI InChI=1S/C22H31N3O7S/c1-13(2)17(26)10-18(14-6-4-3-5-7-14)33-12-16(21(30)24-11-20(28)29)25-19(27)9-8-15(23)22(31)32/h3-7,13,15-16,18H,8-12,23H2,1-2H3,(H,24,30)(H,25,27)(H,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361799

(CHEMBL1938640)Show SMILES CCCCCCCCCCO[C@H]1C=C(COC(C)=O)C(=O)[C@@H]2O[C@@](C)(OC)[C@](C)(OC)O[C@@H]12 |r,t:12| Show InChI InChI=1S/C25H42O8/c1-7-8-9-10-11-12-13-14-15-30-20-16-19(17-31-18(2)26)21(27)23-22(20)32-24(3,28-5)25(4,29-6)33-23/h16,20,22-23H,7-15,17H2,1-6H3/t20-,22-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361800
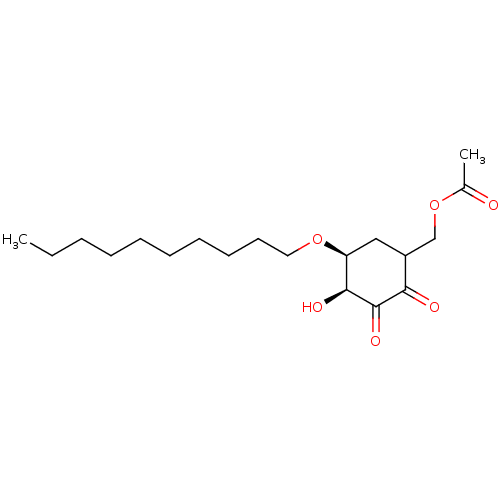
(CHEMBL1938641)Show SMILES CCCCCCCCCCO[C@H]1CC(COC(C)=O)C(=O)C(=O)[C@H]1O |r| Show InChI InChI=1S/C19H32O6/c1-3-4-5-6-7-8-9-10-11-24-16-12-15(13-25-14(2)20)17(21)19(23)18(16)22/h15-16,18,22H,3-13H2,1-2H3/t15?,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088971
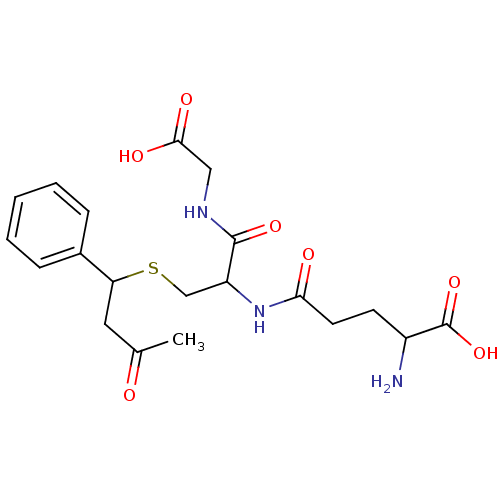
(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(3-oxo-1-...)Show SMILES CC(=O)CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)c1ccccc1 Show InChI InChI=1S/C20H27N3O7S/c1-12(24)9-16(13-5-3-2-4-6-13)31-11-15(19(28)22-10-18(26)27)23-17(25)8-7-14(21)20(29)30/h2-6,14-16H,7-11,21H2,1H3,(H,22,28)(H,23,25)(H,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088976
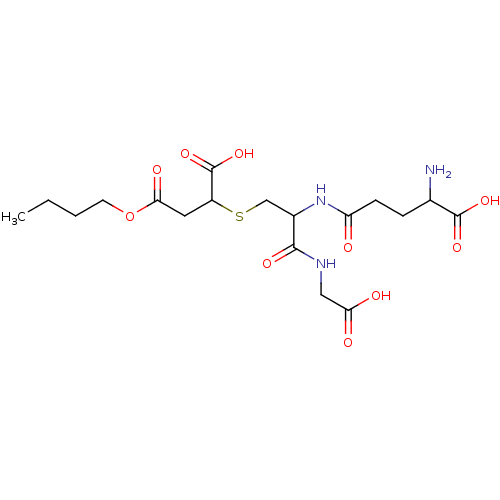
(2-[2-(4-Amino-4-carboxy-butyrylamino)-2-(carboxyme...)Show SMILES CCCCOC(=O)CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C18H29N3O10S/c1-2-3-6-31-15(25)7-12(18(29)30)32-9-11(16(26)20-8-14(23)24)21-13(22)5-4-10(19)17(27)28/h10-12H,2-9,19H2,1H3,(H,20,26)(H,21,22)(H,23,24)(H,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088975
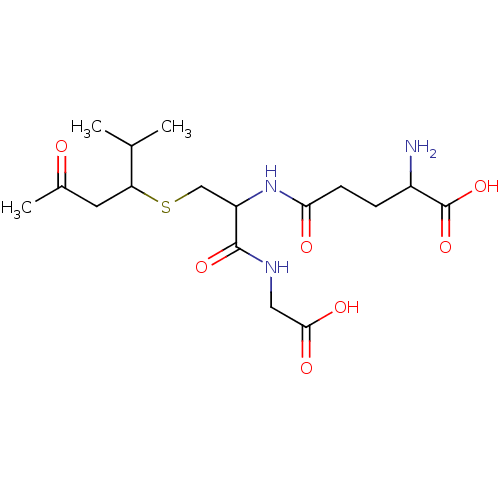
(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(1-isopro...)Show SMILES CC(C)C(CC(C)=O)SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O Show InChI InChI=1S/C17H29N3O7S/c1-9(2)13(6-10(3)21)28-8-12(16(25)19-7-15(23)24)20-14(22)5-4-11(18)17(26)27/h9,11-13H,4-8,18H2,1-3H3,(H,19,25)(H,20,22)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361798
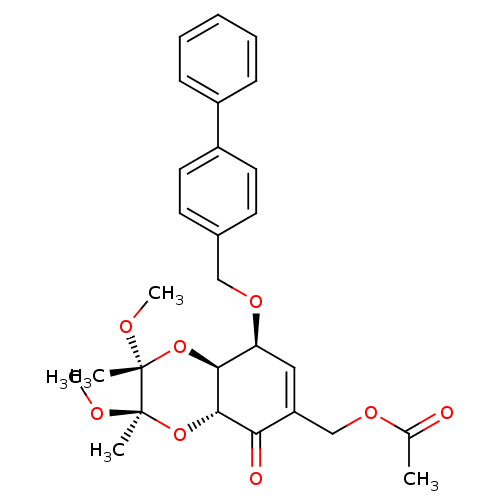
(CHEMBL1938639)Show SMILES CO[C@]1(C)O[C@H]2[C@@H](OCc3ccc(cc3)-c3ccccc3)C=C(COC(C)=O)C(=O)[C@@H]2O[C@@]1(C)OC |r,t:23| Show InChI InChI=1S/C28H32O8/c1-18(29)33-17-22-15-23(25-26(24(22)30)36-28(3,32-5)27(2,31-4)35-25)34-16-19-11-13-21(14-12-19)20-9-7-6-8-10-20/h6-15,23,25-26H,16-17H2,1-5H3/t23-,25-,26-,27+,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361793
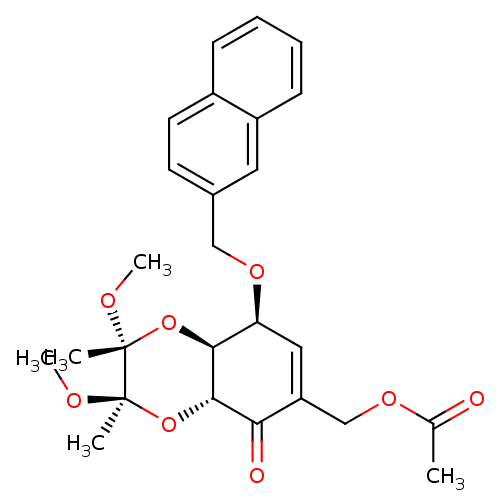
(CHEMBL1938634)Show SMILES CO[C@]1(C)O[C@H]2[C@@H](OCc3ccc4ccccc4c3)C=C(COC(C)=O)C(=O)[C@@H]2O[C@@]1(C)OC |r,t:21| Show InChI InChI=1S/C26H30O8/c1-16(27)31-15-20-13-21(32-14-17-10-11-18-8-6-7-9-19(18)12-17)23-24(22(20)28)34-26(3,30-5)25(2,29-4)33-23/h6-13,21,23-24H,14-15H2,1-5H3/t21-,23-,24-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088968
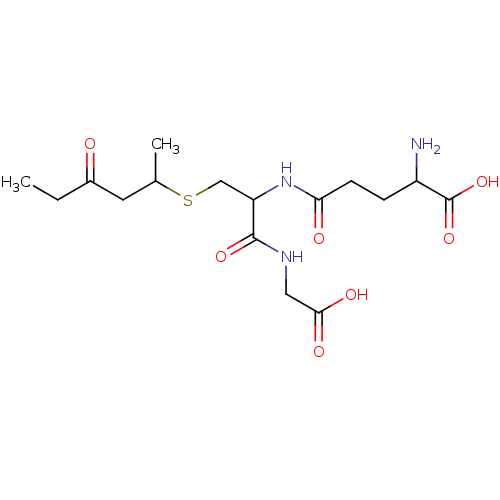
(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(1-methyl...)Show SMILES CCC(=O)CC(C)SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O Show InChI InChI=1S/C16H27N3O7S/c1-3-10(20)6-9(2)27-8-12(15(24)18-7-14(22)23)19-13(21)5-4-11(17)16(25)26/h9,11-12H,3-8,17H2,1-2H3,(H,18,24)(H,19,21)(H,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361795
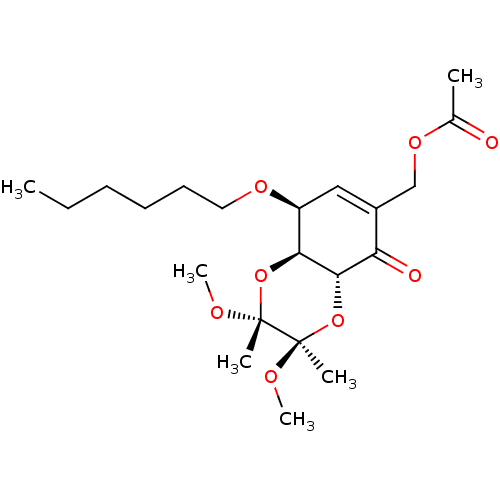
(CHEMBL1938636)Show SMILES CCCCCCO[C@H]1C=C(COC(C)=O)C(=O)[C@@H]2O[C@@](C)(OC)[C@](C)(OC)O[C@@H]12 |r,t:8| Show InChI InChI=1S/C21H34O8/c1-7-8-9-10-11-26-16-12-15(13-27-14(2)22)17(23)19-18(16)28-20(3,24-5)21(4,25-6)29-19/h12,16,18-19H,7-11,13H2,1-6H3/t16-,18-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361797

(CHEMBL1938638)Show SMILES CO[C@]1(C)O[C@H]2[C@@H](OC\C=C\c3ccccc3)C=C(COC(C)=O)C(=O)[C@@H]2O[C@@]1(C)OC |r,t:18| Show InChI InChI=1S/C24H30O8/c1-16(25)30-15-18-14-19(29-13-9-12-17-10-7-6-8-11-17)21-22(20(18)26)32-24(3,28-5)23(2,27-4)31-21/h6-12,14,19,21-22H,13,15H2,1-5H3/b12-9+/t19-,21-,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361794

(CHEMBL1938635)Show SMILES [#6]-[#8][C@]1([#6])[#8]-[#6@H]-2-[#6@@H](-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]=[#6](-[#6]-[#8]-[#6](-[#6])=O)-[#6](=O)-[#6@@H]-2-[#8][C@@]1([#6])[#8]-[#6] |r,t:13| Show InChI InChI=1S/C20H30O8/c1-12(2)8-9-25-15-10-14(11-26-13(3)21)16(22)18-17(15)27-19(4,23-6)20(5,24-7)28-18/h8,10,15,17-18H,9,11H2,1-7H3/t15-,17-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361792
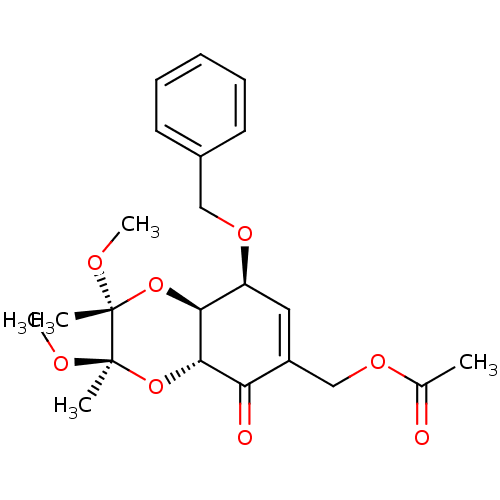
(CHEMBL1938633)Show SMILES CO[C@]1(C)O[C@H]2[C@@H](OCc3ccccc3)C=C(COC(C)=O)C(=O)[C@@H]2O[C@@]1(C)OC |r,t:16| Show InChI InChI=1S/C22H28O8/c1-14(23)27-13-16-11-17(28-12-15-9-7-6-8-10-15)19-20(18(16)24)30-22(3,26-5)21(2,25-4)29-19/h6-11,17,19-20H,12-13H2,1-5H3/t17-,19-,20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361812
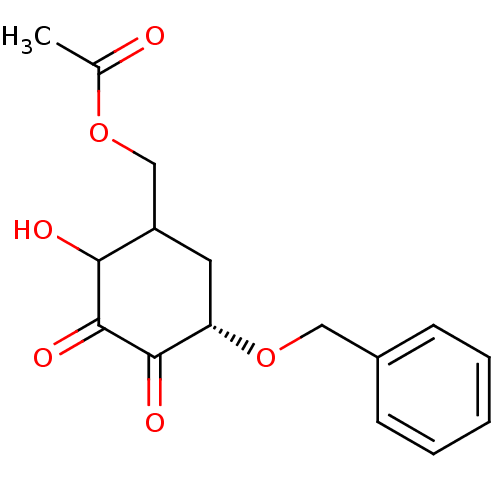
(CHEMBL1938630)Show SMILES CC(=O)OCC1C[C@H](OCc2ccccc2)C(=O)C(=O)C1O |r| Show InChI InChI=1S/C16H18O6/c1-10(17)21-9-12-7-13(15(19)16(20)14(12)18)22-8-11-5-3-2-4-6-11/h2-6,12-14,18H,7-9H2,1H3/t12?,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361811
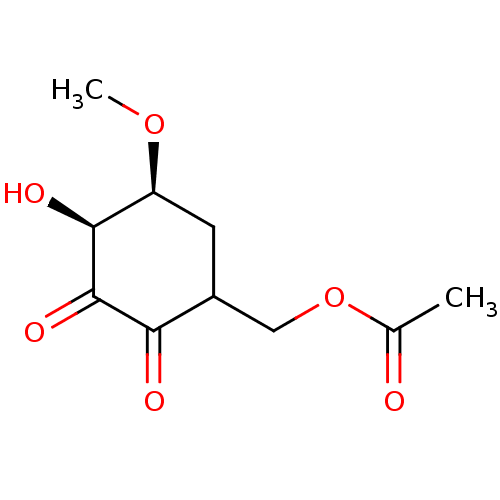
(CHEMBL1938629)Show InChI InChI=1S/C10H14O6/c1-5(11)16-4-6-3-7(15-2)9(13)10(14)8(6)12/h6-7,9,13H,3-4H2,1-2H3/t6?,7-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361809

(CHEMBL1938627)Show SMILES CO[C@@H]1C=C(COC(C)=O)C(=O)[C@@H]2O[C@@](C)(OC)[C@](C)(OC)O[C@@H]12 |r,t:3| Show InChI InChI=1S/C16H24O8/c1-9(17)22-8-10-7-11(19-4)13-14(12(10)18)24-16(3,21-6)15(2,20-5)23-13/h7,11,13-14H,8H2,1-6H3/t11-,13+,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361801

(CHEMBL1938618)Show InChI InChI=1S/C9H12O6/c1-4(10)15-3-5-2-6(11)8(13)9(14)7(5)12/h5-6,8,11,13H,2-3H2,1H3/t5?,6?,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361802

(CHEMBL1938619)Show InChI InChI=1S/C9H12O6/c1-4(10)15-3-5-2-6(11)8(13)9(14)7(5)12/h5-6,8,11,13H,2-3H2,1H3/t5?,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361803

(CHEMBL1938620)Show InChI InChI=1S/C9H12O6/c1-4(10)15-3-5-2-6(11)8(13)9(14)7(5)12/h5-6,8,11,13H,2-3H2,1H3/t5?,6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361804
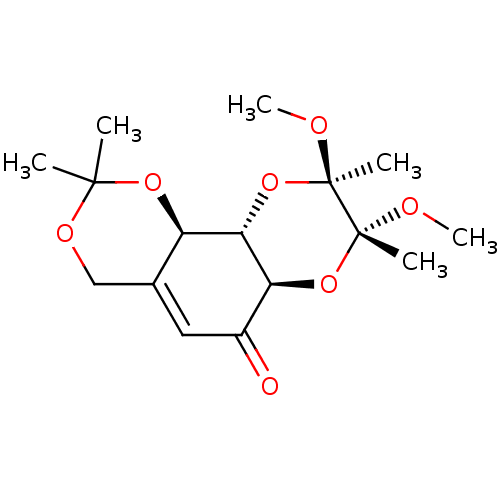
(CHEMBL1938621)Show SMILES CO[C@]1(C)O[C@@H]2[C@@H](O[C@@]1(C)OC)C(=O)C=C1COC(C)(C)O[C@@H]21 |r,t:15| Show InChI InChI=1S/C16H24O7/c1-14(2)20-8-9-7-10(17)12-13(11(9)21-14)23-16(4,19-6)15(3,18-5)22-12/h7,11-13H,8H2,1-6H3/t11-,12+,13+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361815

(CHEMBL1938622)Show SMILES CO[C@]1(C)O[C@H]2[C@H](O)C(CC(=O)[C@@H]2O[C@@]1(C)OC)C=O |r| Show InChI InChI=1S/C13H20O7/c1-12(17-3)13(2,18-4)20-11-9(16)7(6-14)5-8(15)10(11)19-12/h6-7,9-11,16H,5H2,1-4H3/t7?,9-,10+,11+,12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361805
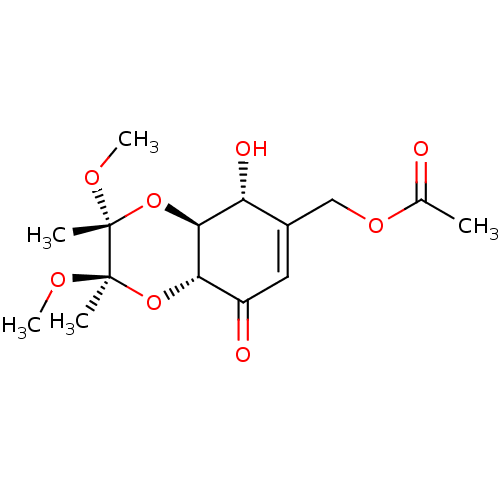
(CHEMBL1938623)Show SMILES CO[C@]1(C)O[C@H]2[C@H](O)C(COC(C)=O)=CC(=O)[C@@H]2O[C@@]1(C)OC |r,c:13| Show InChI InChI=1S/C15H22O8/c1-8(16)21-7-9-6-10(17)12-13(11(9)18)23-15(3,20-5)14(2,19-4)22-12/h6,11-13,18H,7H2,1-5H3/t11-,12+,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361806
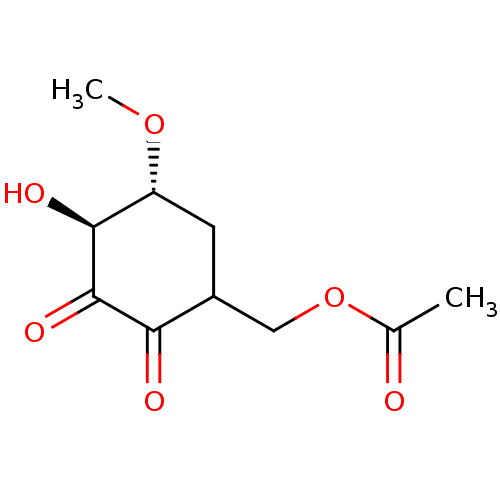
(CHEMBL1938624)Show InChI InChI=1S/C10H14O6/c1-5(11)16-4-6-3-7(15-2)9(13)10(14)8(6)12/h6-7,9,13H,3-4H2,1-2H3/t6?,7-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361807

(CHEMBL1938625)Show SMILES CC(=O)OCC1C[C@@H](OCc2ccccc2)C(=O)C(=O)C1O |r| Show InChI InChI=1S/C16H18O6/c1-10(17)21-9-12-7-13(15(19)16(20)14(12)18)22-8-11-5-3-2-4-6-11/h2-6,12-14,18H,7-9H2,1H3/t12?,13-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361808
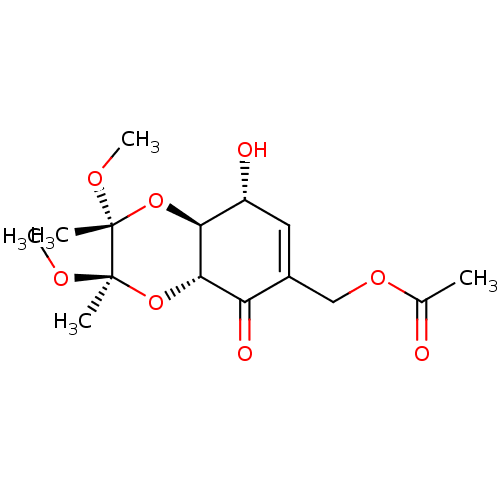
(CHEMBL1938626)Show SMILES CO[C@]1(C)O[C@H]2[C@H](O)C=C(COC(C)=O)C(=O)[C@@H]2O[C@@]1(C)OC |r,t:8| Show InChI InChI=1S/C15H22O8/c1-8(16)21-7-9-6-10(17)12-13(11(9)18)23-15(3,20-5)14(2,19-4)22-12/h6,10,12-13,17H,7H2,1-5H3/t10-,12+,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088970
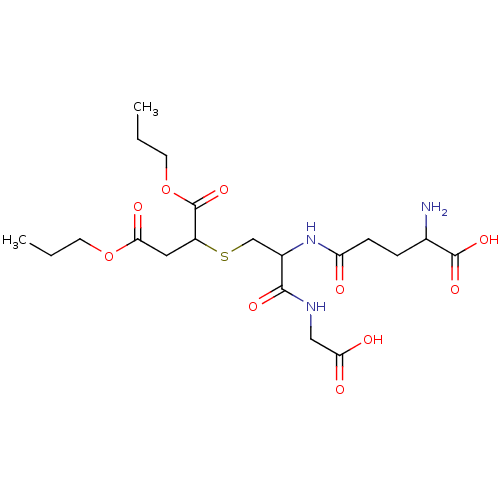
(2-[2-(4-Amino-4-carboxy-butyrylamino)-2-(carboxyme...)Show SMILES CCCOC(=O)CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(=O)OCCC Show InChI InChI=1S/C20H33N3O10S/c1-3-7-32-17(27)9-14(20(31)33-8-4-2)34-11-13(18(28)22-10-16(25)26)23-15(24)6-5-12(21)19(29)30/h12-14H,3-11,21H2,1-2H3,(H,22,28)(H,23,24)(H,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50361796

(CHEMBL1938637)Show SMILES [#6]-[#8][C@]1([#6])[#8]-[#6@H]-2-[#6@@H](-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6]=[#6](-[#6]-[#8]-[#6](-[#6])=O)-[#6](=O)-[#6@@H]-2-[#8][C@@]1([#6])[#8]-[#6] |r,t:18| Show InChI InChI=1S/C25H38O8/c1-16(2)10-9-11-17(3)12-13-30-20-14-19(15-31-18(4)26)21(27)23-22(20)32-24(5,28-7)25(6,29-8)33-23/h10,12,14,20,22-23H,9,11,13,15H2,1-8H3/b17-12+/t20-,22-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry, Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometry |
J Med Chem 54: 8574-81 (2011)
Article DOI: 10.1021/jm201131n
BindingDB Entry DOI: 10.7270/Q26D5TDT |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088973

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(3-oxo-cy...)Show SMILES NC(CCC(=O)NC(CSC1CCCC(=O)C1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C16H25N3O7S/c17-11(16(25)26)4-5-13(21)19-12(15(24)18-7-14(22)23)8-27-10-3-1-2-9(20)6-10/h10-12H,1-8,17H2,(H,18,24)(H,19,21)(H,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A2
(Homo sapiens (Human)) | BDBM50088972

(2-[2-(4-Amino-4-carboxy-butyrylamino)-2-(carboxyme...)Show SMILES COC(=O)CC(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(=O)OC Show InChI InChI=1S/C16H25N3O10S/c1-28-13(23)5-10(16(27)29-2)30-7-9(14(24)18-6-12(21)22)19-11(20)4-3-8(17)15(25)26/h8-10H,3-7,17H2,1-2H3,(H,18,24)(H,19,20)(H,21,22)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wales
Curated by ChEMBL
| Assay Description
The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2 |
Bioorg Med Chem Lett 10: 979-81 (2000)
BindingDB Entry DOI: 10.7270/Q2BZ6589 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data