

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxyacylglutathione hydrolase, mitochondrial (Bos taurus) | BDBM50126960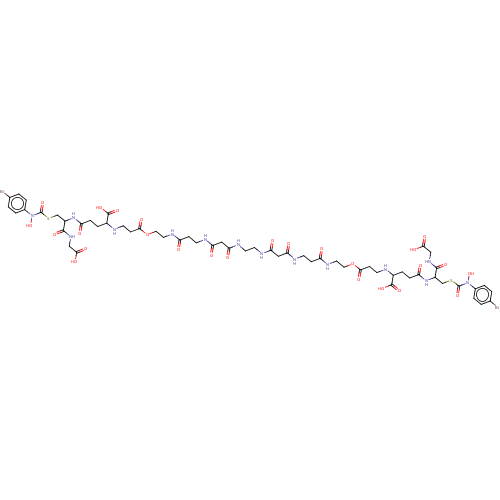 (CHEMBL3629116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of bovine liver glyoxalase 2 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Bos taurus) | BDBM50126961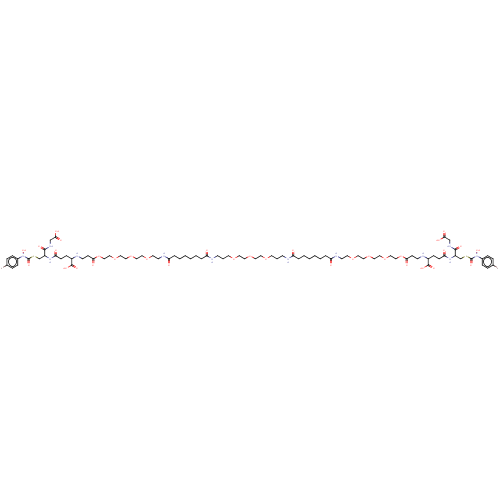 (CHEMBL3629115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of bovine liver glyoxalase 2 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039111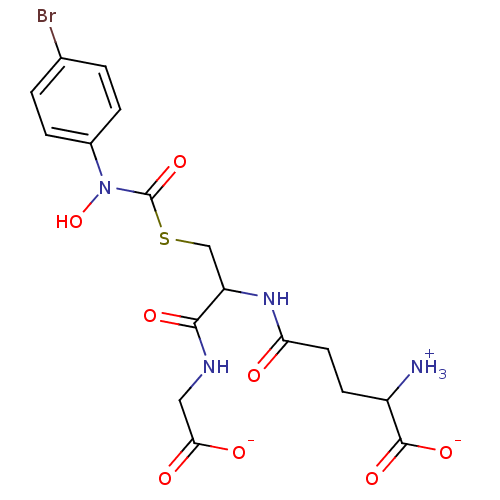 (S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Bos taurus) | BDBM50092824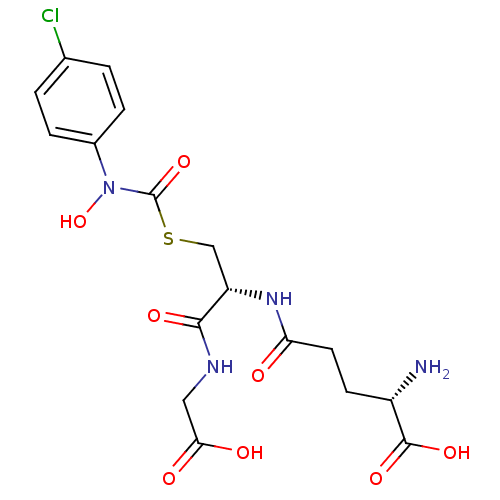 (CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of bovine liver glyoxalase 2 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039108 (S-(N-Hydroxy-N-(4-chlorophenyl)carbamoyl)glutathio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Rattus norvegicus) | BDBM50026371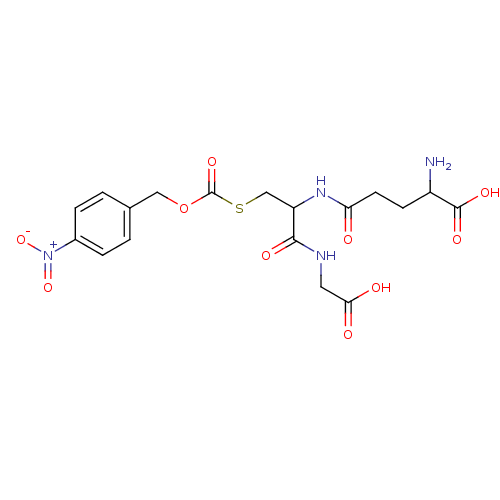 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(4-nitro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards rat liver glyoxalase II | J Med Chem 28: 828-30 (1985) BindingDB Entry DOI: 10.7270/Q2M32TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Rattus norvegicus) | BDBM50026368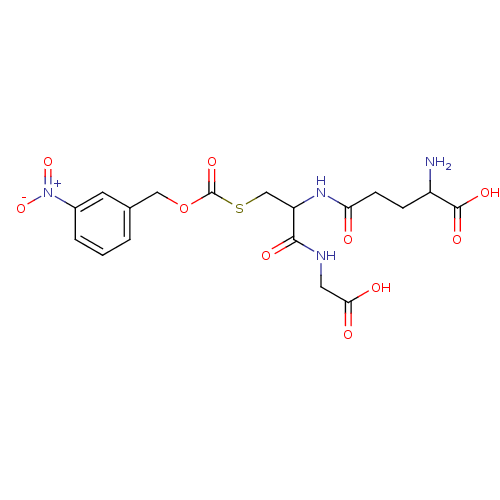 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(3-nitro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards rat liver glyoxalase II | J Med Chem 28: 828-30 (1985) BindingDB Entry DOI: 10.7270/Q2M32TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039113 (S-(N-Hydroxy-N-phenylcarbamoyl)glutathione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constant for the inhibition of the hydrolysis of S-D-lactoylglutathione by glyoxalase II | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Rattus norvegicus) | BDBM50026370 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(2-nitro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards rat liver glyoxalase II | J Med Chem 28: 828-30 (1985) BindingDB Entry DOI: 10.7270/Q2M32TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Rattus norvegicus) | BDBM50026369 (2-Amino-4-[2-benzyloxycarbonylsulfanyl-1-(carboxym...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards rat liver glyoxalase II | J Med Chem 28: 828-30 (1985) BindingDB Entry DOI: 10.7270/Q2M32TRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Rattus norvegicus) | BDBM50028202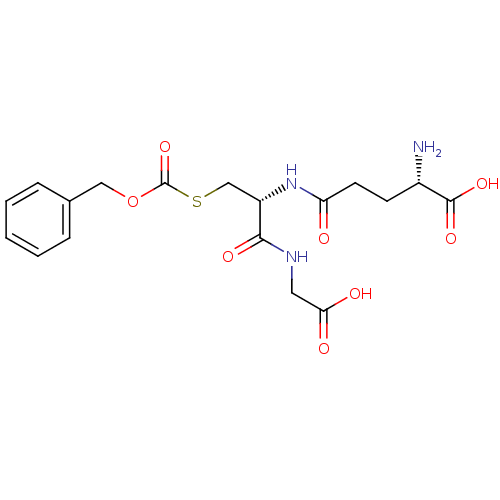 ((S)-2-Amino-4-[(R)-2-benzyloxycarbonylsulfanyl-1-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory activity of the compound against glyoxalase II in rat liver | J Med Chem 26: 1784-5 (1984) BindingDB Entry DOI: 10.7270/Q2SJ1JNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50039110 (S-(N-Methyl-N-hydroxycarbomyl)ethylglutathione) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ... | J Med Chem 37: 2161-6 (1994) BindingDB Entry DOI: 10.7270/Q2TT4RKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxyacylglutathione hydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50582857 (CHEMBL5091922) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Glyoxalase-2 assessed as reduction of substrate level using S-D-lactoylglutathione as substrate by spectrophotometric... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113873 BindingDB Entry DOI: 10.7270/Q2B56PM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||