

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Effective concentration required against L100I mutant HIV-1 reverse transcriptase (as per ref 8 in the article) | Bioorg Med Chem Lett 11: 2799-802 (2001) BindingDB Entry DOI: 10.7270/Q2J103P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102265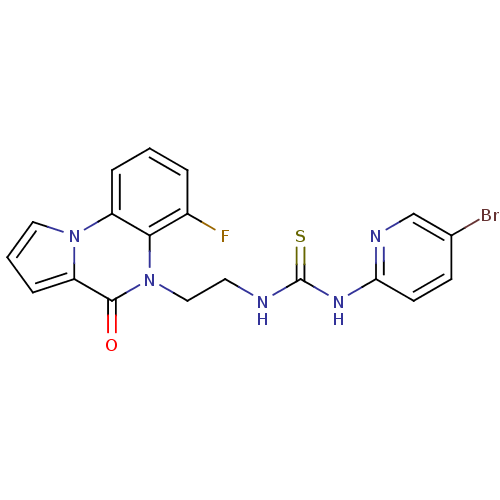 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102271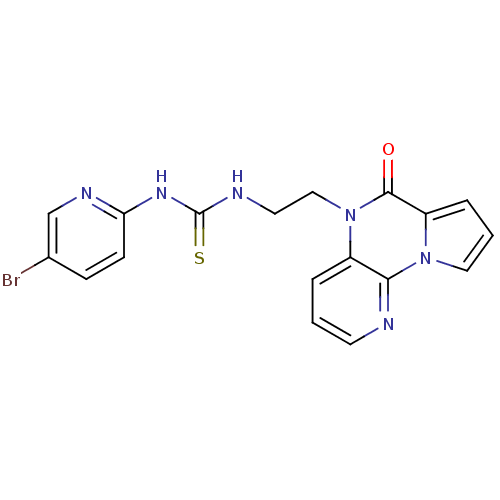 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-oxo-4H-5,9,9b-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061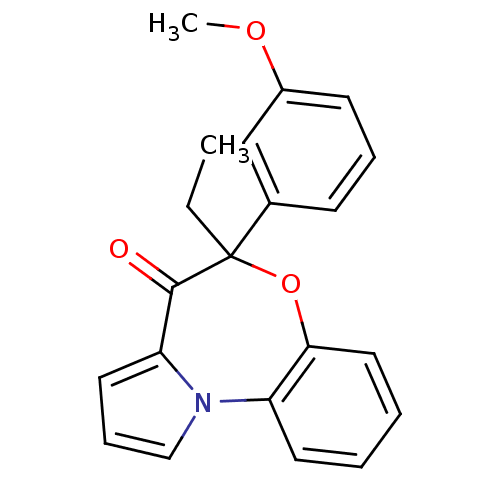 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution V106A | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution L100I | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102268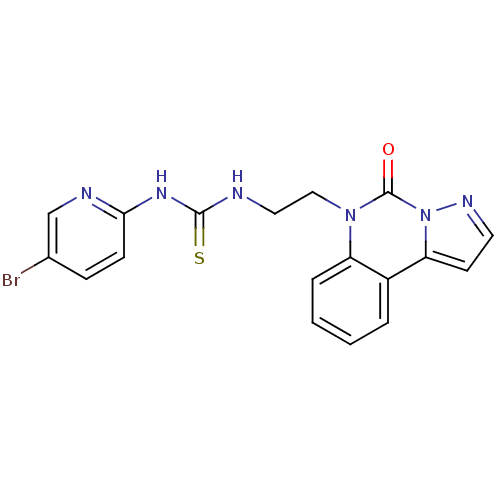 (1-(5-Bromo-pyridin-2-yl)-3-[2-(5-oxo-pyrazolo[1,5-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102265 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50285269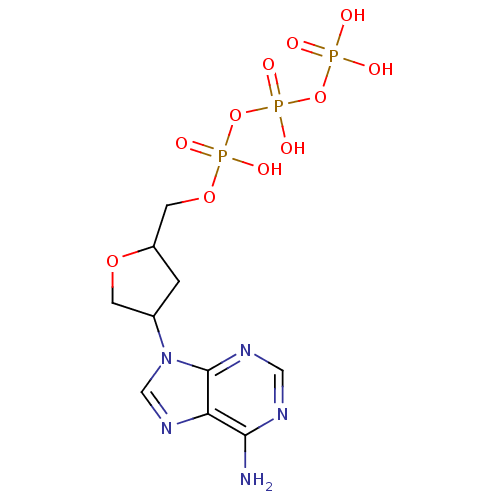 ((S,S)-isodideoxyadenosinetriphosphate | CHEMBL7247...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against HIV reverse transcriptase (HIV RT) | Bioorg Med Chem Lett 5: 2235-2238 (1995) Article DOI: 10.1016/0960-894X(95)00386-8 BindingDB Entry DOI: 10.7270/Q27H1JKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50285269 ((S,S)-isodideoxyadenosinetriphosphate | CHEMBL7247...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against HIV reverse transcriptase | Bioorg Med Chem Lett 7: 3195-3198 (1997) Article DOI: 10.1016/S0960-894X(97)10183-4 BindingDB Entry DOI: 10.7270/Q2NZ885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Effective concentration required against wild type HIV-1 reverse transcriptase (as per ref 6 in the article) | Bioorg Med Chem Lett 11: 2799-802 (2001) BindingDB Entry DOI: 10.7270/Q2J103P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity towards L100I mutant HIV-1 reverse transcriptase (as per ref 10 in the article) | Bioorg Med Chem Lett 11: 2799-802 (2001) BindingDB Entry DOI: 10.7270/Q2J103P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102276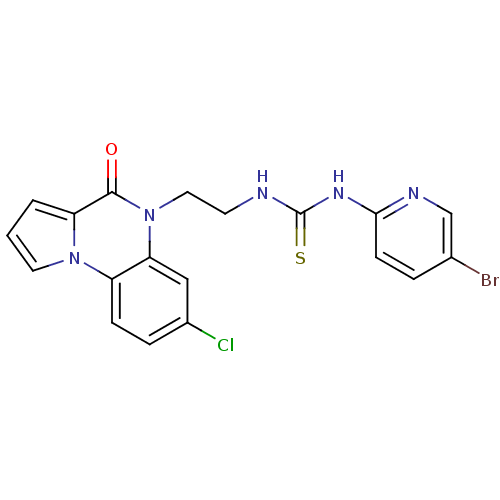 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7-chloro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102276 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7-chloro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102268 (1-(5-Bromo-pyridin-2-yl)-3-[2-(5-oxo-pyrazolo[1,5-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution K103N | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082055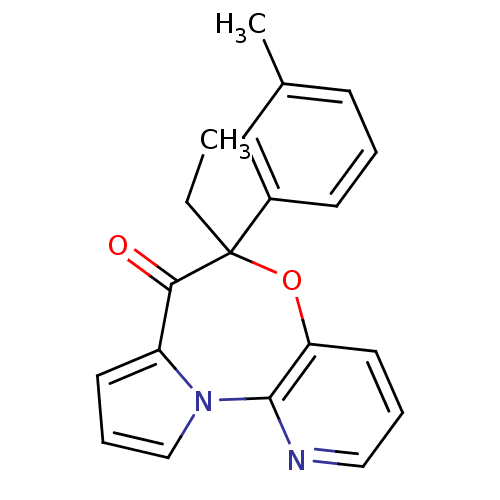 (5-Ethyl-5-m-tolyl-6-oxa-10,10b-diaza-benzo[e]azule...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 wild type reverse transcriptase (RT) | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102277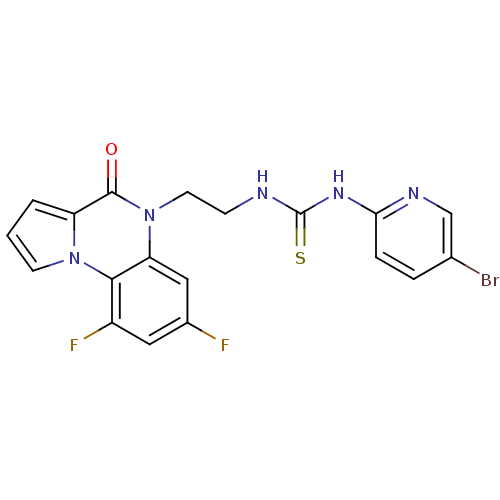 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,9-difluoro-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution K103N | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082060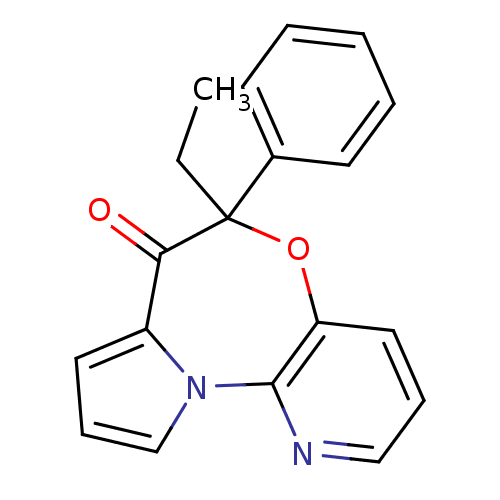 (5-Ethyl-5-phenyl-6-oxa-10,10b-diaza-benzo[e]azulen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 wild type reverse transcriptase (RT) | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164642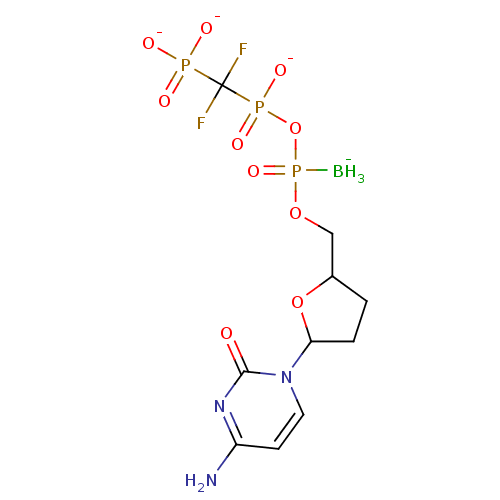 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164639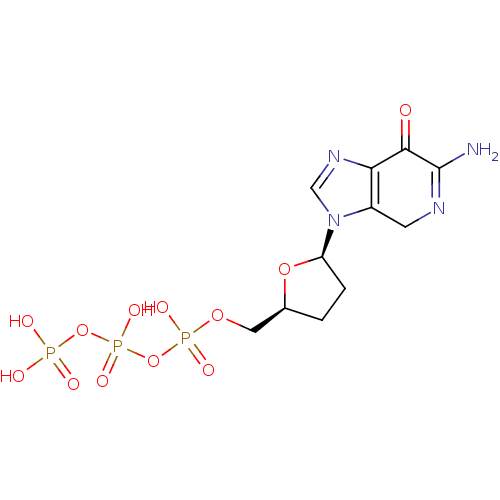 (2'-3'-dideoxy-7-deaza-guaninetriphosphate | CHEMBL...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to wild type HIV-1 p66/p51 reverse transcriptase/nucleic acid/dTTP ternary complex using poly(rA)/oligo(dT) as templates in presence... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102266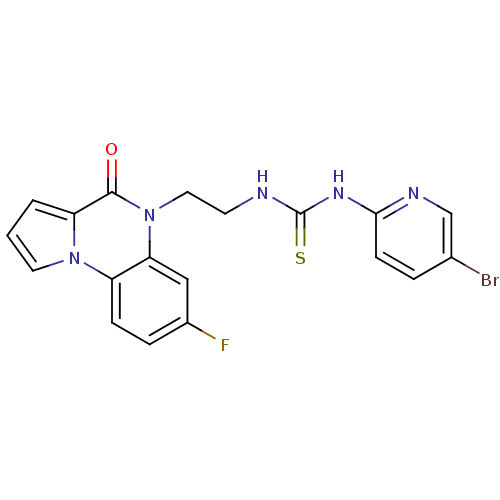 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102273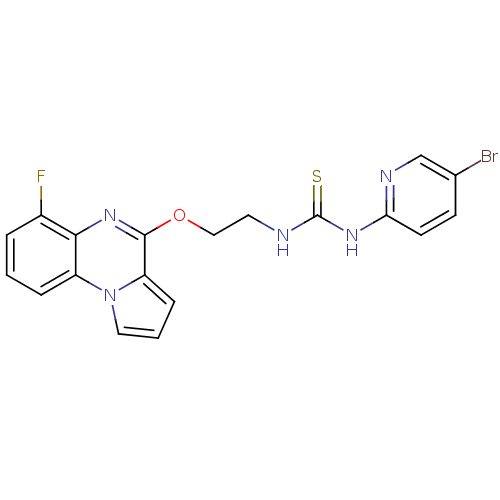 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-pyrrolo[1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082062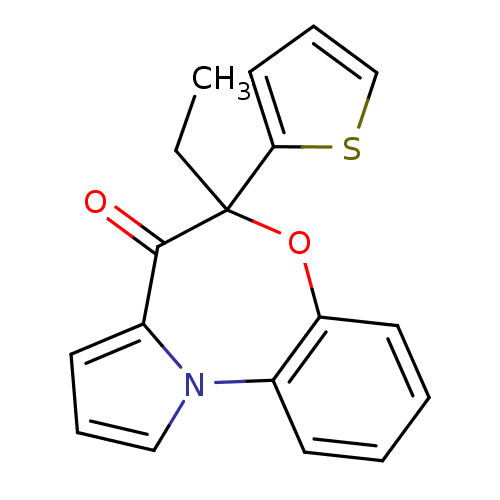 (5-Ethyl-5-thiophen-2-yl-6-oxa-10b-aza-benzo[e]azul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 wild type reverse transcriptase (RT) | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082055 (5-Ethyl-5-m-tolyl-6-oxa-10,10b-diaza-benzo[e]azule...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution K103N | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102277 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,9-difluoro-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to free form of wild type HIV-1 p66/p51 reverse transcriptase using poly(rA)/oligo(dT) as templates in presence of [3H]dTTP | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164652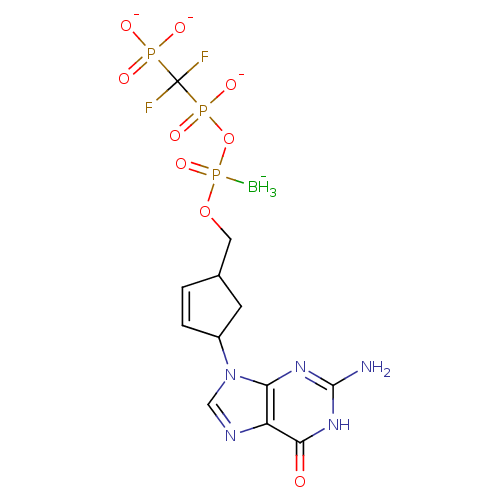 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50556159 (CHEMBL4784247) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to wild type HIV-1 p66/p51 reverse transcriptase/nucleic acid binary complex using poly(rA)/oligo(dT) as templates in presence of [3... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112696 BindingDB Entry DOI: 10.7270/Q21C21HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164654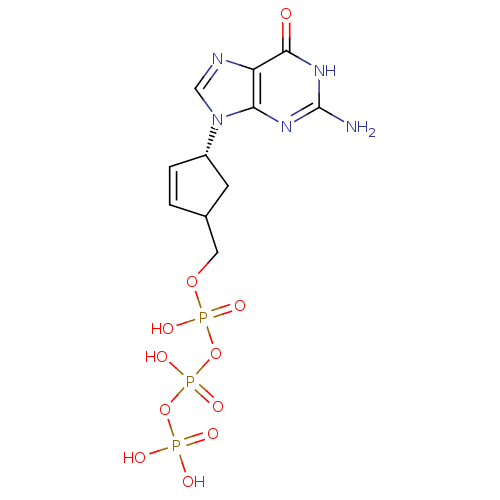 (CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropuri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102266 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102270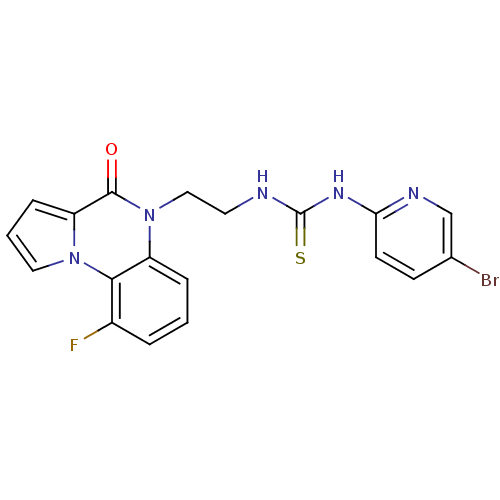 (1-(5-Bromo-pyridin-2-yl)-3-[2-(9-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082060 (5-Ethyl-5-phenyl-6-oxa-10,10b-diaza-benzo[e]azulen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution L100I | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082055 (5-Ethyl-5-m-tolyl-6-oxa-10,10b-diaza-benzo[e]azule...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution L100I | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164637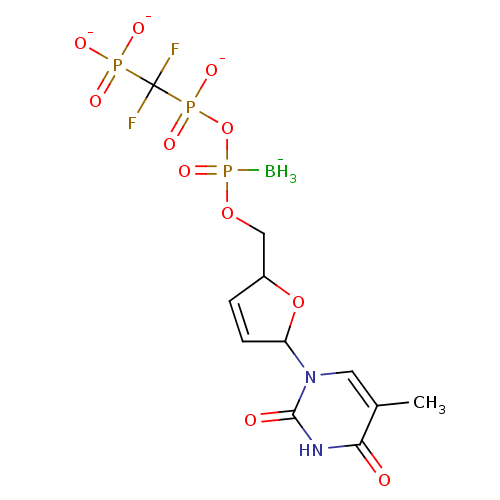 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082056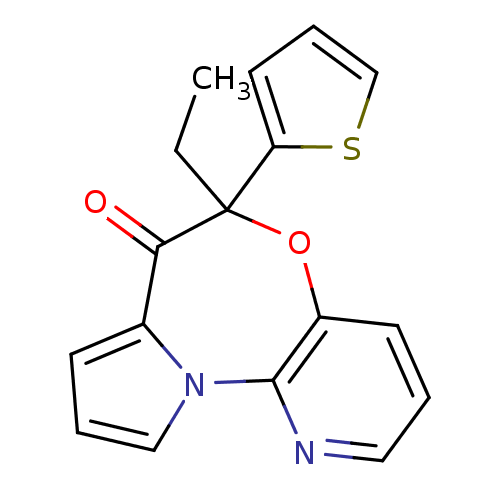 (5-Ethyl-5-thiophen-2-yl-6-oxa-10,10b-diaza-benzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution K103N | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164638 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164647 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102271 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-oxo-4H-5,9,9b-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50145605 (4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102267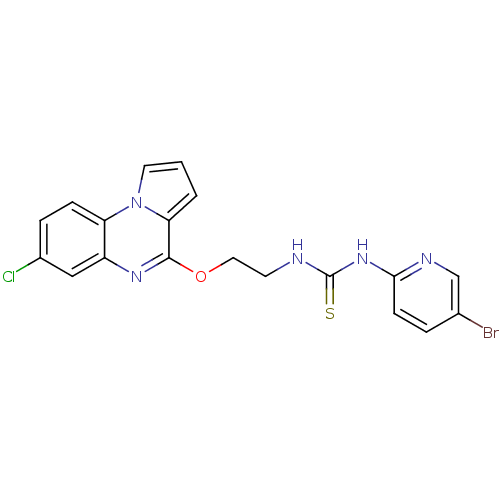 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7-chloro-pyrrolo[1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102273 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-pyrrolo[1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102275 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-oxo-4H-pyrrolo[1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 wild type reverse transcriptase (RT) | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102270 (1-(5-Bromo-pyridin-2-yl)-3-[2-(9-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164648 (2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 3750 total ) | Next | Last >> |