

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36548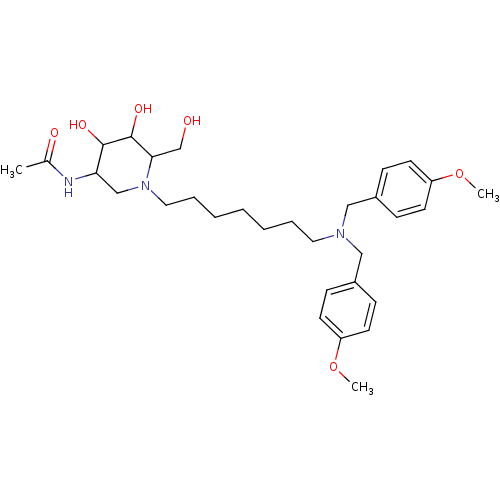 (N-[1-(7-{bis[(4-methoxyphenyl)methyl]amino}heptyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | -53.2 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36547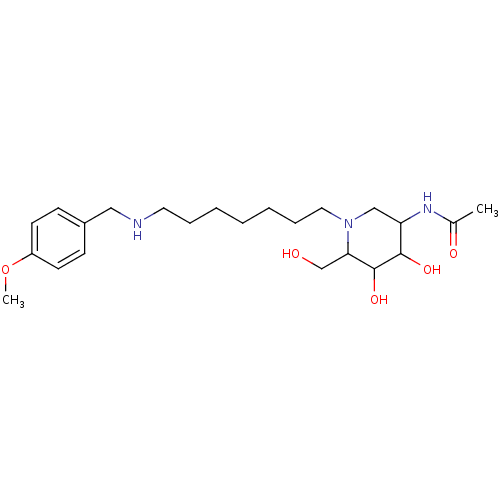 (N-[4,5-dihydroxy-6-(hydroxymethyl)-1-(7-{[(4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | -51.8 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36546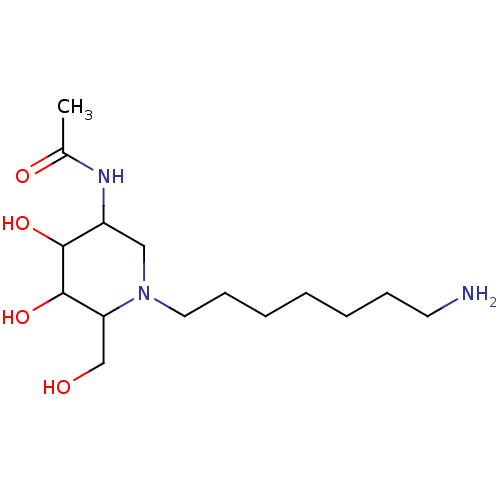 (N-[1-(7-aminoheptyl)-4,5-dihydroxy-6-(hydroxymethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -50.4 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40762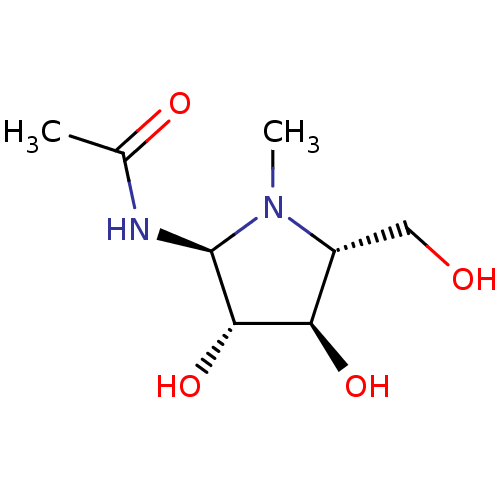 (Iminocyclitol, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36549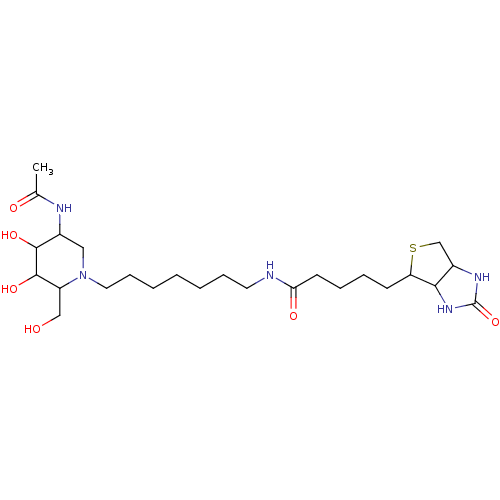 (N-{7-[5-acetamido-3,4-dihydroxy-2-(hydroxymethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26.7 | -44.0 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40761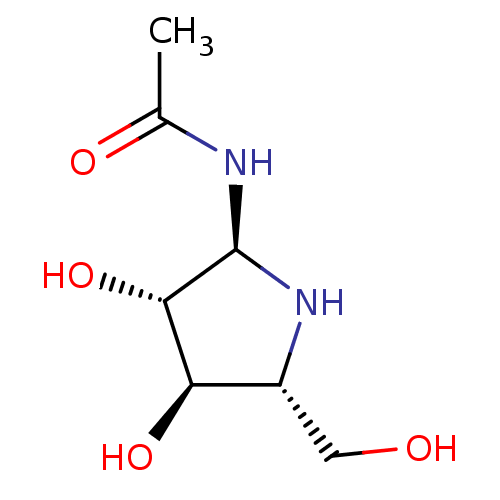 (Iminocyclitol, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40760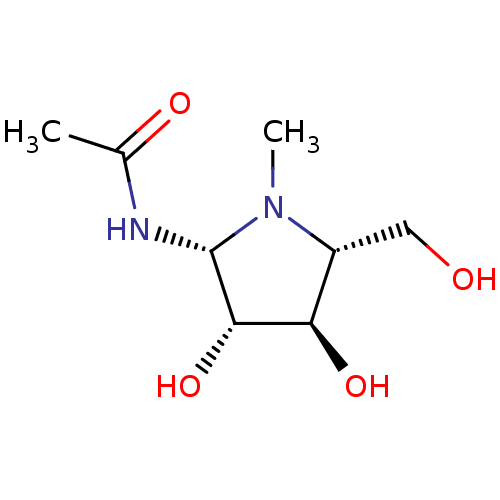 (Iminocyclitol, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513954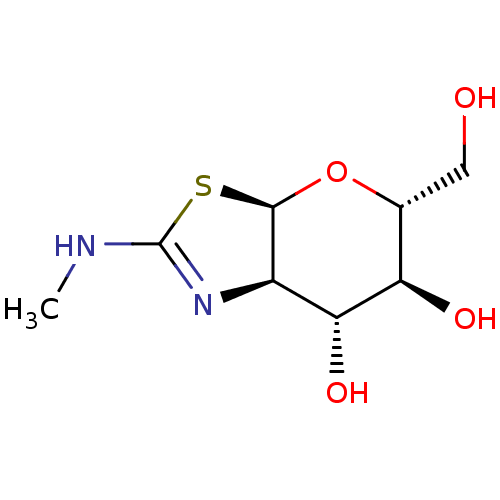 (CHEMBL4527453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl 2-acetamido-2-deoxy-beta-D-glucopyranoside as subs... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50327038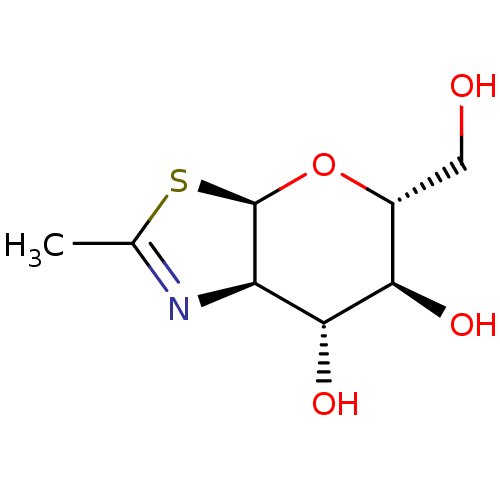 ((3aR,5R,6S,7R,7aR)-5-(hydroxymethyl)-2-methyl-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of human HexB | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40759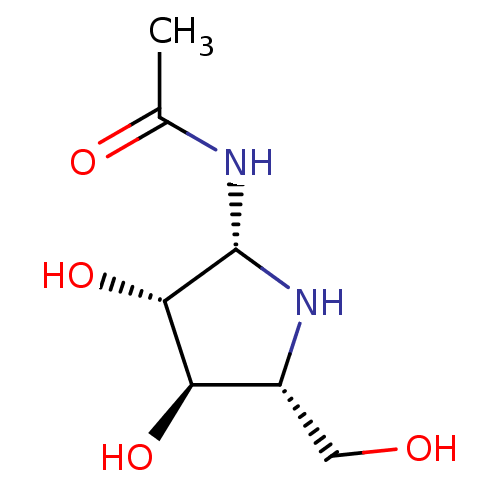 (Iminocyclitol, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50327038 ((3aR,5R,6S,7R,7aR)-5-(hydroxymethyl)-2-methyl-5,6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Competitive inhibition of human beta-N-acetyl-D-hexosaminidase-B using 4-Methylumbelliferyl N-acetyl-beta-D-glucosaminide as substrate assessed as re... | ACS Med Chem Lett 4: 527-31 (2013) Article DOI: 10.1021/ml300475m BindingDB Entry DOI: 10.7270/Q2HX1GMT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50182804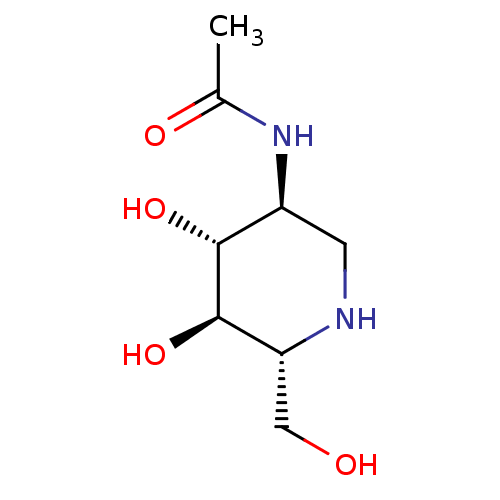 (2-ACETAMIDO-1,2-DIDEOXYNOJIRMYCIN | CHEMBL382689 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 540 | -36.4 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205423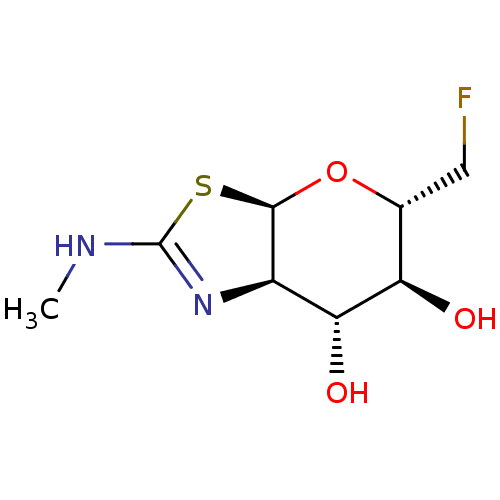 (US9243020, 17 | US9815861, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40763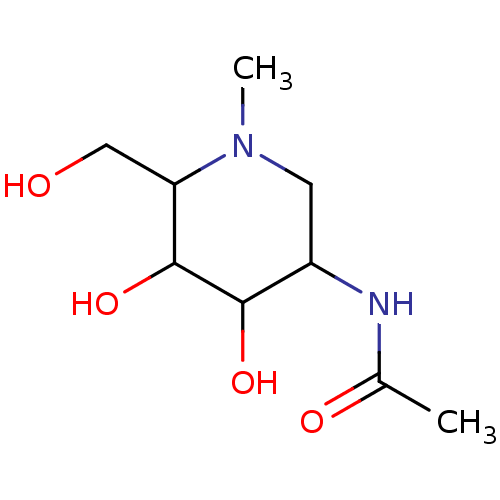 (Iminocyclitol, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50182804 (2-ACETAMIDO-1,2-DIDEOXYNOJIRMYCIN | CHEMBL382689 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507470 (CHEMBL4448965) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513934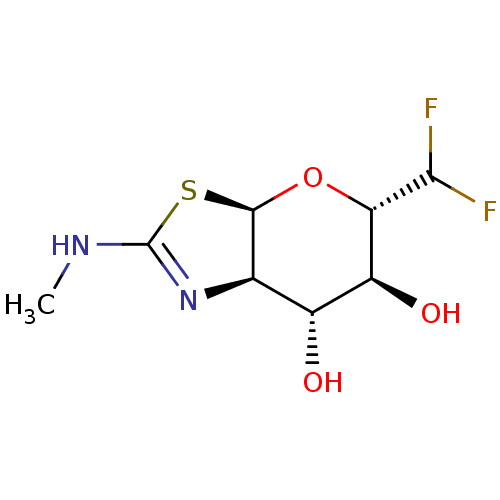 (CHEMBL4443587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513958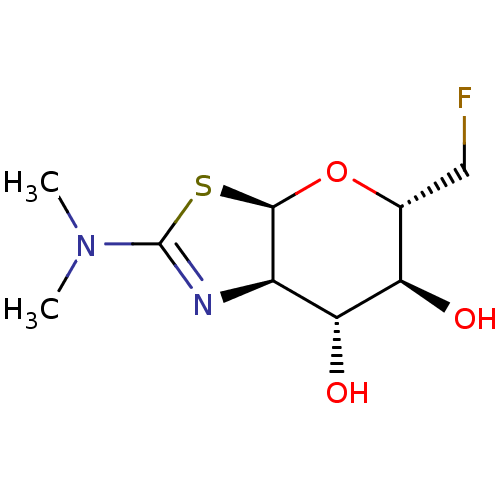 (CHEMBL4473252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507475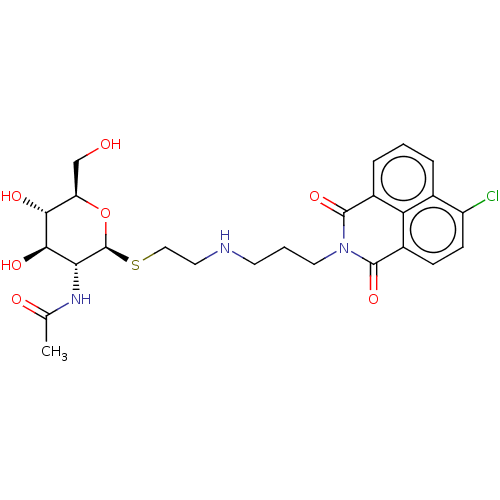 (CHEMBL4435797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507463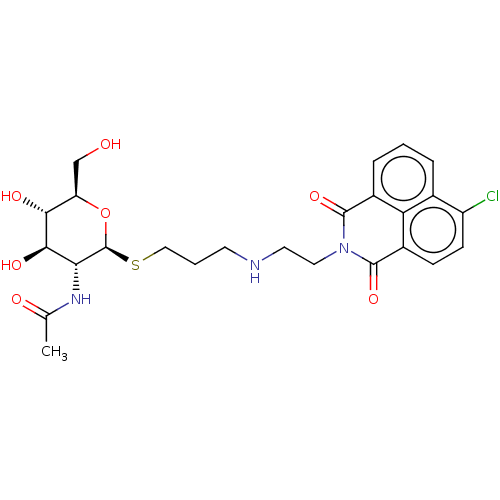 (CHEMBL4452208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36550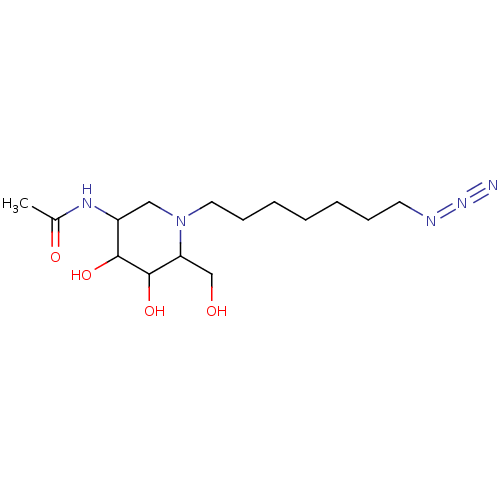 (N-[1-(7-azidoheptyl)-4,5-dihydroxy-6-(hydroxymethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507469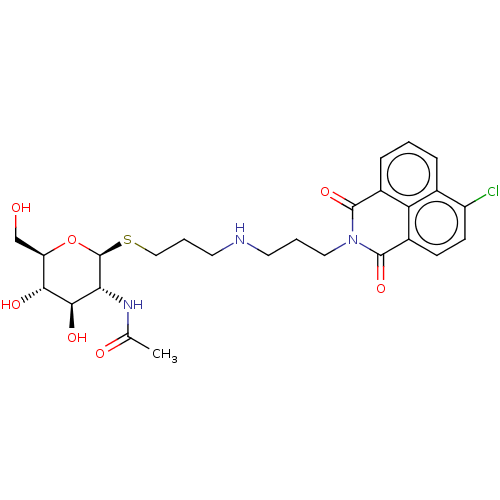 (CHEMBL4454357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507472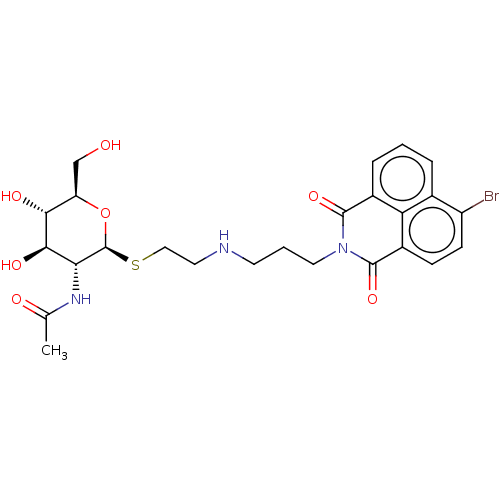 (CHEMBL4548064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507462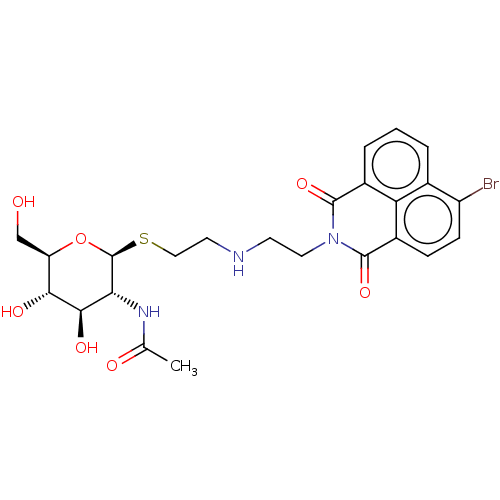 (CHEMBL4548738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513955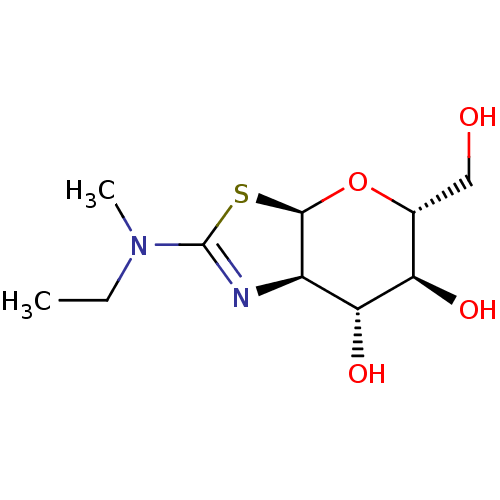 (CHEMBL4483089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205425 (US9243020, 20 | US9815861, Example 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507476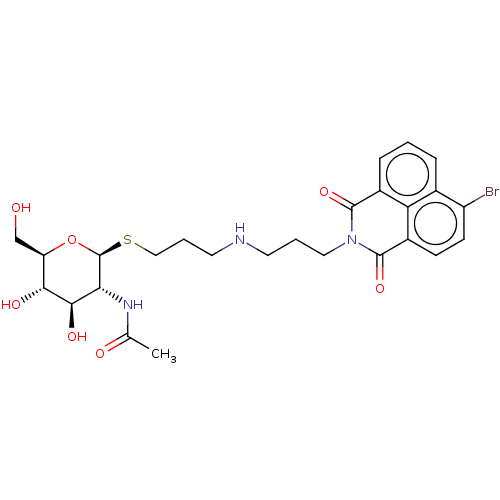 (CHEMBL4555514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM141965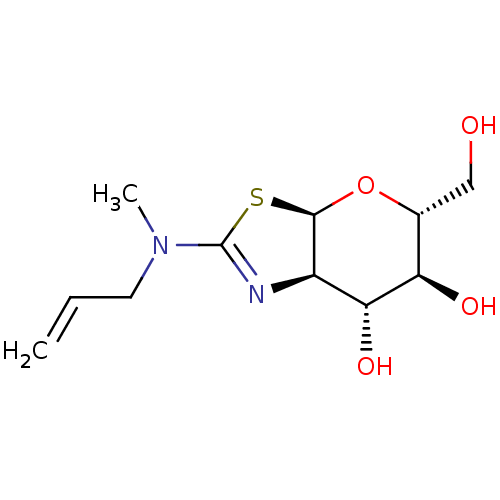 (US8927507, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM40765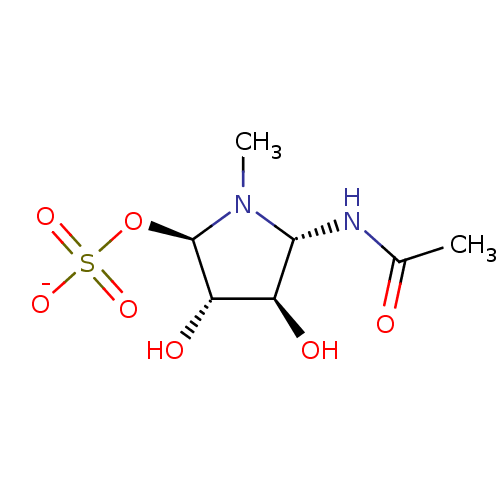 (Iminocyclitol, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Hexosaminidase activity assay was measured by fluorometry, VersaFluor Fluorometer from Bio-Rad. | Chem Biol 8: 701-11 (2001) Article DOI: 10.1016/S1074-5521(01)00045-X BindingDB Entry DOI: 10.7270/Q28C9TNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50507471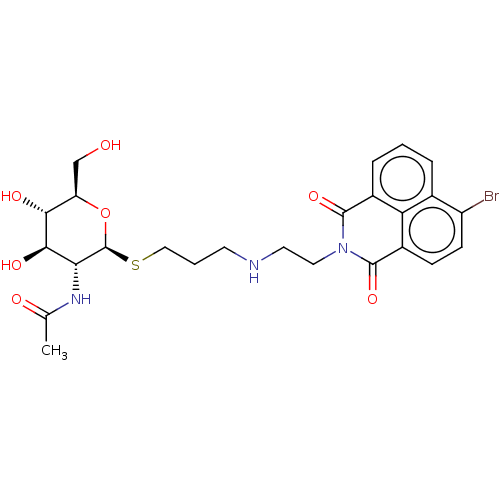 (CHEMBL4540913) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Agricultural University Curated by ChEMBL | Assay Description Inhibition of recombinant human His6-tagged HexB expressed in Pichia pastoris using varying levels of 4-Mu-GlcNAc as substrate preincubated for 10 mi... | ACS Med Chem Lett 9: 1241-1246 (2018) Article DOI: 10.1021/acsmedchemlett.8b00406 BindingDB Entry DOI: 10.7270/Q2ZS30VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205422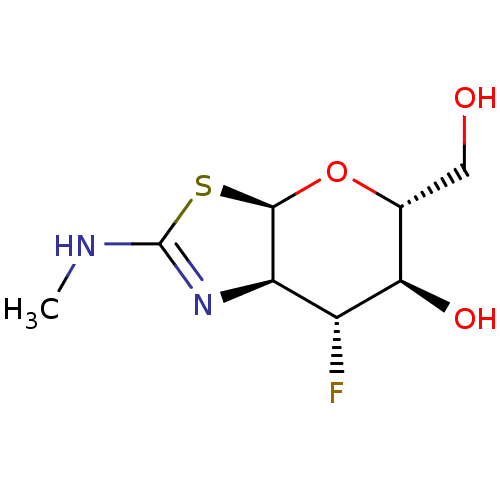 (US9243020, 9 | US9815861, Example 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513949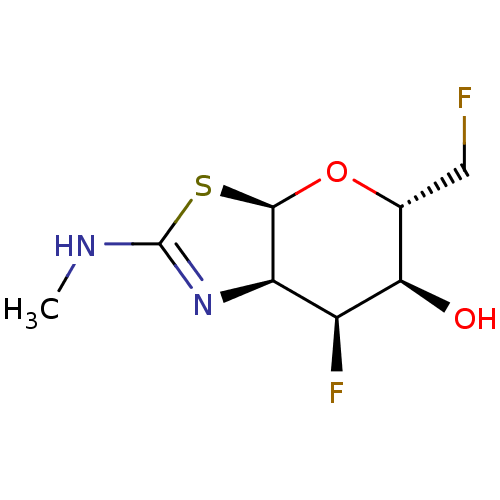 (CHEMBL4476839) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513960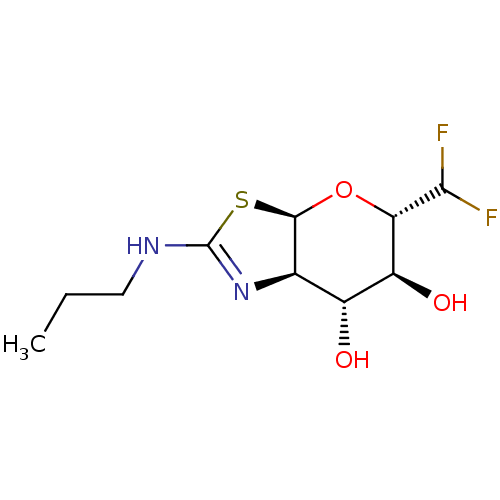 (CHEMBL4561925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205420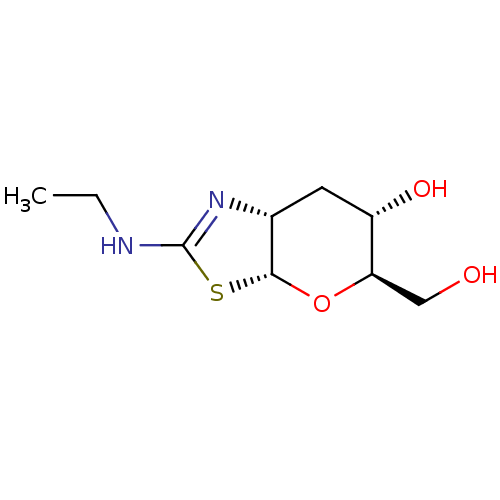 (US9243020, 2 | US9815861, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50323697 ((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513957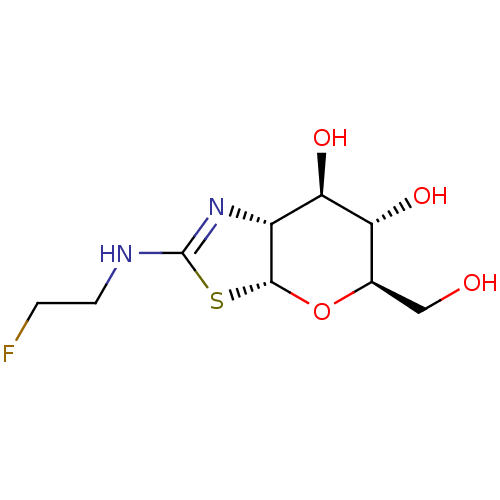 (CHEMBL4456515) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513965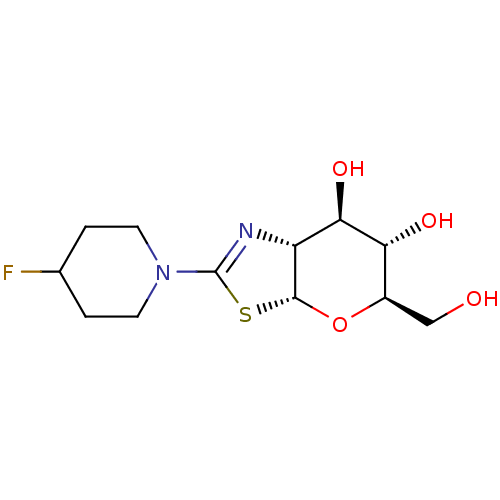 (CHEMBL4578331) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513964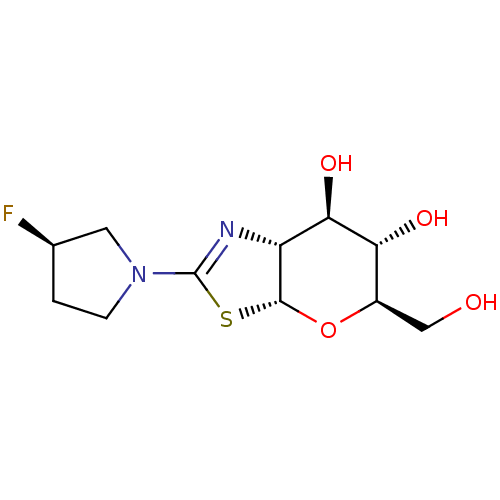 (CHEMBL4454942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205428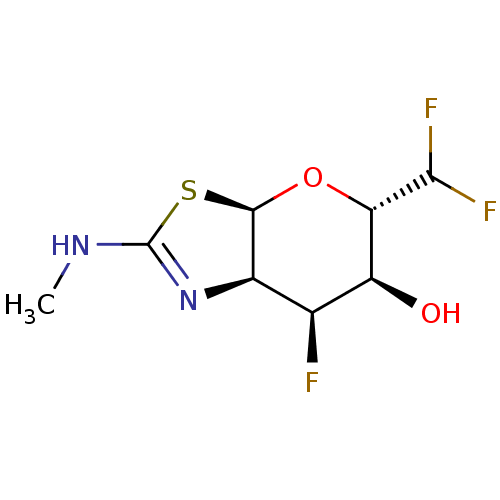 (US9243020, 33 | US9815861, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513931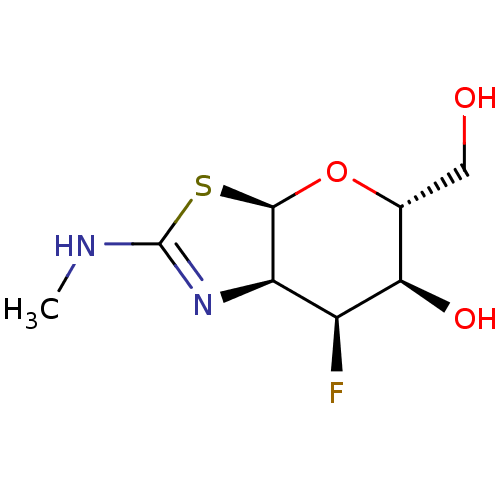 (CHEMBL4444446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513959 (CHEMBL4465511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM142083 (US8933040, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM205426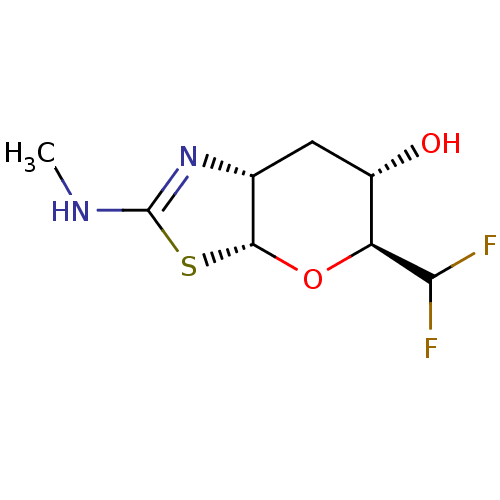 (US9243020, 27 | US9815861, Example 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513956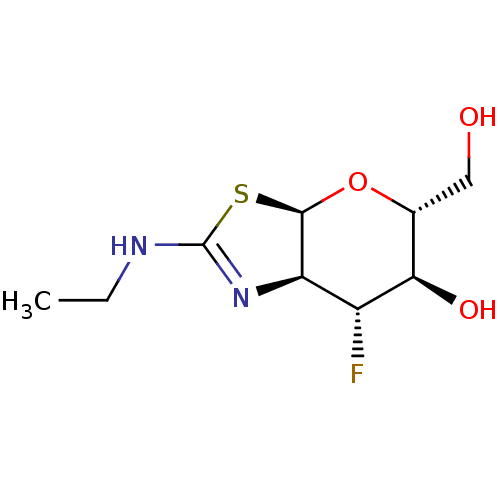 (CHEMBL4448352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513937 (CHEMBL4520749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513941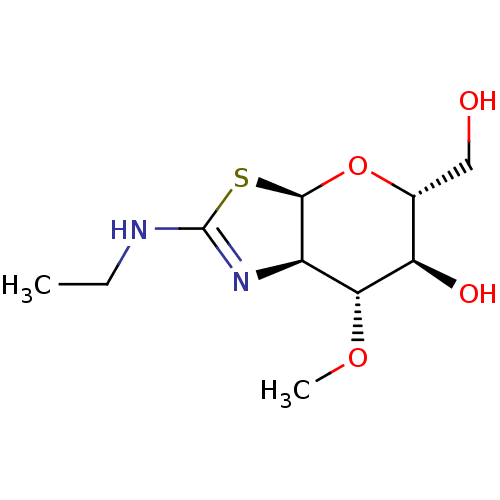 (CHEMBL4519186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513953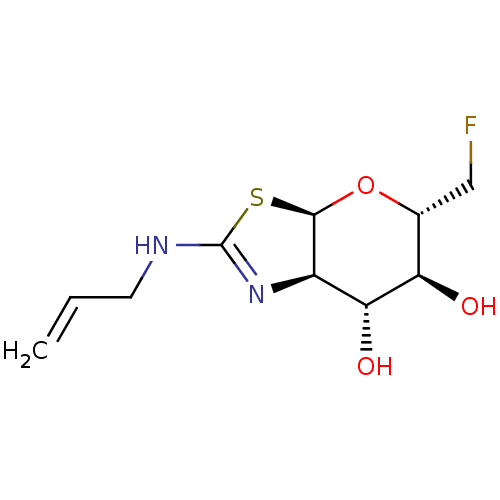 (CHEMBL4449194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513952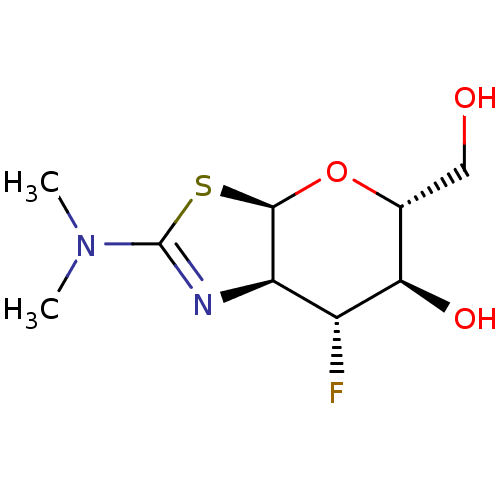 (CHEMBL4576476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513951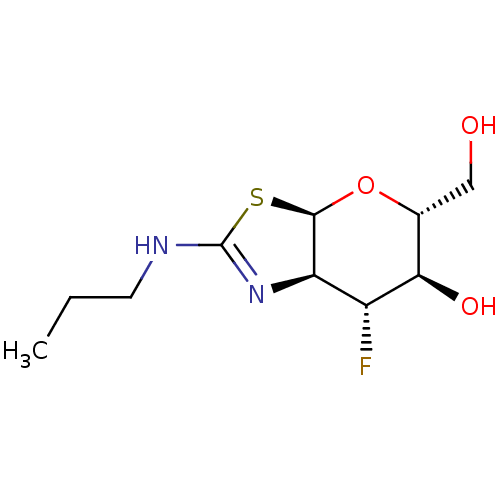 (CHEMBL4583010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM50513938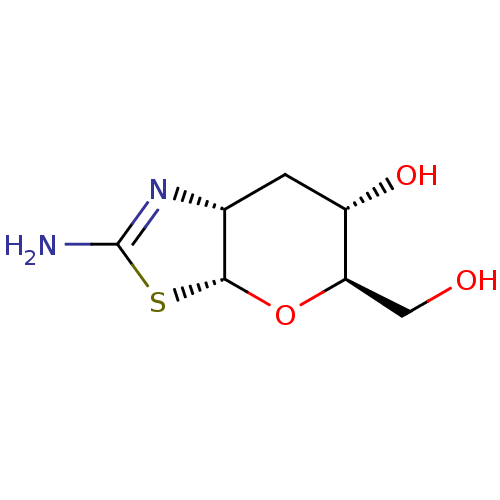 (CHEMBL4552866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... | J Med Chem 62: 10062-10097 (2019) Article DOI: 10.1021/acs.jmedchem.9b01090 BindingDB Entry DOI: 10.7270/Q21G0QMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |