

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8977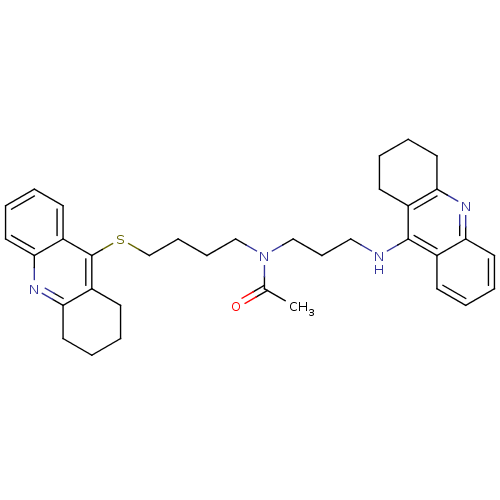 (CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8975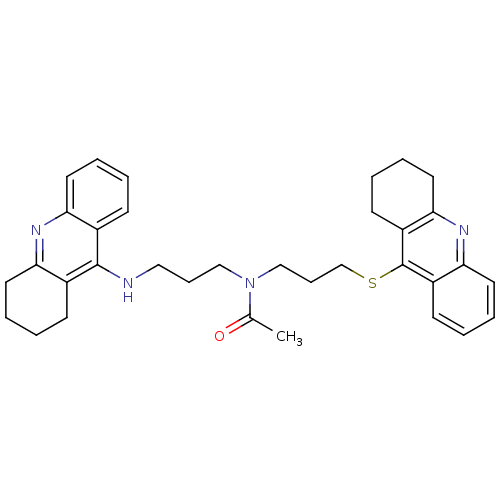 (CHEMBL179192 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8971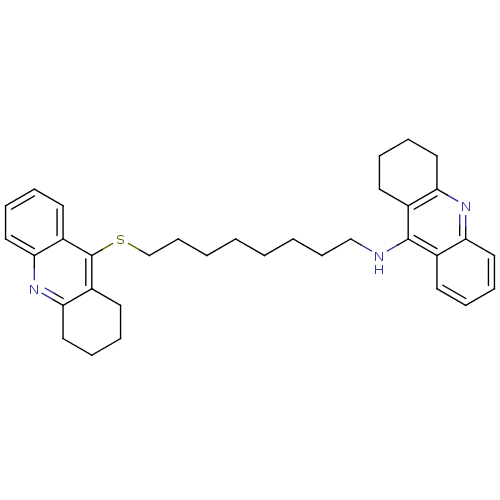 (CHEMBL129108 | N-[8-(1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8976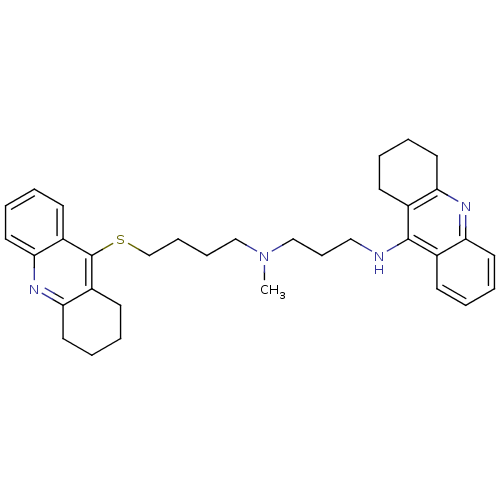 (CHEMBL175555 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8967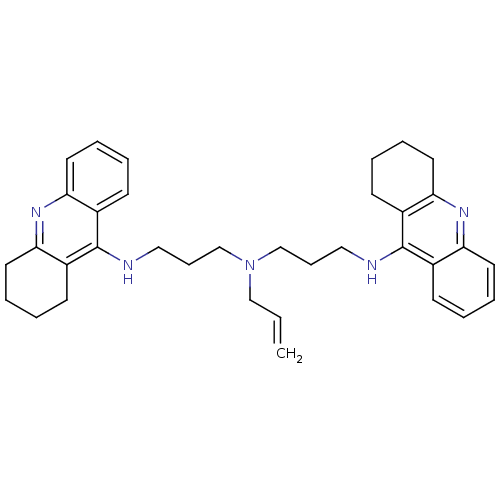 (N,N-Bis[3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8966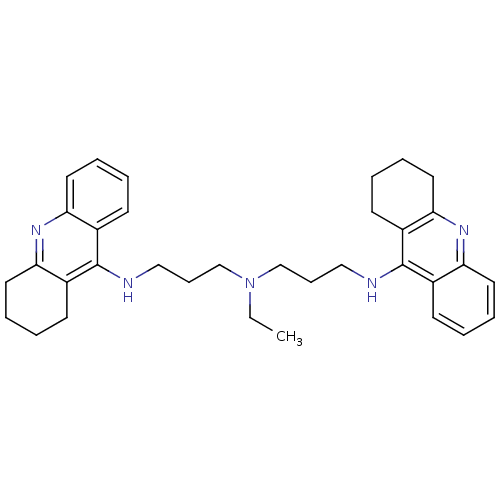 (N,N-Bis[3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232997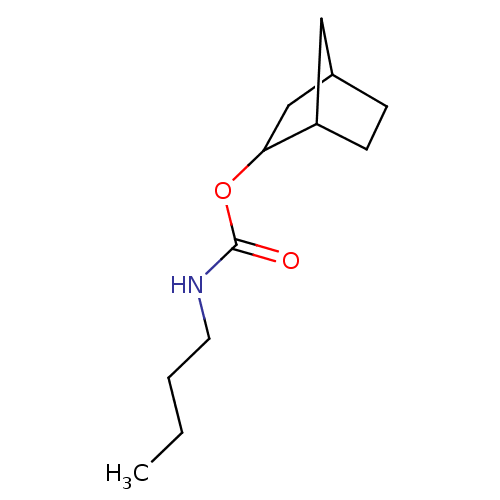 ((S)-(-)-endo-2-norbornyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8964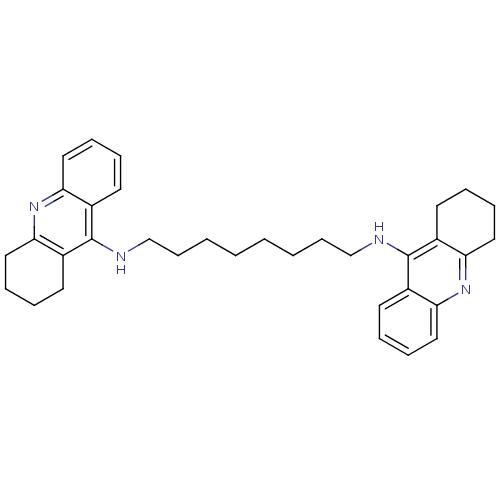 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8969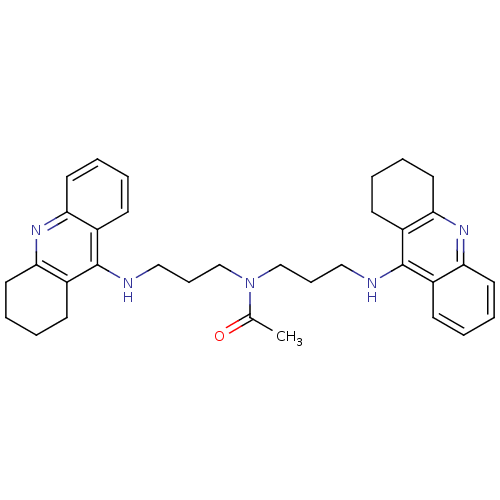 (CHEMBL131410 | N,N-bis[3-(1,2,3,4-tetrahydroacridi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8972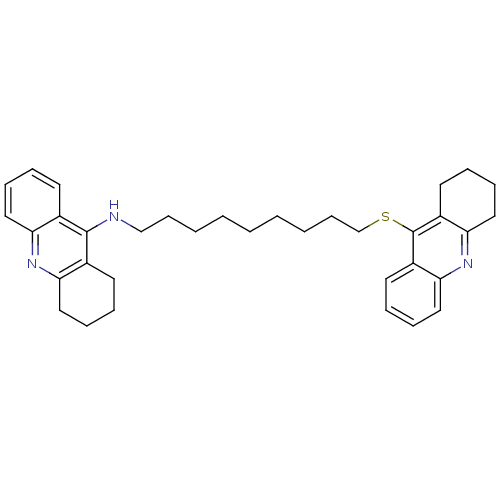 (N-(1,2,3,4-Tetrahydroacridin-9-yl)-9-[(1,2,3,4-tet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232998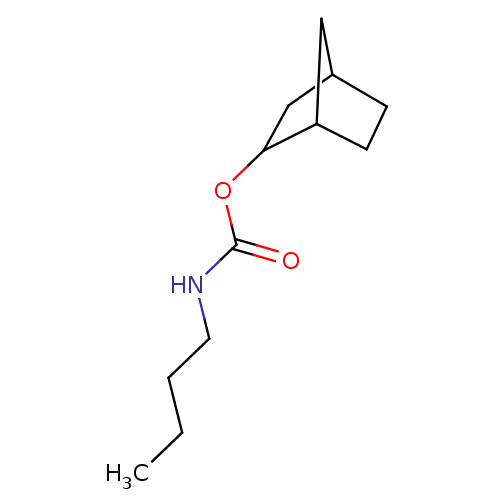 ((R)-(+)-endo-2-norbornyl-N-n-butylcarbamate | rac-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8968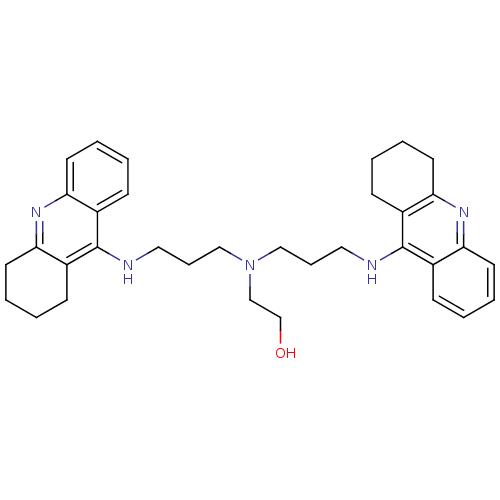 (2-{bis[3-(1,2,3,4-tetrahydroacridin-9-ylamino)prop...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8974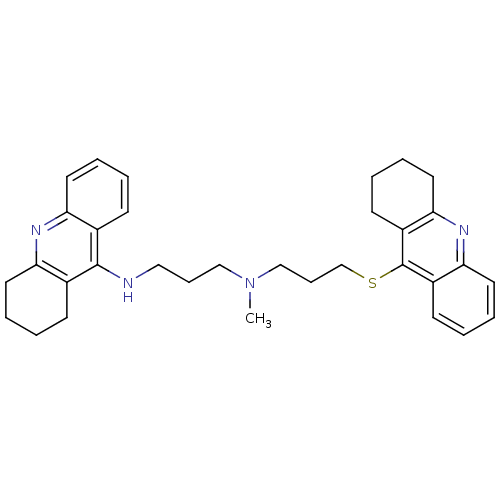 (CHEMBL367067 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232998 ((R)-(+)-endo-2-norbornyl-N-n-butylcarbamate | rac-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8965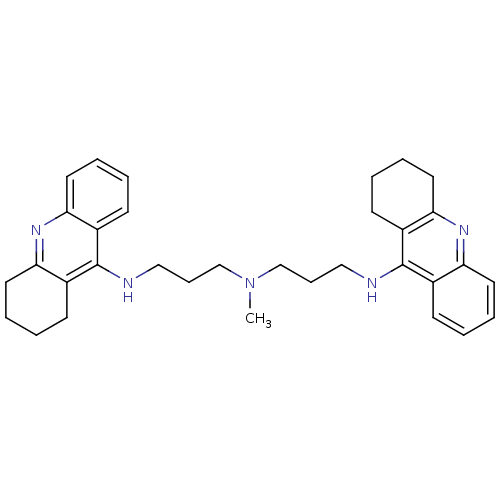 (CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016848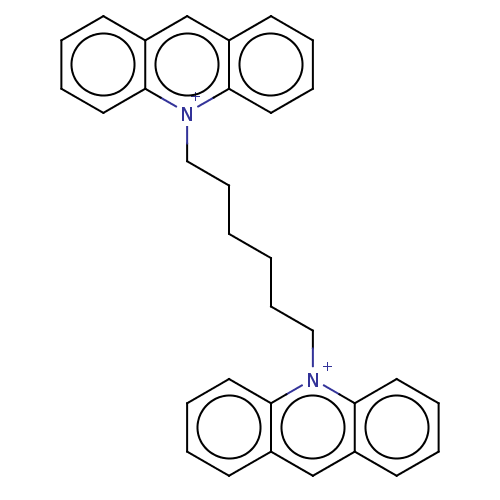 (CHEMBL3276408) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135653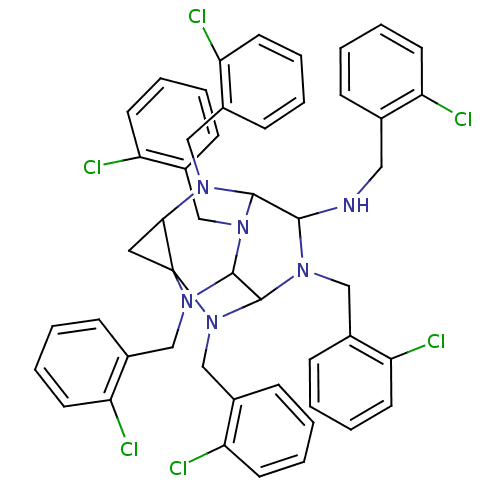 (2,4,6,8,10,12-hexa(2-chloro phenylmethyl)-2,4,6,8,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016871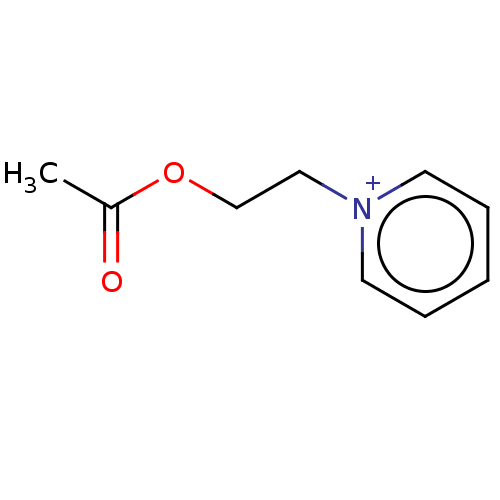 (CHEMBL3276432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016875 (CHEMBL3276431) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232995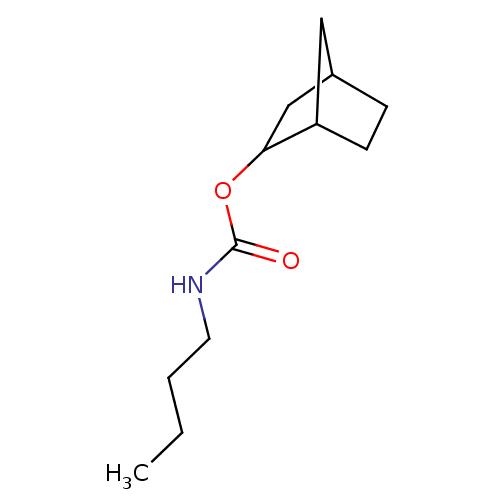 ((R)-(+)-exo-2-norbonyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8958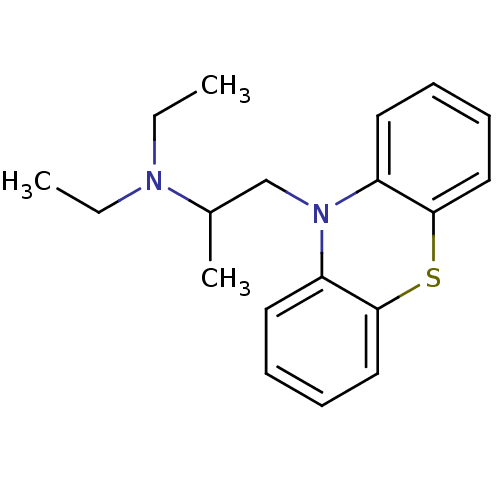 (10-(2-diethylaminopropyl)phenothiazine | CHEMBL120...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50072037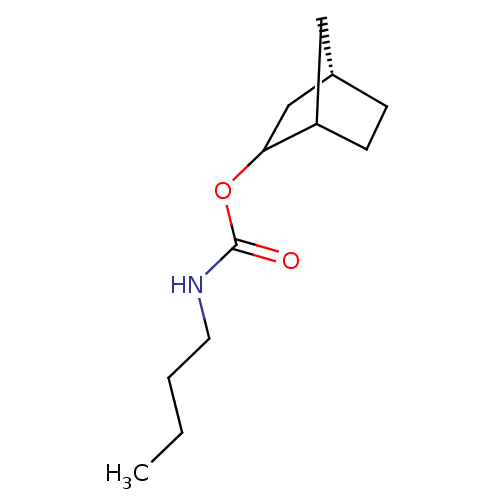 (Butyl-carbamic acid (S)-bicyclo[2.2.1]hept-2-yl es...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Compound was evaluated for irreversible inhibition of Horse serum Butyrylcholinesterase | Bioorg Med Chem Lett 8: 2747-50 (1999) BindingDB Entry DOI: 10.7270/Q2WD4133 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50072037 (Butyl-carbamic acid (S)-bicyclo[2.2.1]hept-2-yl es...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Compound was evaluated for irreversible inhibition of Horse serum Butyrylcholinesterase | Bioorg Med Chem Lett 8: 2747-50 (1999) BindingDB Entry DOI: 10.7270/Q2WD4133 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016848 (CHEMBL3276408) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 25 | -44.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8973 (N-(1,2,3,4-Tetrahydroacridin-9-yl)-N-[8-(1,2,3,4-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016849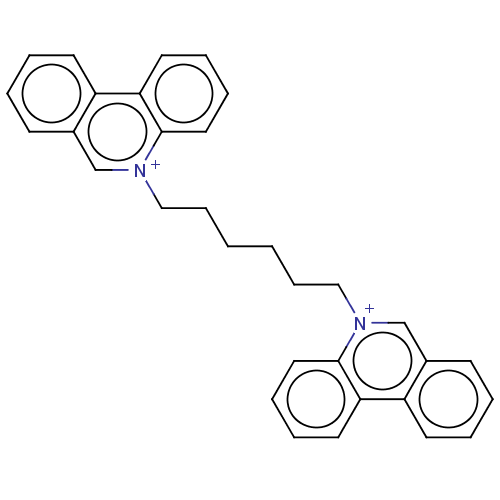 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232999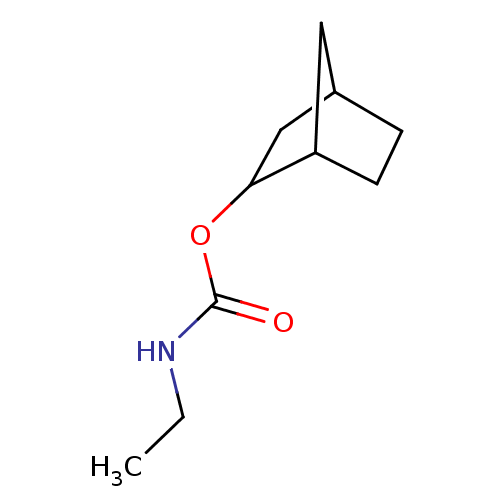 (rac-(±)-exo-2-norbornyl carbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10525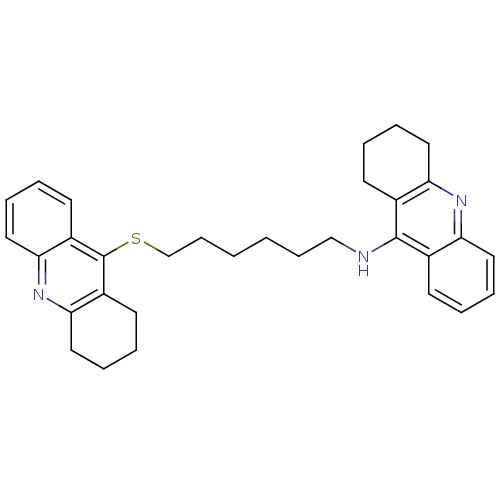 (N-[6-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)hexyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8970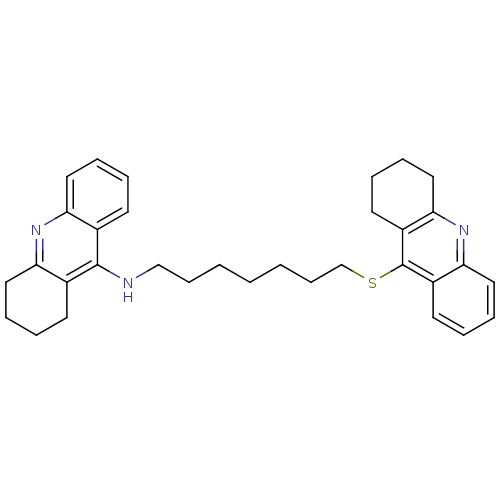 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8970 (CHEMBL51197 | N-[7-(1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | -42.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10526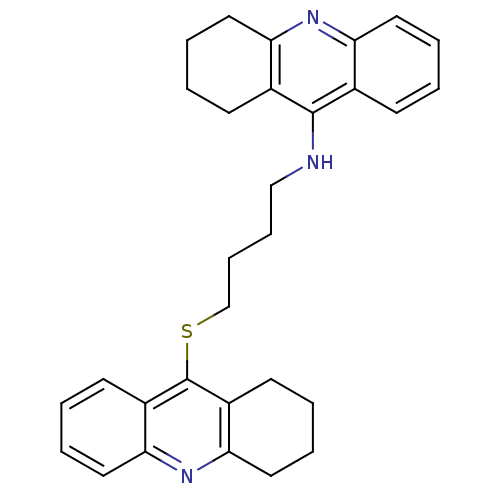 (N-[4-(1,2,3,4-tetrahydroacridin-9-ylsulfanyl)butyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10524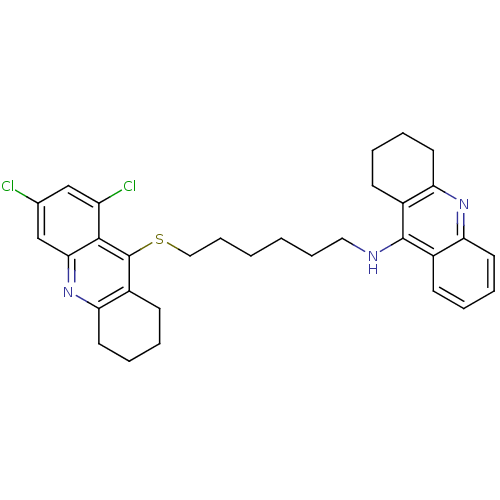 (N-{6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10516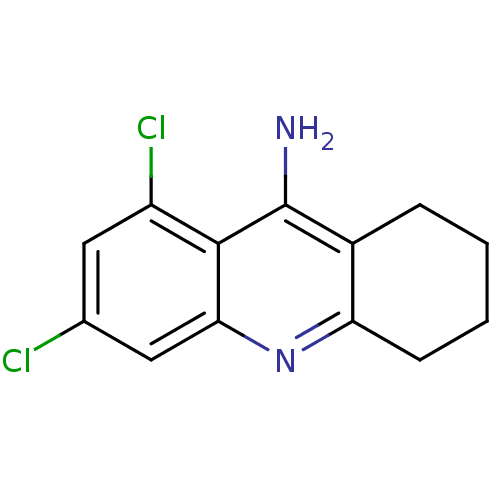 (6,8-dichloro-1,2,3,4-tetrahydroacridin-9-amine | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM232996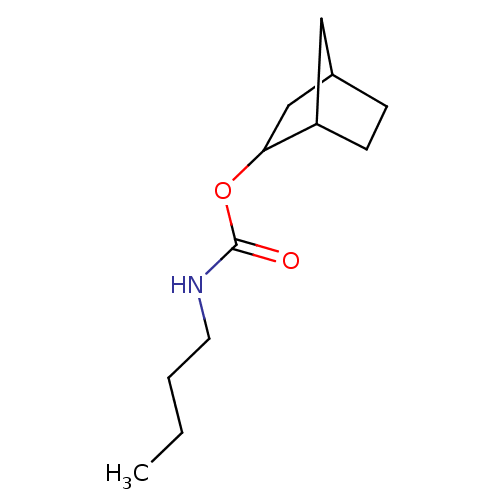 ((S)-(-)-exo-2-norbonyl-N-n-butylcarbamate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Chung Shan Medical University Hospital | Assay Description BChE inhibitions by the carbamate inhibitors were assayed by the Ellman method [Ellman et al., Biochem. Pharm., 7:88-95]. BChE-catalyzed hydrolysis o... | J Enzyme Inhib Med Chem 25: 13-20 (2010) Article DOI: 10.3109/14756360902888200 BindingDB Entry DOI: 10.7270/Q2C8286J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016828 (CHEMBL3276418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016851 (CHEMBL3276412) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50135654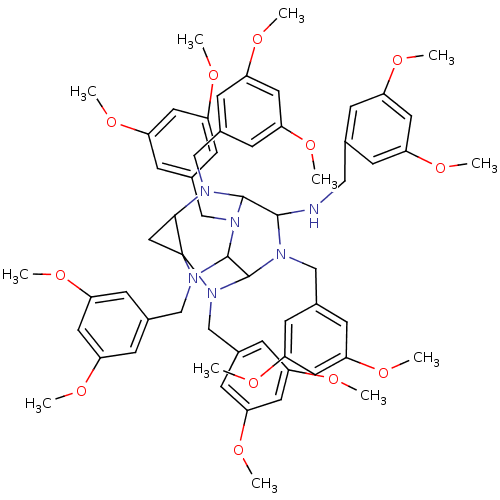 (2,4,6,8,10,12-hexa(3,5-dimethoxyphenylmethyl)-2,4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chung-Hsing University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Butyrylcholinesterase | Bioorg Med Chem Lett 13: 2887-90 (2003) BindingDB Entry DOI: 10.7270/Q29C6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016827 (CHEMBL3276417) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10522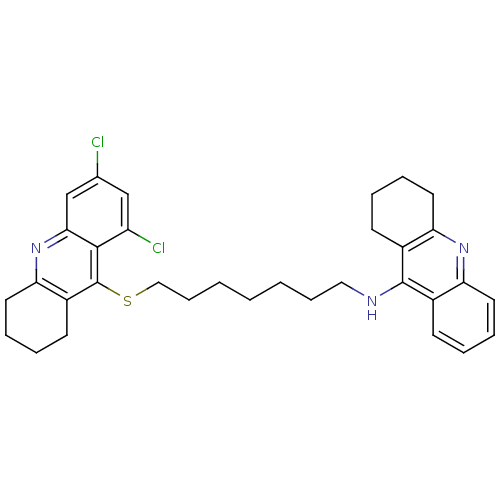 (N-{7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | -40.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016849 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016870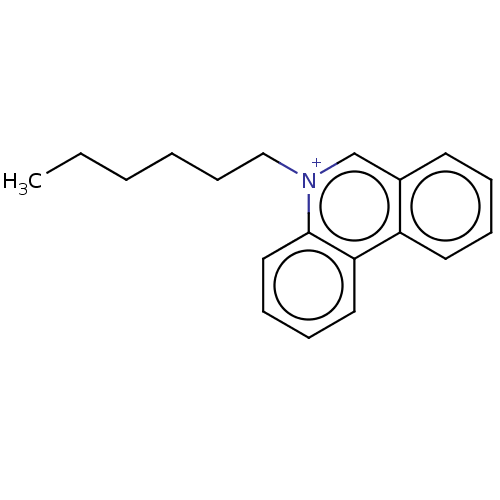 (CHEMBL3276416) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016872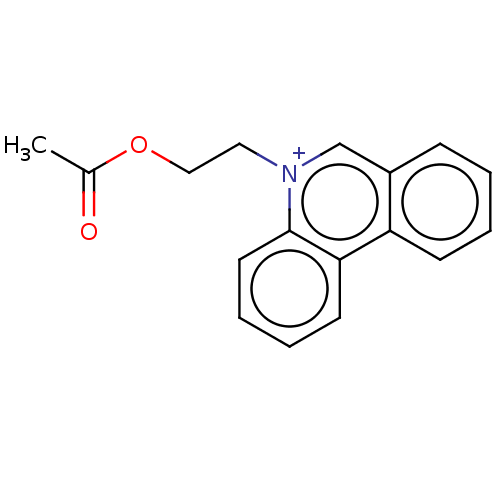 (CHEMBL3276421) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10519 (CHEMBL51350 | N-{7-[(6,8-dichloro-1,2,3,4-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | -38.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita degli Studi di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | Bioorg Med Chem Lett 11: 1779-82 (2001) Article DOI: 10.1016/s0960-894x(01)00294-3 BindingDB Entry DOI: 10.7270/Q2DZ06JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016851 (CHEMBL3276412) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1776 total ) | Next | Last >> |