 Found 719 hits Enz. Inhib. hit(s) with Target = 'Arginase-1'
Found 719 hits Enz. Inhib. hit(s) with Target = 'Arginase-1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arginase-1
(Homo sapiens (Human)) | BDBM50561046

(CHEMBL4790798) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561045

(CHEMBL4758805)Show SMILES NC(CCCCB(O)O)(CCCN1CCC(O)(CC1)c1ccc(Cl)cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 using L-arginine as substrate after 60 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316603
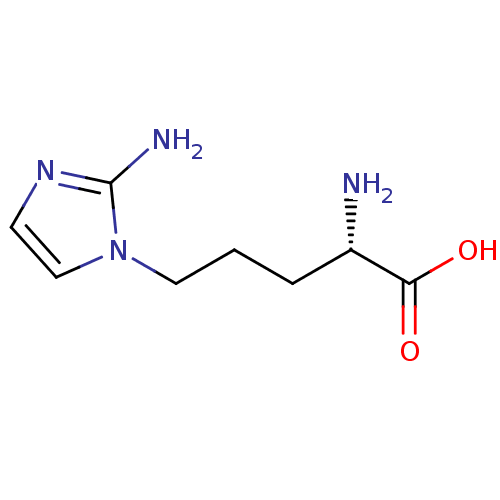
(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50375814
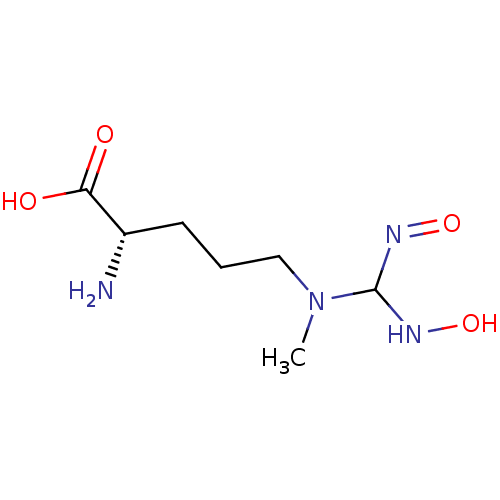
(CHEMBL260628)Show InChI InChI=1S/C7H16N4O4/c1-11(7(9-14)10-15)4-2-3-5(8)6(12)13/h5,7,9,14H,2-4,8H2,1H3,(H,12,13)/t5-,7?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver arginase |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50230418
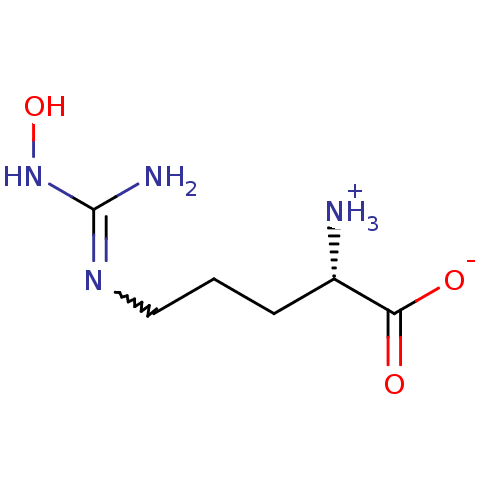
(CHEMBL260629 | N(gamma)-hydroxy-L-arginine | N-OME...)Show SMILES NC(NO)=NCCC[C@H]([NH3+])C([O-])=O |r,w:4.4| Show InChI InChI=1S/C6H14N4O3/c7-4(5(11)12)2-1-3-9-6(8)10-13/h4,13H,1-3,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver arginase |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Rattus norvegicus) | BDBM50354832

(CHEMBL1834160)Show InChI InChI=1S/C7H13NO3/c8-6(7(10)11)4-2-1-3-5-9/h5-6H,1-4,8H2,(H,10,11)/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50553165
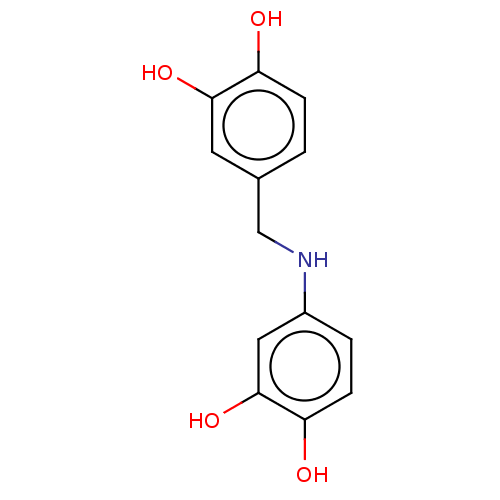
(CHEMBL4754674) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50553165

(CHEMBL4754674) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50553165

(CHEMBL4754674) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | CHEMBL5287570

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50045936
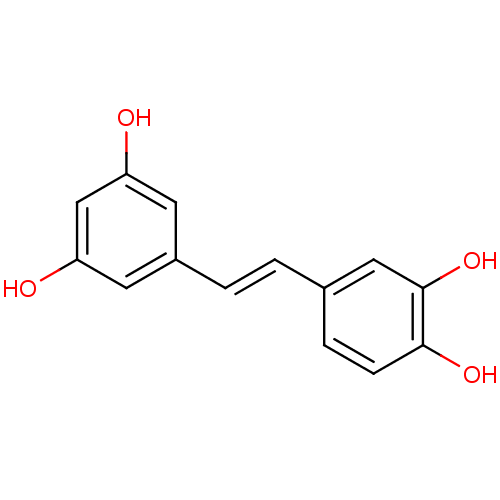
((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...)Show InChI InChI=1S/C14H12O4/c15-11-5-10(6-12(16)8-11)2-1-9-3-4-13(17)14(18)7-9/h1-8,15-18H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Cornish-Bowden plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50045936

((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...)Show InChI InChI=1S/C14H12O4/c15-11-5-10(6-12(16)8-11)2-1-9-3-4-13(17)14(18)7-9/h1-8,15-18H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50045936

((E)-4-(3,5-dihydroxystyryl)benzene-1,2-diol | (E)-...)Show InChI InChI=1S/C14H12O4/c15-11-5-10(6-12(16)8-11)2-1-9-3-4-13(17)14(18)7-9/h1-8,15-18H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of recombinant bovine liver ARGI using L-arginine as substrate incubated for 60 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00011f
BindingDB Entry DOI: 10.7270/Q261140V |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50462601
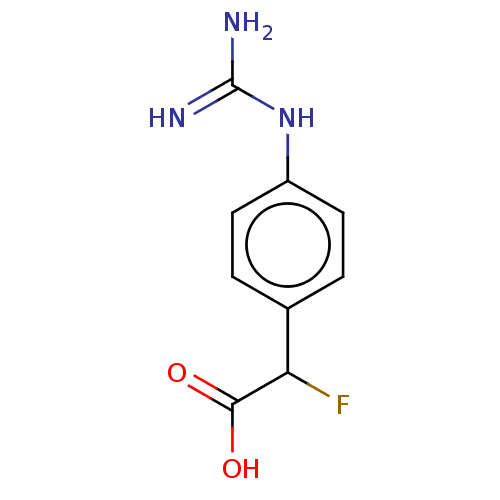
(CHEMBL4250607)Show InChI InChI=1S/C9H10FN3O2/c10-7(8(14)15)5-1-3-6(4-2-5)13-9(11)12/h1-4,7H,(H,14,15)(H4,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316607
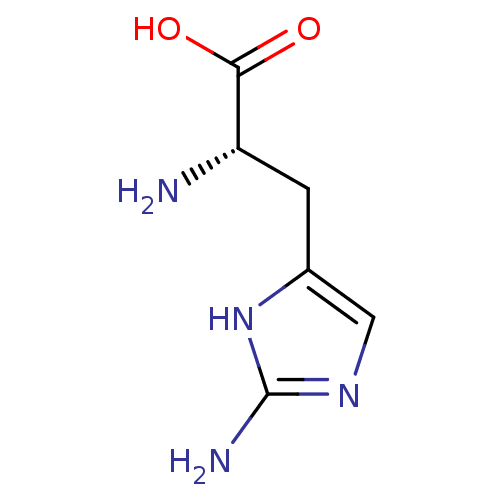
(2-amino-L-histidine | CHEMBL1099167 | L-2-aminohis...)Show InChI InChI=1S/C6H10N4O2/c7-4(5(11)12)1-3-2-9-6(8)10-3/h2,4H,1,7H2,(H,11,12)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arginase-1
(Homo sapiens (Human)) | BDBM50462600
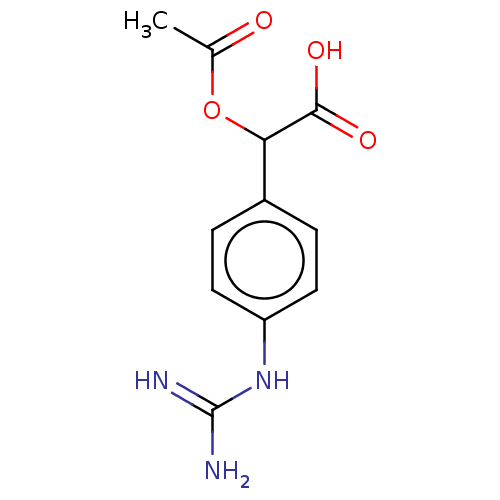
(CHEMBL4238387)Show InChI InChI=1S/C11H13N3O4/c1-6(15)18-9(10(16)17)7-2-4-8(5-3-7)14-11(12)13/h2-5,9H,1H3,(H,16,17)(H4,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316604
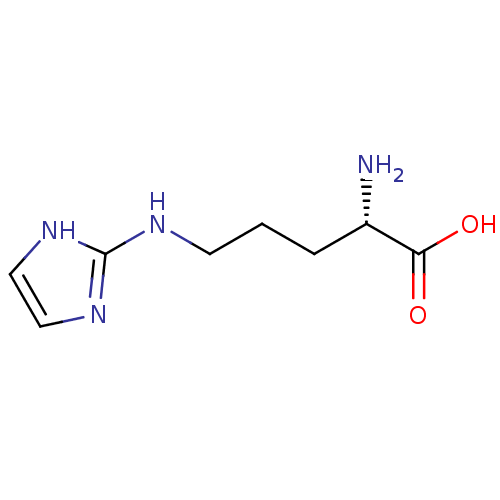
((S)-2-amino-5-(imidazol-2-ylamino)pentanoic acid |...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-3-10-8-11-4-5-12-8/h4-6H,1-3,9H2,(H,13,14)(H2,10,11,12)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316606

((2S)-2-amino-4-(2-amino-1H-imidazol-5-yl)butanoic ...)Show InChI InChI=1S/C7H12N4O2/c8-5(6(12)13)2-1-4-3-10-7(9)11-4/h3,5H,1-2,8H2,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arginase-1
(Homo sapiens (Human)) | BDBM50316608
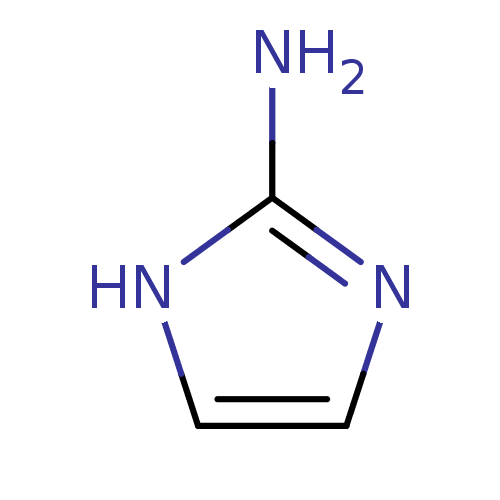
(1H-Imidazol-2-yl-ammonium | 1H-Imidazol-2-ylamine ...)Show InChI InChI=1S/C3H5N3/c4-3-5-1-2-6-3/h1-2H,(H3,4,5,6) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
Article
PubMed
| 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316603

(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| | 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316605
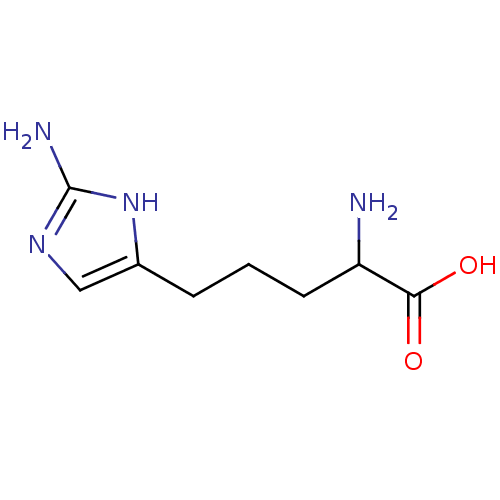
(2-amino-5-(2-aminoimidazol-4-yl)pentanoic acid | C...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)3-1-2-5-4-11-8(10)12-5/h4,6H,1-3,9H2,(H,13,14)(H3,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length arginase 1 expressed in Escherichia coli BL21(DE3) by fixed point assay |
J Med Chem 53: 4266-76 (2010)
Article DOI: 10.1021/jm100306a
BindingDB Entry DOI: 10.7270/Q2SQ90JK |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561047
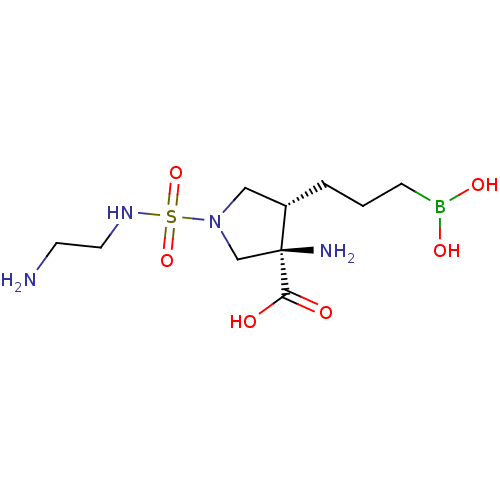
(CHEMBL4764455)Show SMILES NCCNS(=O)(=O)N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561040

(CHEMBL4754637)Show SMILES N[C@@H](C1CCCC1)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561042
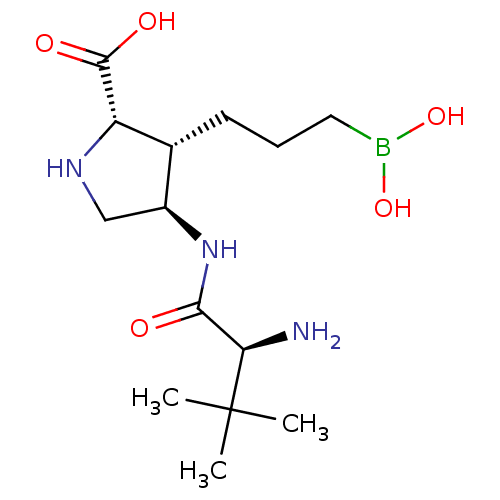
(CHEMBL4748950)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50595731
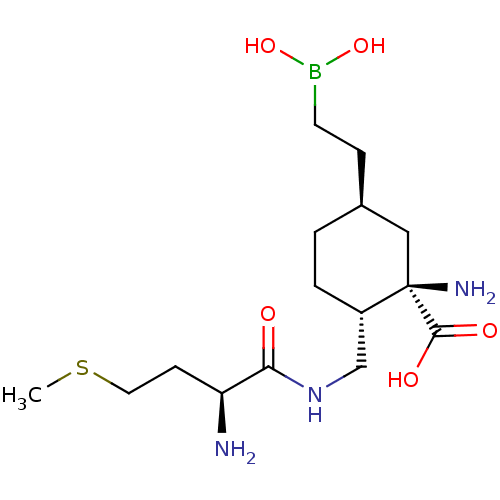
(CHEMBL5170454)Show SMILES CSCC[C@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561051

(CHEMBL4749434)Show SMILES C[C@H](N)C(=O)N1C[C@@H](CCB(O)O)C[C@@](N)([C@@H]1C)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561052

(CHEMBL4755855)Show SMILES C[C@@H]1N(C[C@@H](CCB(O)O)C[C@]1(N)C(O)=O)C(=O)CN |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561053
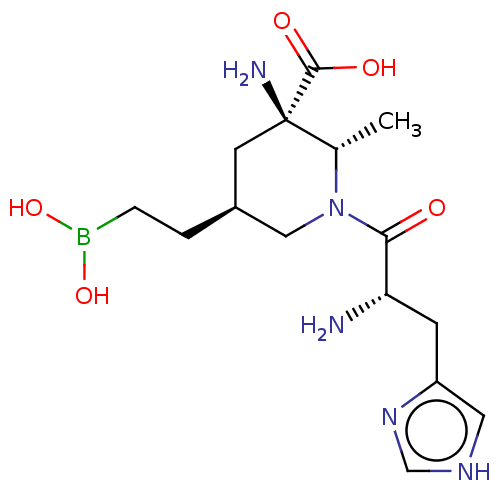
(CHEMBL4750602)Show SMILES C[C@@H]1N(C[C@@H](CCB(O)O)C[C@]1(N)C(O)=O)C(=O)[C@@H](N)Cc1c[nH]cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50595729

(CHEMBL5176739)Show SMILES NCC(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50595730

(CHEMBL5171566)Show SMILES CC(C)C[C@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561049
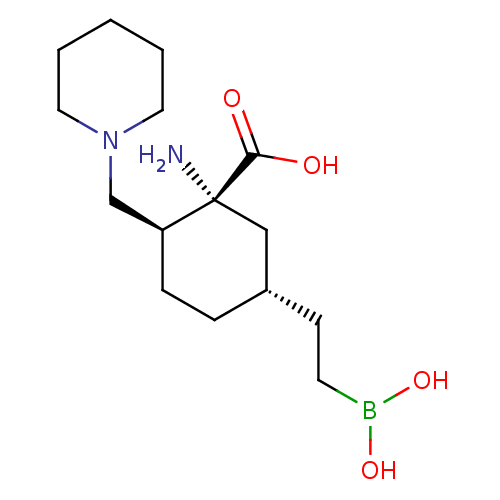
(CHEMBL4793482)Show SMILES N[C@@]1(C[C@H](CCB(O)O)CC[C@H]1CN1CCCCC1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50595723

(CHEMBL5193387)Show SMILES C[C@@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50595727
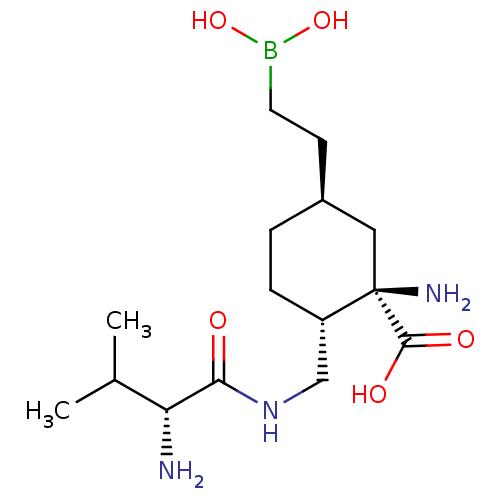
(CHEMBL5184299)Show SMILES CC(C)[C@@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561042

(CHEMBL4748950)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50509011

(CHEMBL4538713)Show SMILES N[C@H](CN1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C17H28BN3O4/c19-15(9-13-5-2-1-3-6-13)11-21-10-14(7-4-8-18(24)25)17(20,12-21)16(22)23/h1-3,5-6,14-15,24-25H,4,7-12,19-20H2,(H,22,23)/t14-,15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... |
J Med Chem 62: 8164-8177 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00931
BindingDB Entry DOI: 10.7270/Q2X92FM9 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561041
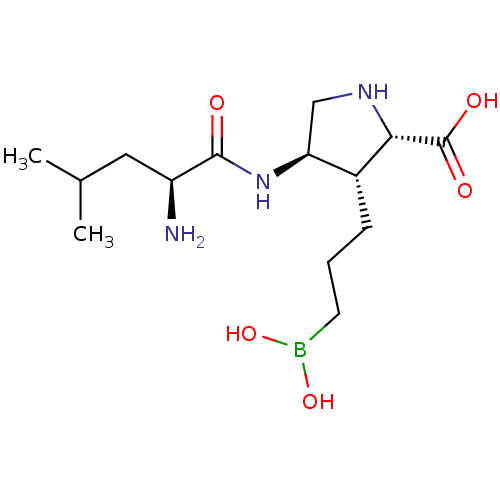
(CHEMBL4791224)Show SMILES CC(C)C[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579614

(CHEMBL4871970)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)CCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577469
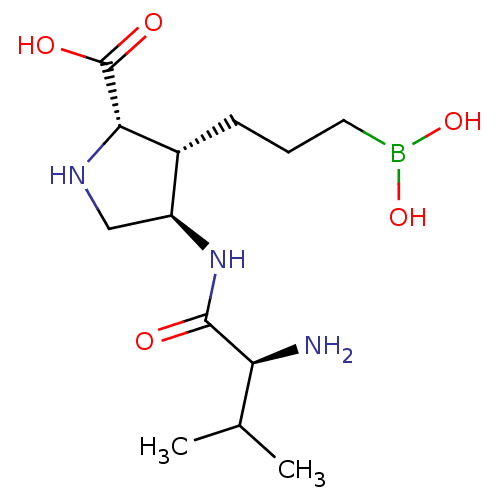
(CHEMBL4850213)Show SMILES CC(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579610

(CHEMBL4873520)Show SMILES [H][C@]12CN[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:9:8:7.6.5:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50509016
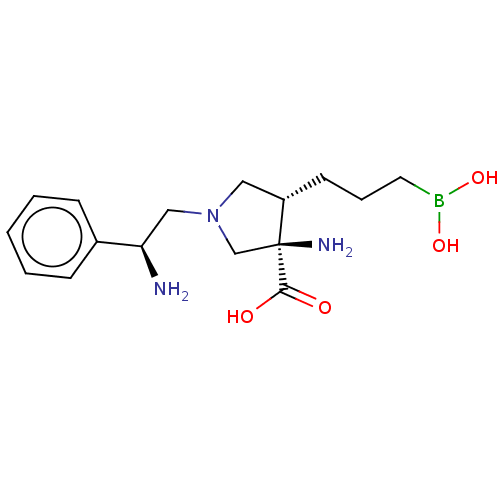
(CHEMBL4450972)Show SMILES N[C@H](CN1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C16H26BN3O4/c18-14(12-5-2-1-3-6-12)10-20-9-13(7-4-8-17(23)24)16(19,11-20)15(21)22/h1-3,5-6,13-14,23-24H,4,7-11,18-19H2,(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... |
J Med Chem 62: 8164-8177 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00931
BindingDB Entry DOI: 10.7270/Q2X92FM9 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50509014

(CHEMBL4557975)Show SMILES N[C@]1(CN(C[C@H]2CCCCN2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C14H28BN3O4/c16-14(13(19)20)10-18(9-12-5-1-2-7-17-12)8-11(14)4-3-6-15(21)22/h11-12,17,21-22H,1-10,16H2,(H,19,20)/t11-,12+,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
New England Discovery Partners
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs... |
J Med Chem 62: 8164-8177 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00931
BindingDB Entry DOI: 10.7270/Q2X92FM9 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561035
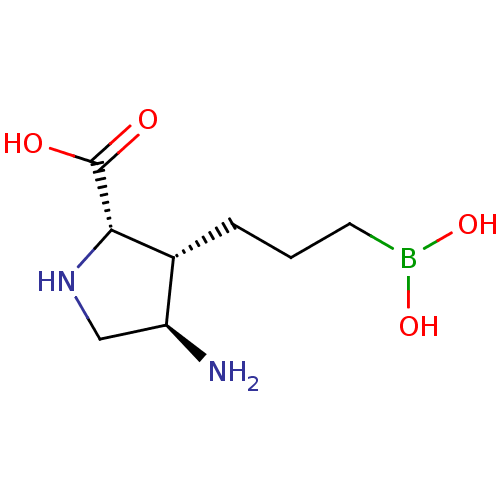
(CHEMBL4763578) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM568474
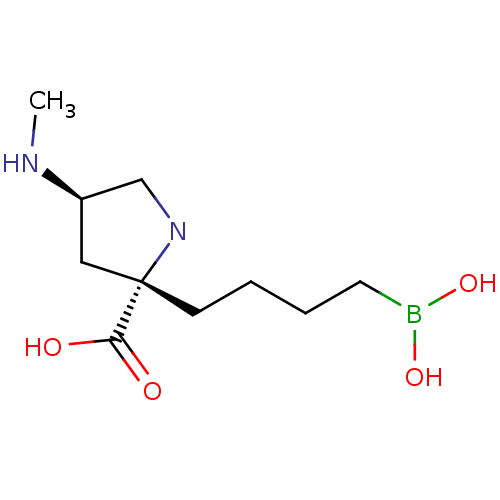
(US11420984, Example 24)Show SMILES CN[C@H]1CN[C@@](CCCCB(O)O)(C1)C(O)=O |r,$;;;;N;;;;;;;;;;;;$| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243GV |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561039

(CHEMBL4749355 | US11420984, Example 23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579616

(CHEMBL4845730)Show SMILES [H][C@]12CN[C@H](C(=O)OC)[C@@]1(CCCB(O)O)CCCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561035

(CHEMBL4763578) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg1 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM642313
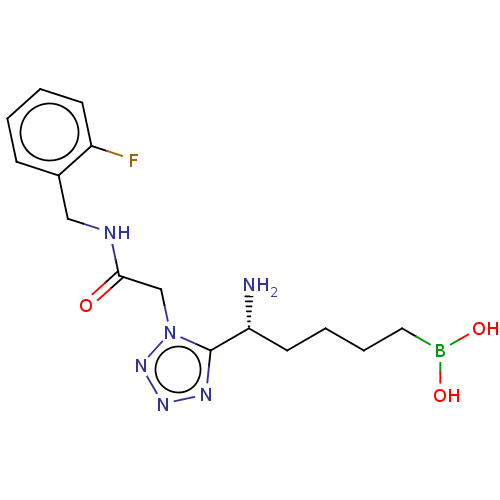
((+)-(R)-(5-amino-5-(1-(2-((2- fluorobenzyl)amino)-...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579611
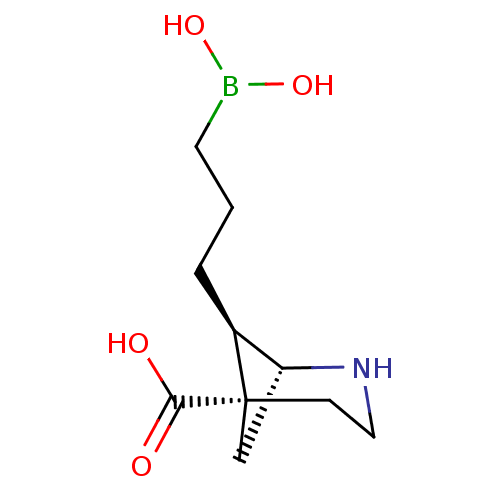
(CHEMBL4871097)Show SMILES [H][C@]12C[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:8:7:2:4.5.6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538536
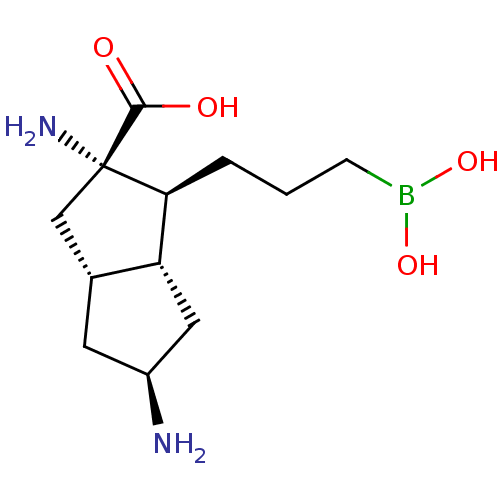
(CHEMBL4639893)Show SMILES [H][C@]12C[C@H](N)C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C12H23BN2O4/c14-8-4-7-6-12(15,11(16)17)10(9(7)5-8)2-1-3-13(18)19/h7-10,18-19H,1-6,14-15H2,(H,16,17)/t7-,8+,9-,10+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561036
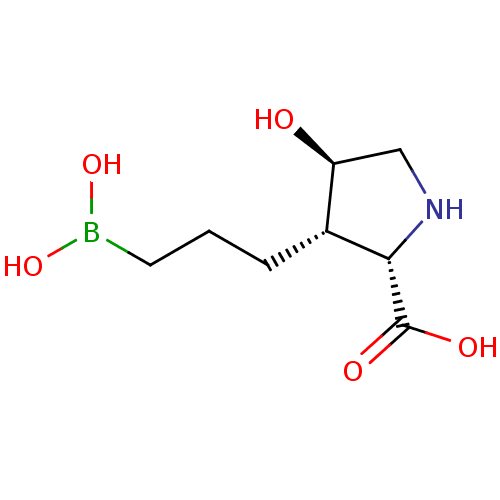
(CHEMBL4778978)Show SMILES OB(O)CCC[C@@H]1[C@@H](O)CN[C@@H]1C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data