

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176394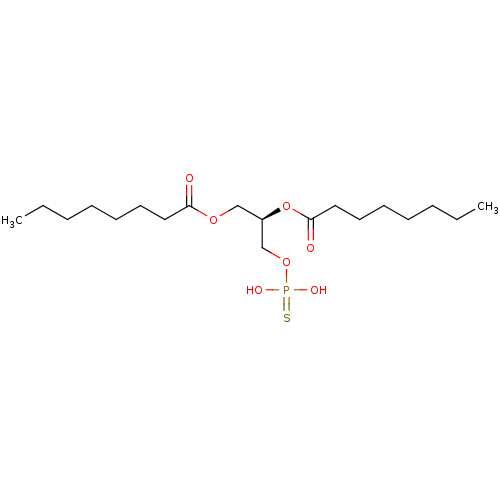 (CHEMBL202361 | octanoic acid (R)-2-octanoyloxy-3-t...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1/3 (Rattus norvegicus) | BDBM50496697 (CHEMBL3218460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Antagonist activity at LPAR1/LPAR3 in rat glioma C62B cells assessed as inhibition of LPA-induced reduction in isoproterenol-stimulated [3H]cAMP accu... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557828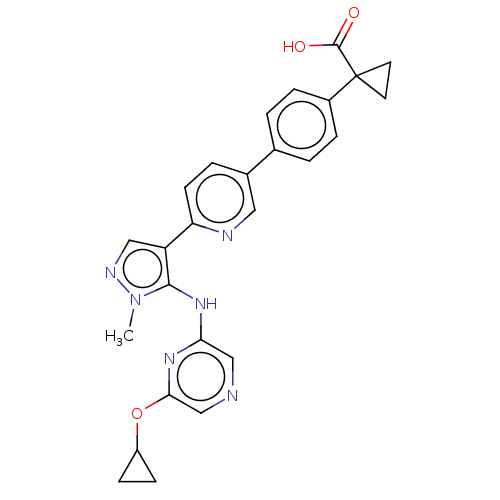 (1-[4-[6-[5-[[6-(cyclopropoxy)pyrazin-2-yl]amino]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557838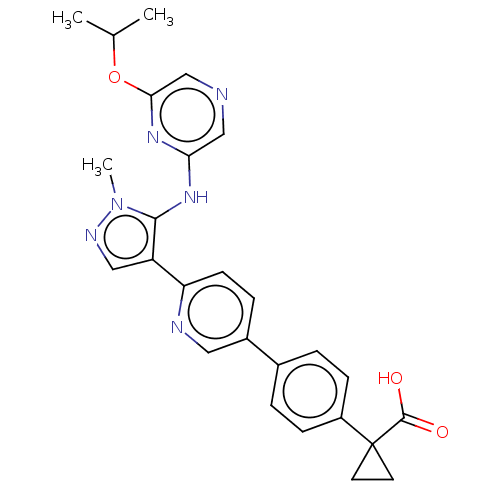 (1-[4-[6-[5-[(6-isopropoxypyrazin-2-yl)amino]-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170847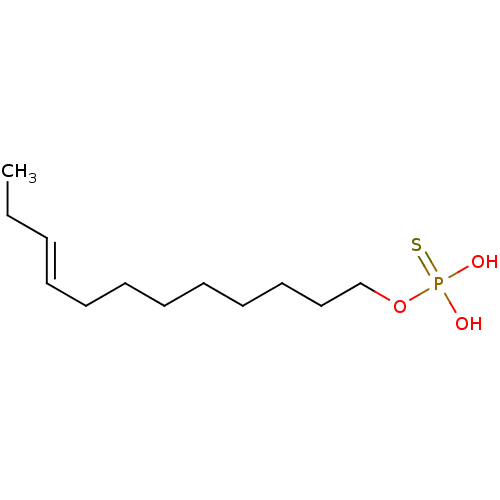 (CHEMBL190484 | Thiophosphoric acid (E)-dodec-9-eny...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170852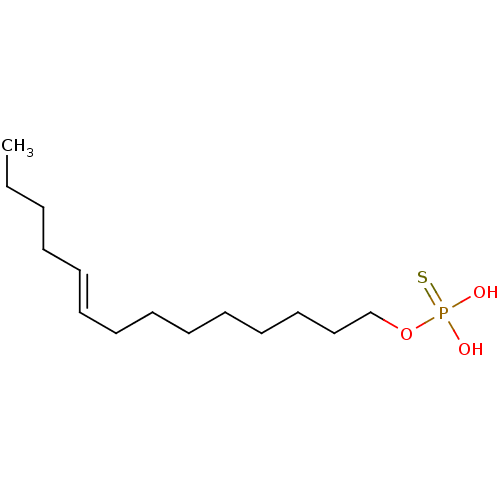 (CHEMBL187402 | Thiophosphoric acid (E)-tetradec-9-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170860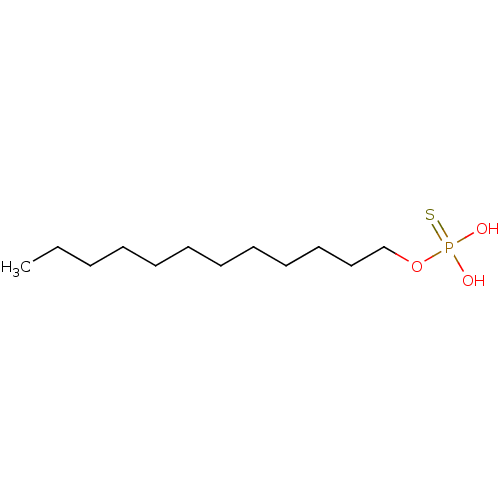 (CHEMBL191365 | Thiophosphoric acid dodecyl ester) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557845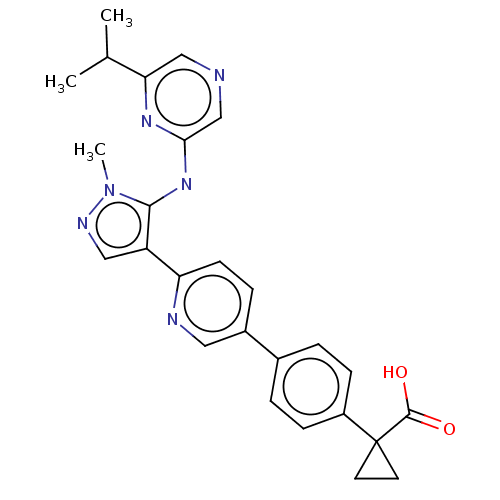 (US11365185, Example 27B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146232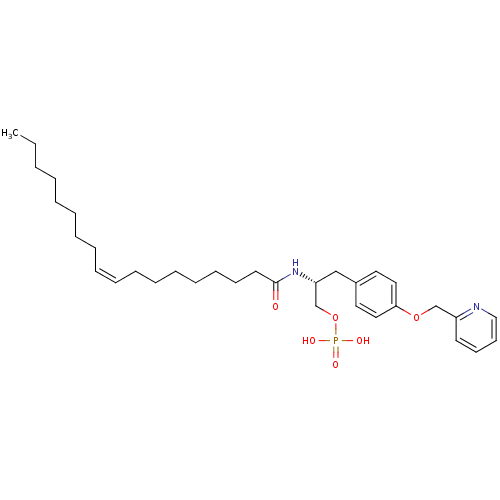 (CHEMBL440696 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146232 (CHEMBL440696 | Phosphoric acid mono-{(R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description In vitro ability to antagonize LPA-evoked [35S]-GTP-gammaS binding to lysophosphatidic acid receptor 1 in HEK293T cell lines | Bioorg Med Chem Lett 14: 2735-40 (2004) Article DOI: 10.1016/j.bmcl.2004.03.076 BindingDB Entry DOI: 10.7270/Q2DF6QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557844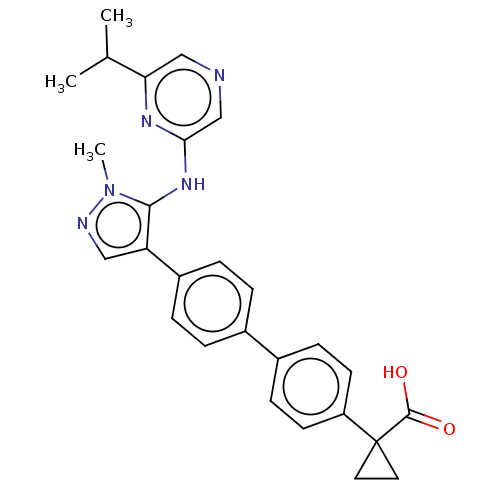 (1-[4-[4-[5-[(6-isopropylpyrazin-2-yl)amino]-1-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150007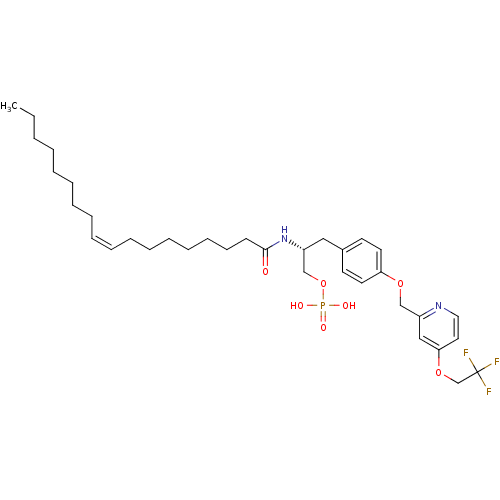 (CHEMBL183221 | Phosphoric acid mono-((R)-2-((Z)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557836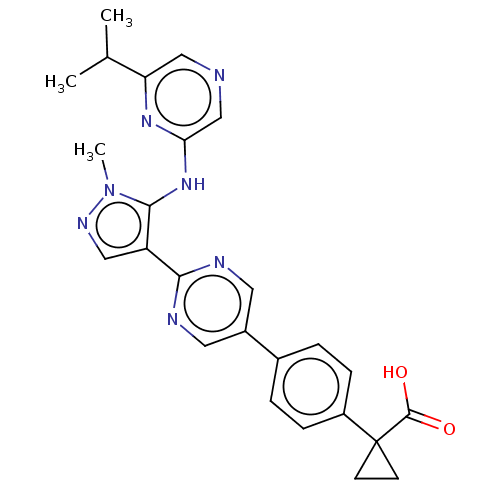 (1-[4-[2-[5-[(6-isopropylpyrazin-2-yl)amino]-1-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149996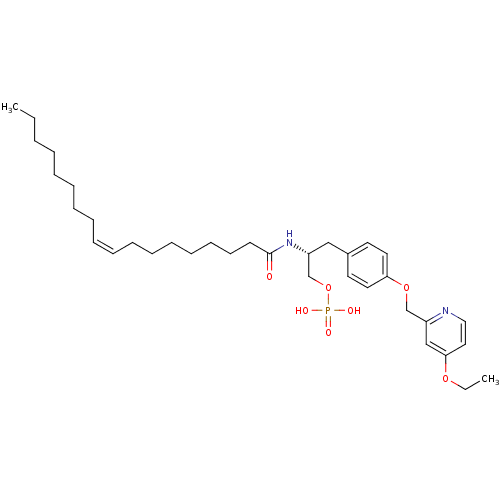 (CHEMBL183143 | Phosphoric acid mono-[3-[4-(4-ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170858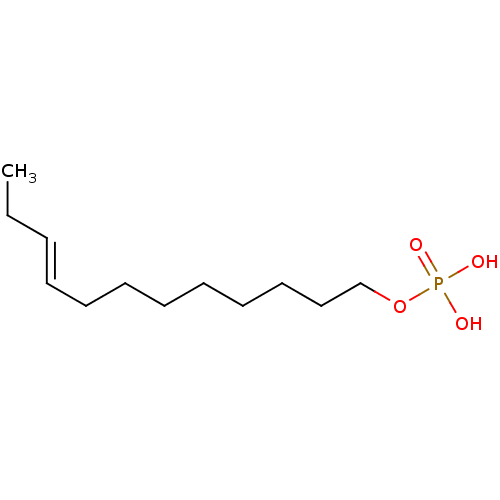 (CHEMBL190349 | Phosphoric acid mono-((E)-dodec-9-e...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557839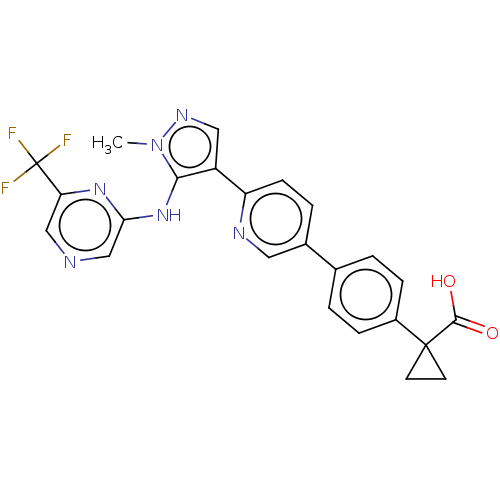 (1-[4-[6-[1-methyl-5-[[6-(trifluoromethyl)pyrazin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50149997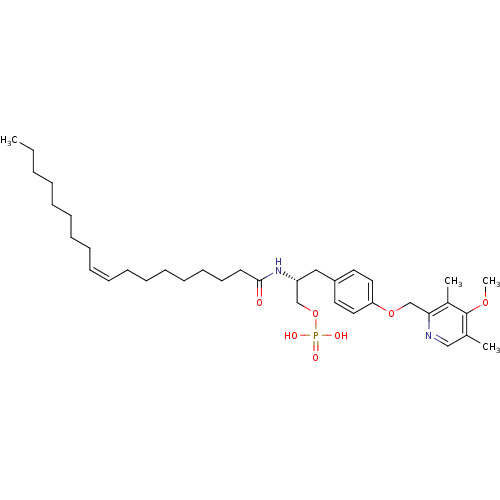 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557831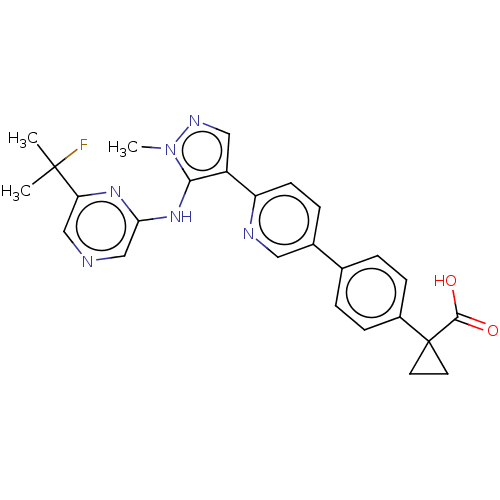 (1-[4-[6-[5-[[6-(1-fluoro-1-methyl-ethyl)pyrazin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557843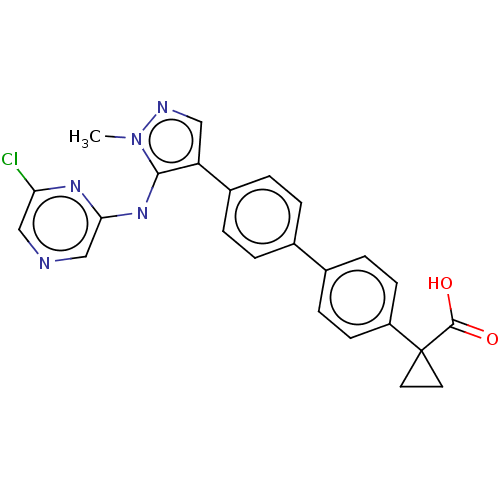 (US11365185, Example 25B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146240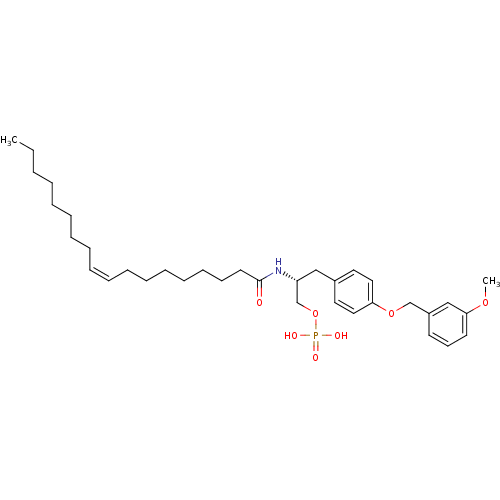 (CHEMBL91058 | Phosphoric acid mono-[(R)-3-[4-(3-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description In vitro ability to antagonize LPA-evoked [35S]-GTP-gammaS binding to lysophosphatidic acid receptor 1 in HEK293T cell lines | Bioorg Med Chem Lett 14: 2735-40 (2004) Article DOI: 10.1016/j.bmcl.2004.03.076 BindingDB Entry DOI: 10.7270/Q2DF6QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557829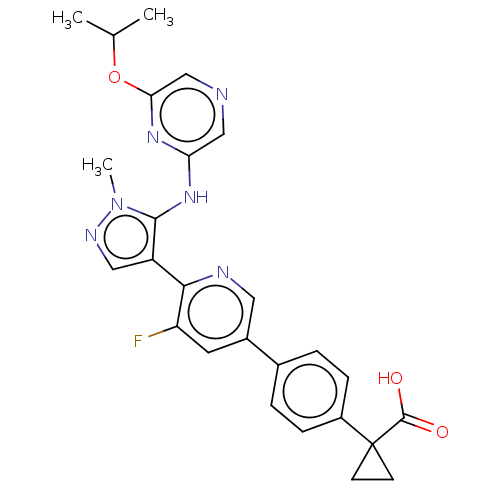 (1-[4-[5-fluoro-6-[5-[(6-isopropoxypyrazin-2-yl)ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150000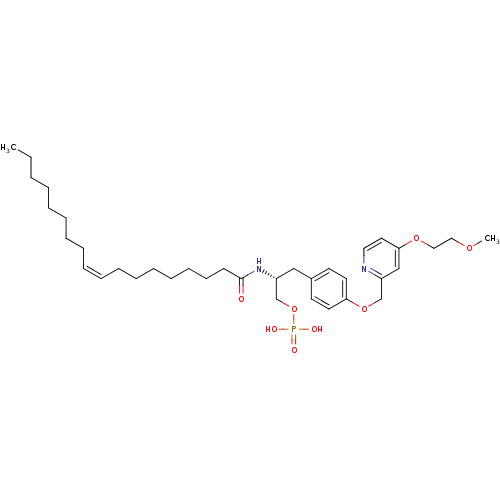 (CHEMBL182446 | Phosphoric acid mono-[(R)-3-{4-[4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149997 (CHEMBL362053 | Phosphoric acid mono-[3-[4-(4-metho...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50149996 (CHEMBL183143 | Phosphoric acid mono-[3-[4-(4-ethox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176391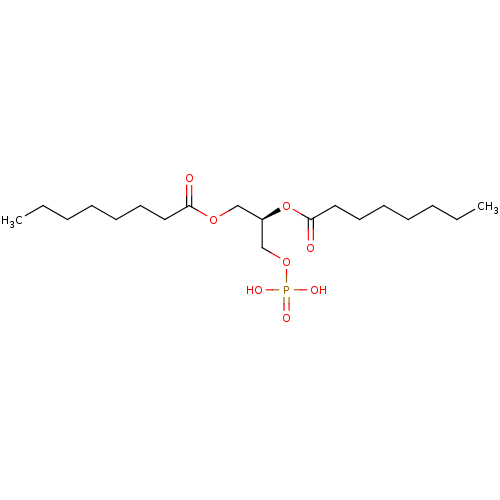 (CHEMBL202185 | octanoic acid (R)-2-octanoyloxy-3-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170851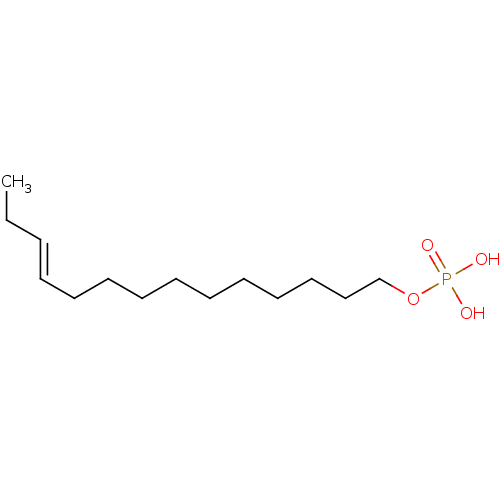 (CHEMBL188591 | Phosphoric acid mono-((E)-tetradec-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557830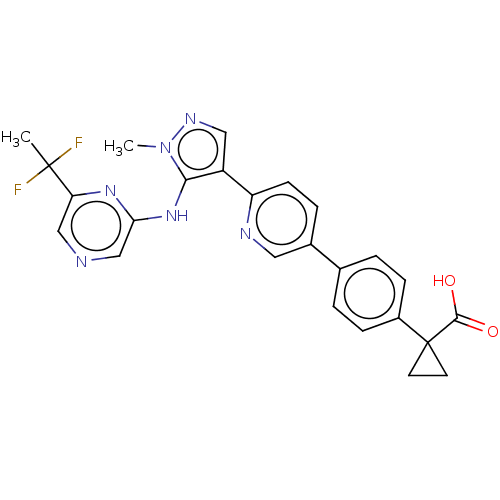 (1-[4-[6-[5-[[6-(1,1-difluoroethyl)pyrazin-2-yl]ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557821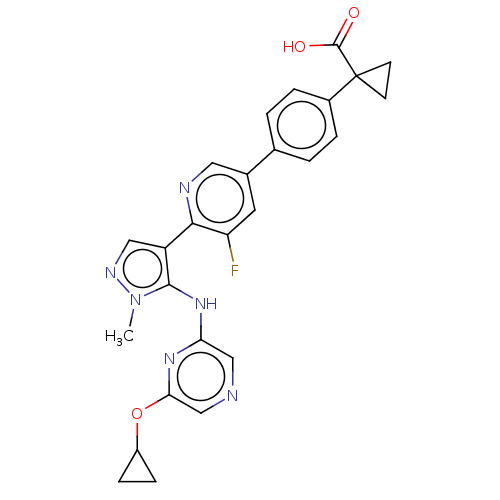 (1-[4-[6-[5-[[6-(cyclopropoxy)pyrazin-2-yl]amino]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 44.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557842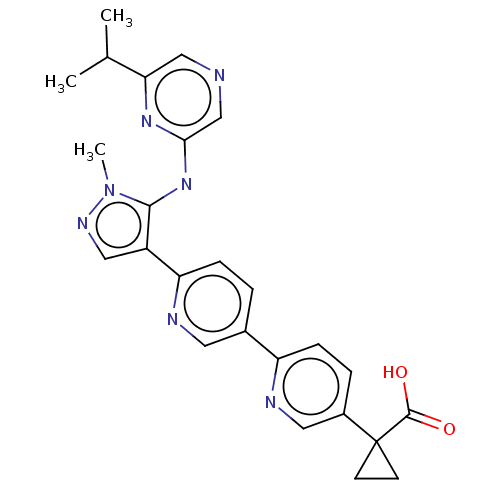 (US11365185, Example 24B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50496698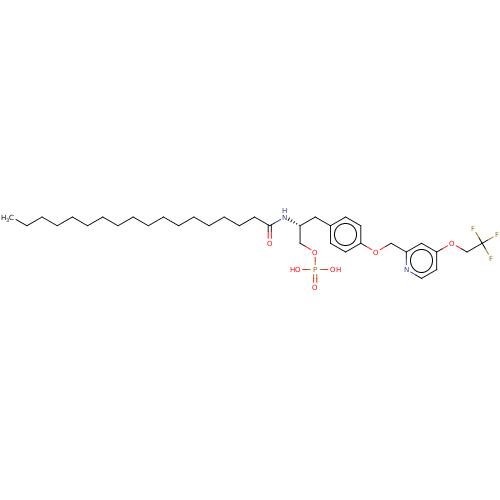 (CHEMBL3218459) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at N-terminal 3XHA-tagged human LPA3 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]G... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150007 (CHEMBL183221 | Phosphoric acid mono-((R)-2-((Z)-oc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50304580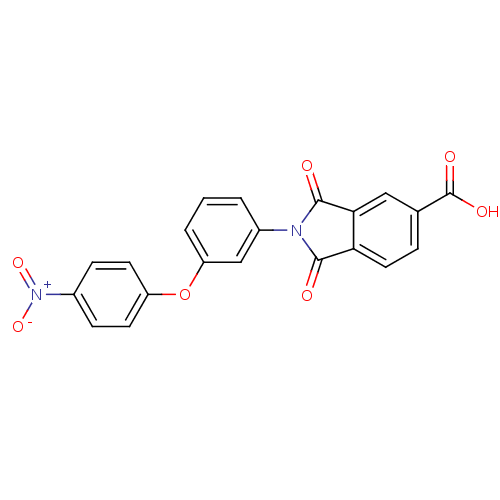 (2-(3-(4-nitrophenoxy)phenyl)-1,3-dioxoisoindoline-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Binding affinity to LPA1 | Bioorg Med Chem 17: 7457-64 (2009) Article DOI: 10.1016/j.bmc.2009.09.022 BindingDB Entry DOI: 10.7270/Q2GB244M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170857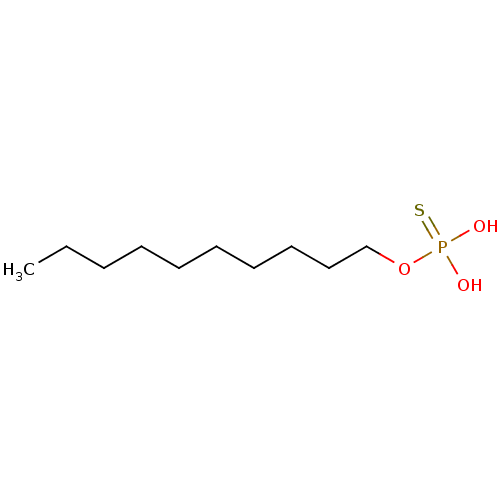 (CHEMBL187795 | Thiophosphoric acid decyl ester) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50271763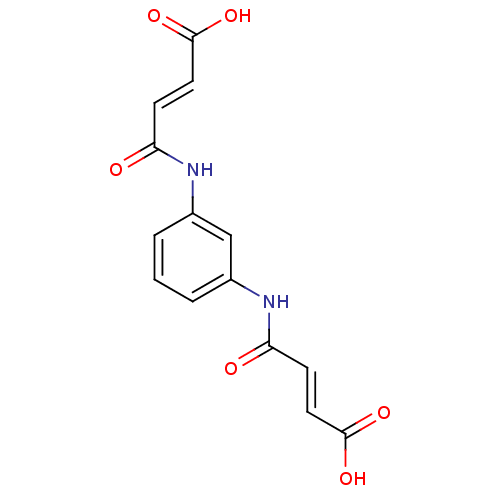 (3-[3-(3-Carboxy-acryloylamino)-phenylcarbamoyl]-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Memphis Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor (unknown origin) expressed in rat RH7777 cells assessed as inhibition of LPA-induced intracellular calcium conce... | Bioorg Med Chem 16: 6207-17 (2008) Article DOI: 10.1016/j.bmc.2008.04.035 BindingDB Entry DOI: 10.7270/Q270817C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176397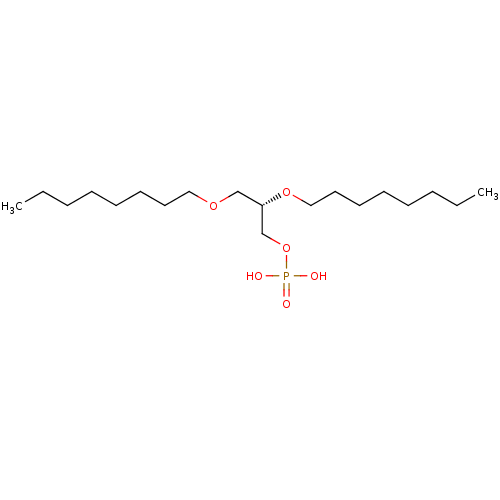 (CHEMBL203986 | nonanoic acid (S)-2-nonanoyloxy-3-p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557823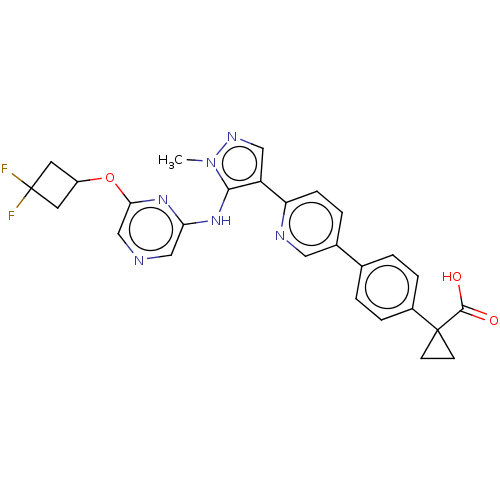 (1-[4-[6-[5-[[6-(3,3-difluorocyclobutoxy)pyrazin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 51.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557834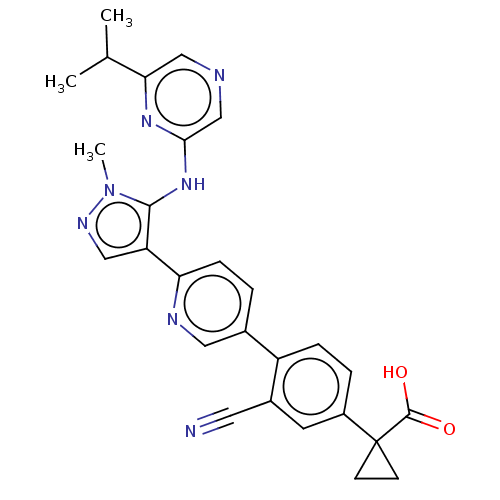 (1-[3-cyano-4-[6-[5-[(6-isopropylpyrazin-2-yl)amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 53.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170843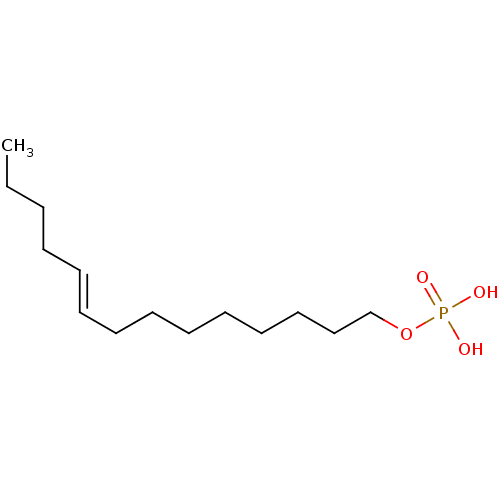 (CHEMBL190430 | Phosphoric acid mono-((E)-tetradec-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50193515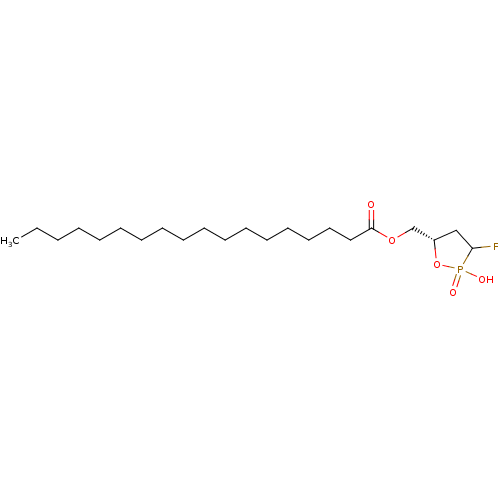 (1-fluoro-(3S)-hydroxyl-4-oleoyloxylbutane 1,3-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Utah Curated by ChEMBL | Assay Description Activity at human LPA1 receptor expressed in RH7777 cells by calcium mobilization assay | J Med Chem 49: 5309-15 (2006) Article DOI: 10.1021/jm060351+ BindingDB Entry DOI: 10.7270/Q2VM4BWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50150014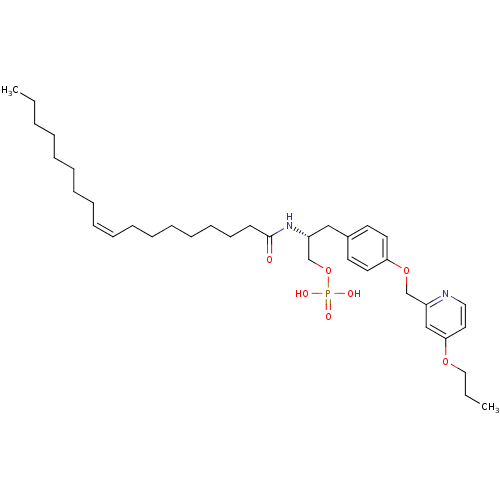 (CHEMBL291229 | Phosphoric acid mono-{2-((Z)-(R)-oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 1 (LPA1) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557825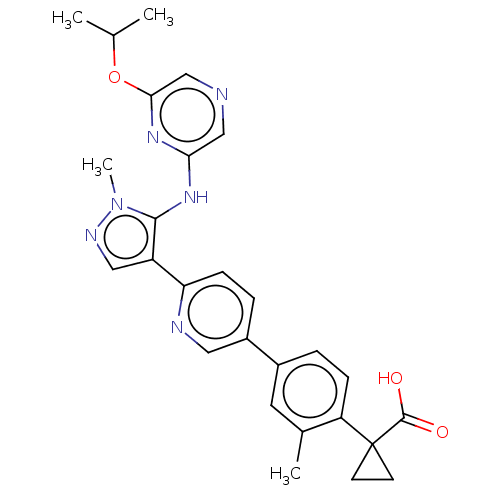 (1-[4-[6-[5-[(6-isopropoxypyrazin-2-yl)amino]-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 72.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50146238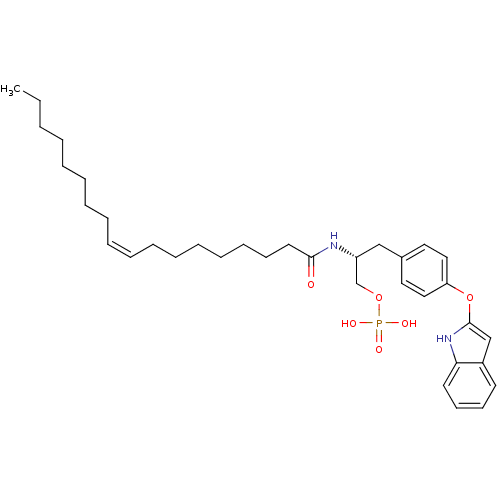 (CHEMBL314554 | Phosphoric acid mono-[(R)-3-[4-(1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description In vitro ability to antagonize LPA-evoked [35S]-GTP-gammaS binding to lysophosphatidic acid receptor 1 in HEK293T cell lines | Bioorg Med Chem Lett 14: 2735-40 (2004) Article DOI: 10.1016/j.bmcl.2004.03.076 BindingDB Entry DOI: 10.7270/Q2DF6QN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50170849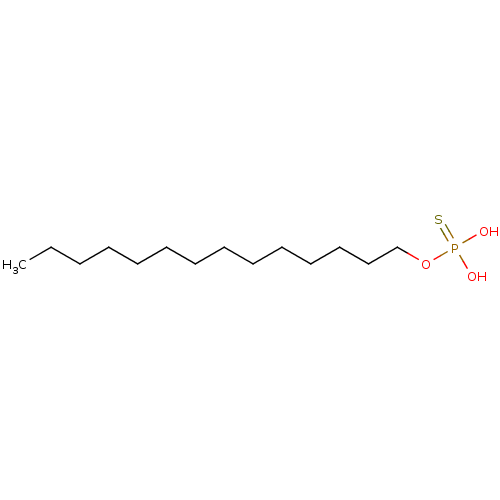 (CHEMBL189296 | Thiophosphoric acid tetradecyl este...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Binding affinity for Lysophosphatidic acid receptor 3 expressed in RH7777 rat hepatoma cells | J Med Chem 48: 4919-30 (2005) Article DOI: 10.1021/jm049609r BindingDB Entry DOI: 10.7270/Q2HD7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557822 (1-[4-[5-fluoro-6-[5-[[6-(1-fluoro-1-methyl-ethyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 80.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Rattus norvegicus) | BDBM50177339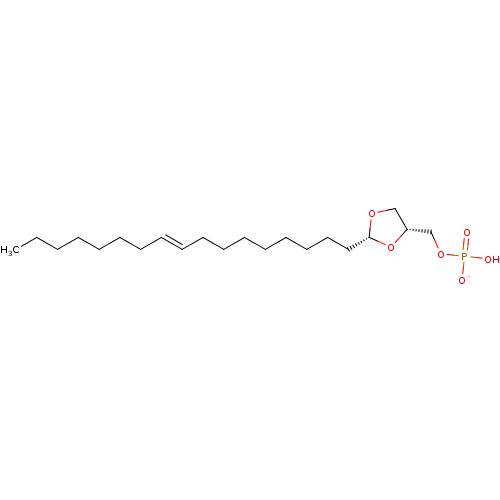 (CHEMBL436763 | potassium ((2R,4R)-2-(heptadec-9-en...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor in RH7777 rat hepatoma cell line | Bioorg Med Chem Lett 16: 451-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.096 BindingDB Entry DOI: 10.7270/Q2Z89C01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50496698 (CHEMBL3218459) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Competitive antagonist activity at human LPA1 receptor overexpressed in CHO cells assessed as inhibition of LPA-induced [35S]GTPgammaS binding by liq... | Medchemcomm 2: 325-330 (2011) Article DOI: 10.1039/c0md00273a BindingDB Entry DOI: 10.7270/Q2FN1943 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557833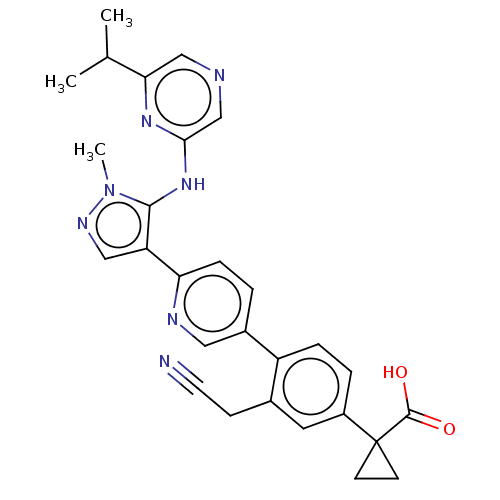 (1-[3-(cyanomethyl)-4-[6-[5-[(6-isopropylpyrazin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 94.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50150010 (CHEMBL265967 | Phosphoric acid mono-[(R)-3-[4-(4-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Curated by ChEMBL | Assay Description Binding affinity towards Lysophosphatidic acid 3 (LPA3) receptor | Bioorg Med Chem Lett 14: 4069-74 (2004) Article DOI: 10.1016/j.bmcl.2004.05.023 BindingDB Entry DOI: 10.7270/Q2930SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM557824 (1-[4-[6-[5-[[6-(1-methoxy-1-methyl-ethyl)pyrazin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of a compound to inhibit binding of a ligand (1-(4′-(4-(((benzyloxy)carbonyl)amino)-3-methylisoxazol-5-yl)-[1,1′-biphenyl]-4-... | Citation and Details BindingDB Entry DOI: 10.7270/Q26M3B1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 3 (Homo sapiens (Human)) | BDBM50176392 (CHEMBL379248 | thiophosphoric acid (R)-2-octanoyla...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Activity at LPA3 receptor transfected RH7777 cells | Bioorg Med Chem Lett 16: 633-40 (2005) Article DOI: 10.1016/j.bmcl.2005.10.031 BindingDB Entry DOI: 10.7270/Q2ZC82DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 890 total ) | Next | Last >> |