 Found 102 hits Enz. Inhib. hit(s) with Target = 'Alpha-mannosidase 2'
Found 102 hits Enz. Inhib. hit(s) with Target = 'Alpha-mannosidase 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM84868
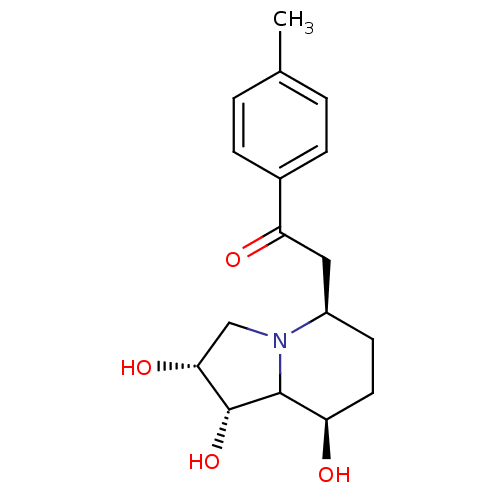
(Swainsonine derivative, 3)Show SMILES Cc1ccc(cc1)C(=O)C[C@H]1CC[C@@H](O)C2[C@H](O)[C@H](O)CN12 |r| Show InChI InChI=1S/C17H23NO4/c1-10-2-4-11(5-3-10)14(20)8-12-6-7-13(19)16-17(22)15(21)9-18(12)16/h2-5,12-13,15-17,19,21-22H,6-9H2,1H3/t12-,13-,15-,16?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | -48.9 | 29 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto
| Assay Description
Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... |
Chembiochem 11: 673-80 (2010)
Article DOI: 10.1002/cbic.200900750
BindingDB Entry DOI: 10.7270/Q2WS8RRM |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM84869

(Swainsonine derivative, 4)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)C[C@H]1CC[C@@H](O)C2[C@H](O)[C@H](O)CN12 |r| Show InChI InChI=1S/C20H29NO4/c1-20(2,3)13-6-4-12(5-7-13)16(23)10-14-8-9-15(22)18-19(25)17(24)11-21(14)18/h4-7,14-15,17-19,22,24-25H,8-11H2,1-3H3/t14-,15-,17-,18?,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | -48.9 | 29 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto
| Assay Description
Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... |
Chembiochem 11: 673-80 (2010)
Article DOI: 10.1002/cbic.200900750
BindingDB Entry DOI: 10.7270/Q2WS8RRM |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM84867
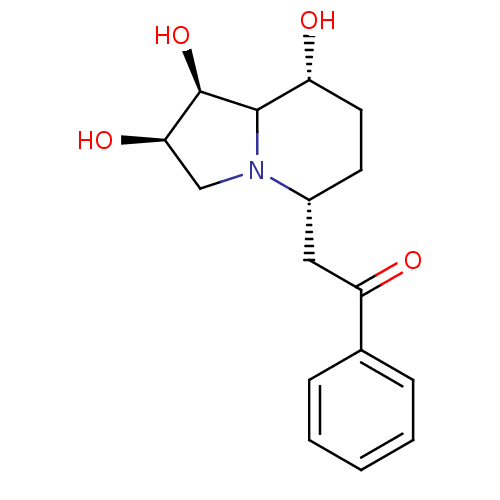
(Swainsonine derivative, 2)Show SMILES O[C@@H]1CN2C([C@@H]1O)[C@H](O)CC[C@@H]2CC(=O)c1ccccc1 |r| Show InChI InChI=1S/C16H21NO4/c18-12-7-6-11(17-9-14(20)16(21)15(12)17)8-13(19)10-4-2-1-3-5-10/h1-5,11-12,14-16,18,20-21H,6-9H2/t11-,12-,14-,15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | -48.8 | 30 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto
| Assay Description
Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... |
Chembiochem 11: 673-80 (2010)
Article DOI: 10.1002/cbic.200900750
BindingDB Entry DOI: 10.7270/Q2WS8RRM |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM50168995
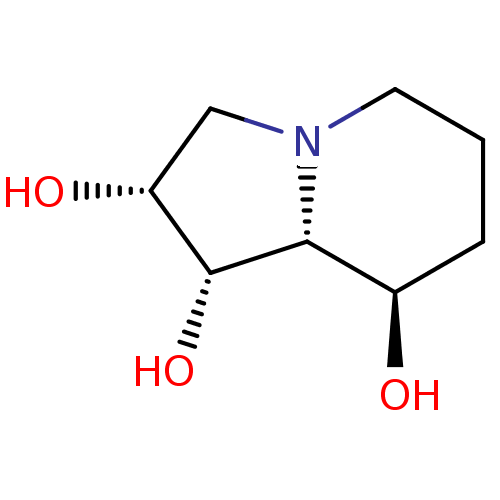
((-)-swainsonine | (1S,2R,8R,8aR)-Octahydro-indoliz...)Show InChI InChI=1S/C8H15NO3/c10-5-2-1-3-9-4-6(11)8(12)7(5)9/h5-8,10-12H,1-4H2/t5-,6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.6 | 37 | n/a | n/a | n/a | n/a | 5.75 | 25 |
University of Toronto
| Assay Description
Inhibition of dGMII was measured at pH 5.75. Determination of the IC50 values (concentrations of inhibitor at which 50% of activity remains) was car... |
Chembiochem 11: 673-80 (2010)
Article DOI: 10.1002/cbic.200900750
BindingDB Entry DOI: 10.7270/Q2WS8RRM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM50078117
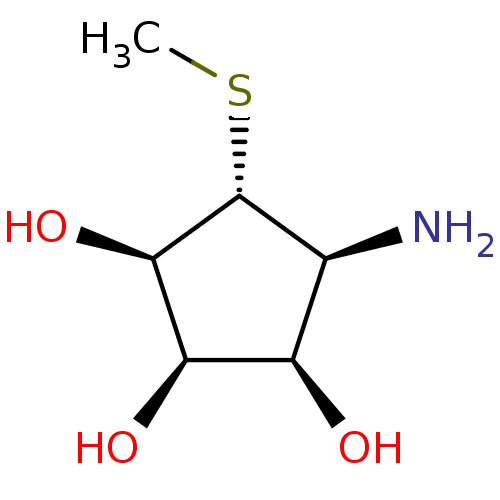
((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...)Show SMILES CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO3S/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
| Assay Description
The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... |
Chembiochem 10: 268-77 (2009)
Article DOI: 10.1002/cbic.200800538
BindingDB Entry DOI: 10.7270/Q2DZ06T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84465
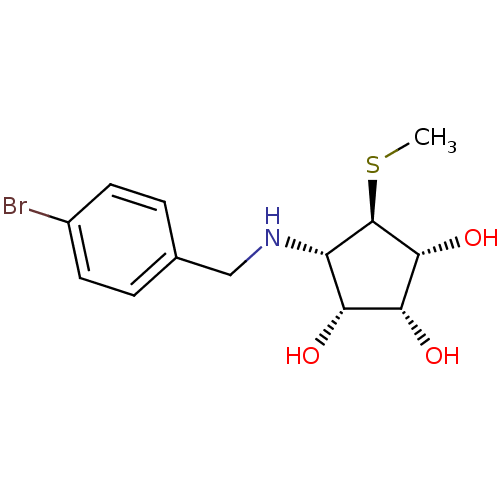
(Benzylation of mannostatin A, 1e)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Br)cc1 |r| Show InChI InChI=1S/C13H18BrNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -43.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM50088625

((1R,2R,3R,4S,5R)-4-Amino-5-methoxy-cyclopentane-1,...)Show SMILES CO[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO4/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
| Assay Description
The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... |
Chembiochem 10: 268-77 (2009)
Article DOI: 10.1002/cbic.200800538
BindingDB Entry DOI: 10.7270/Q2DZ06T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50078117

((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...)Show SMILES CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO3S/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 90 | -41.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84464
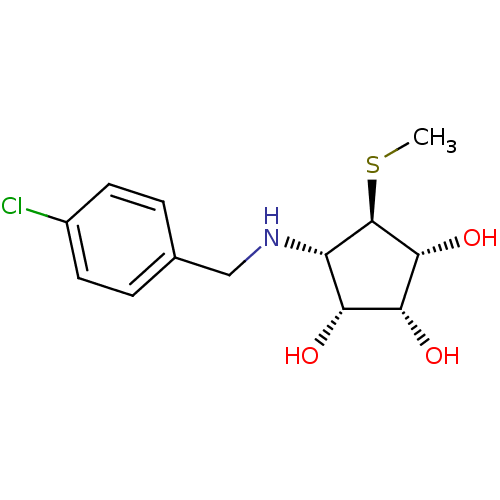
(Benzylation of mannostatin A, 1d)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -41.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84466
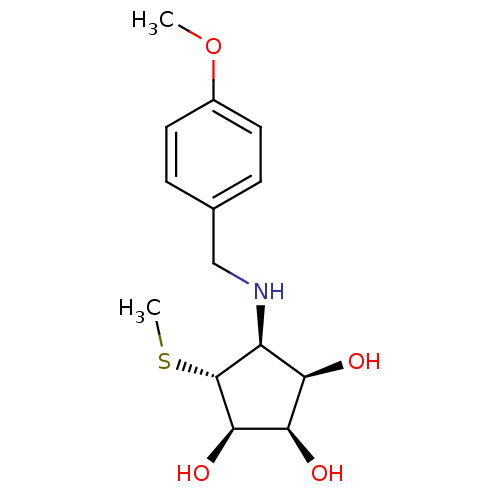
(Benzylation of mannostatin A, 1f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@@H]2SC)cc1 |r| Show InChI InChI=1S/C14H21NO4S/c1-19-9-5-3-8(4-6-9)7-15-10-11(16)12(17)13(18)14(10)20-2/h3-6,10-18H,7H2,1-2H3/t10-,11+,12+,13+,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -41.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84462
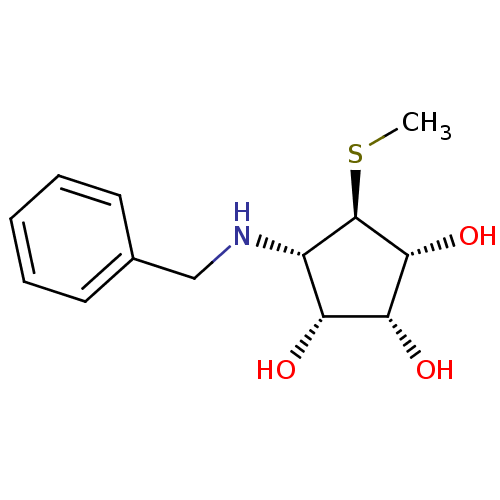
(Benzylation of mannostatin A, 1b)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccccc1 |r| Show InChI InChI=1S/C13H19NO3S/c1-18-13-9(10(15)11(16)12(13)17)14-7-8-5-3-2-4-6-8/h2-6,9-17H,7H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84467

(Benzylation of mannostatin A, 1g)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(cc1)C(=O)OCC=C |r| Show InChI InChI=1S/C17H23NO5S/c1-3-8-23-17(22)11-6-4-10(5-7-11)9-18-12-13(19)14(20)15(21)16(12)24-2/h3-7,12-16,18-21H,1,8-9H2,2H3/t12-,13+,14+,15+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM84614

(Mannostatin B, 2)Show SMILES CS(=O)[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C6H13NO4S/c1-12(11)6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
| Assay Description
The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... |
Chembiochem 10: 268-77 (2009)
Article DOI: 10.1002/cbic.200800538
BindingDB Entry DOI: 10.7270/Q2DZ06T7 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84474
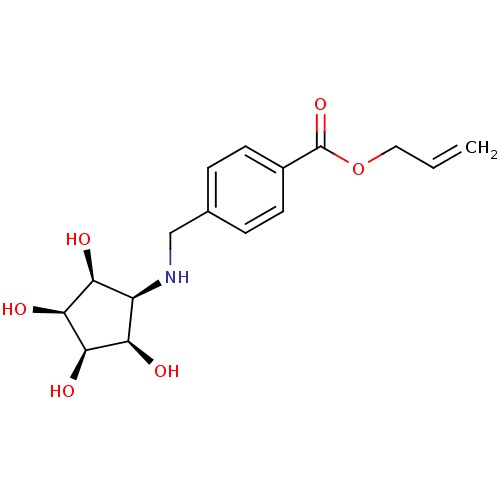
(Aminocyclopentitetrol, 2g)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(cc2)C(=O)OCC=C)[C@H]1O |r| Show InChI InChI=1S/C16H21NO6/c1-2-7-23-16(22)10-5-3-9(4-6-10)8-17-11-12(18)14(20)15(21)13(11)19/h2-6,11-15,17-21H,1,7-8H2/t11-,12+,13-,14+,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | -40.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84463
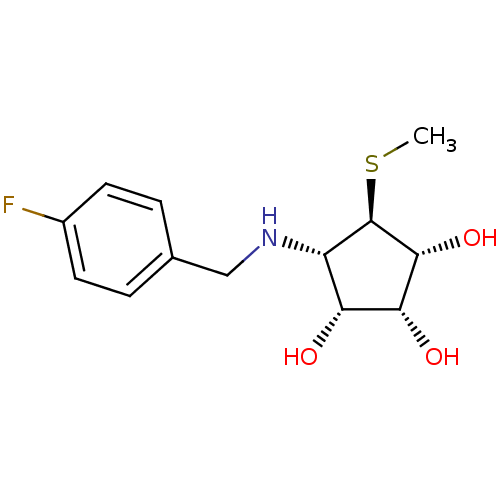
(Benzylation of mannostatin A, 1c)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H18FNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | -40.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50078117

((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...)Show SMILES CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO3S/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 210 | -39.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Drosophila melanogaster (Fruit fly)) | BDBM84615

(Mannostatin analogue, 4a)Show SMILES N[C@@H]1[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2/t1-,2-,3-,4+,5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
| Assay Description
The rate of hydrolysis catalyzed by drosophila golgi alpha-mannosidase II (dGMII)and in the presence of different concentration of inhibitor was meas... |
Chembiochem 10: 268-77 (2009)
Article DOI: 10.1002/cbic.200800538
BindingDB Entry DOI: 10.7270/Q2DZ06T7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84472
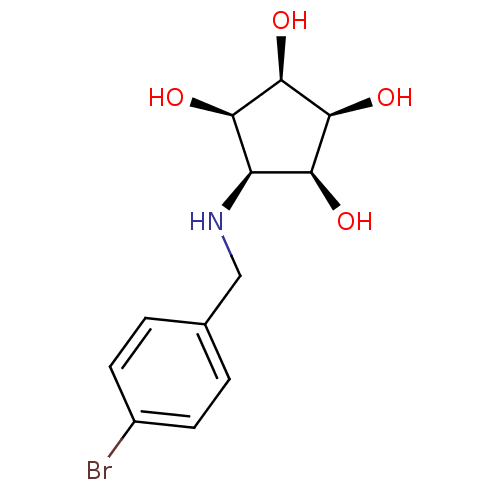
(Aminocyclopentitetrol, 2e)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Br)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16BrNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -38.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84470
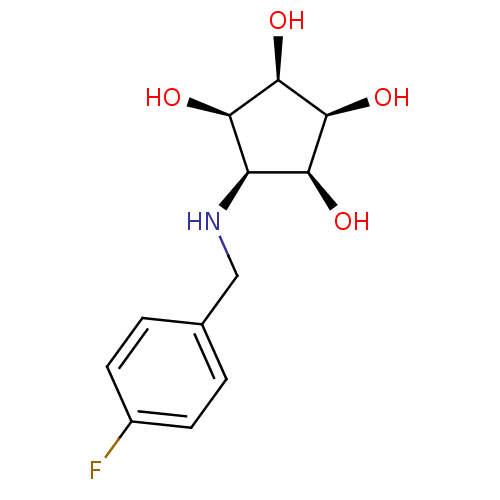
(Aminocyclopentitetrol, 2c)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(F)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16FNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -38.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84469
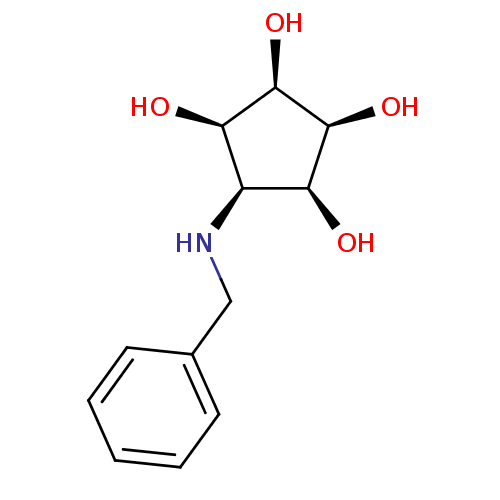
(Aminocyclopentitetrol, 2b)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C12H17NO4/c14-9-8(10(15)12(17)11(9)16)13-6-7-4-2-1-3-5-7/h1-5,8-17H,6H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 450 | -37.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84473
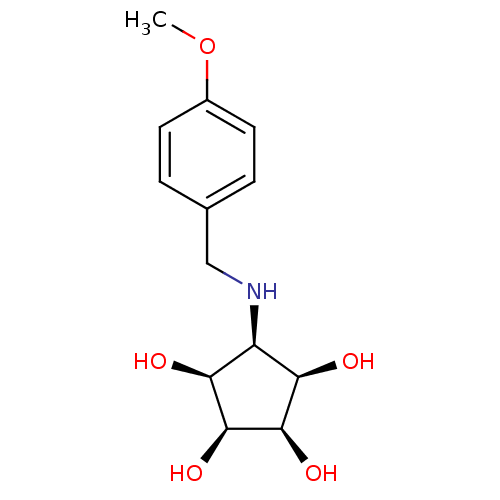
(Aminocyclopentitetrol, 2f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19NO5/c1-19-8-4-2-7(3-5-8)6-14-9-10(15)12(17)13(18)11(9)16/h2-5,9-18H,6H2,1H3/t9-,10+,11-,12+,13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 480 | -37.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84465

(Benzylation of mannostatin A, 1e)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Br)cc1 |r| Show InChI InChI=1S/C13H18BrNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | -37.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84466

(Benzylation of mannostatin A, 1f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@@H]2SC)cc1 |r| Show InChI InChI=1S/C14H21NO4S/c1-19-9-5-3-8(4-6-9)7-15-10-11(16)12(17)13(18)14(10)20-2/h3-6,10-18H,7H2,1-2H3/t10-,11+,12+,13+,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | -37.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84463

(Benzylation of mannostatin A, 1c)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H18FNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | -37.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84471
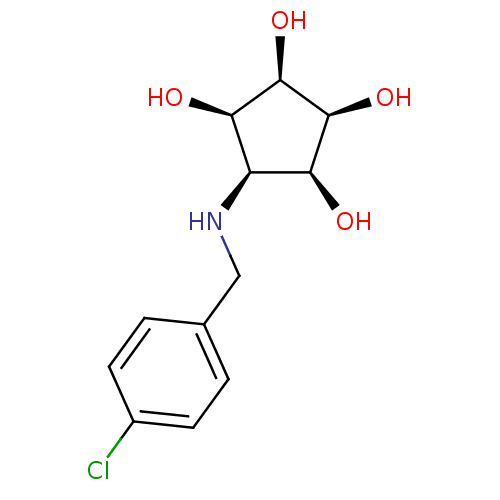
(Aminocyclopentitetrol, 2d)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Cl)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16ClNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | -36.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84462

(Benzylation of mannostatin A, 1b)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccccc1 |r| Show InChI InChI=1S/C13H19NO3S/c1-18-13-9(10(15)11(16)12(13)17)14-7-8-5-3-2-4-6-8/h2-6,9-17H,7H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 880 | -36.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84464

(Benzylation of mannostatin A, 1d)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | -35.9 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84467

(Benzylation of mannostatin A, 1g)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(cc1)C(=O)OCC=C |r| Show InChI InChI=1S/C17H23NO5S/c1-3-8-23-17(22)11-6-4-10(5-7-11)9-18-12-13(19)14(20)15(21)16(12)24-2/h3-7,12-16,18-21H,1,8-9H2,2H3/t12-,13+,14+,15+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.22E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402966
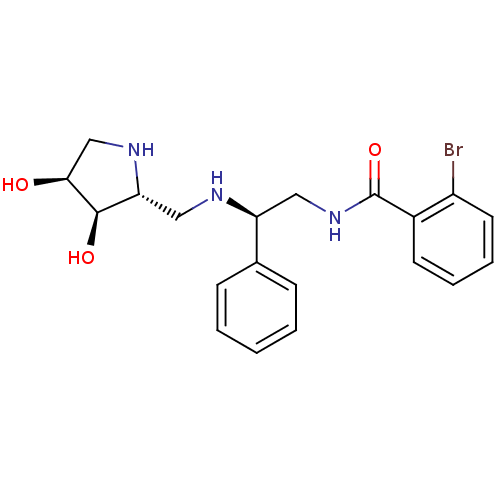
(CHEMBL2206824)Show SMILES O[C@H]1CN[C@H](CN[C@@H](CNC(=O)c2ccccc2Br)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H24BrN3O3/c21-15-9-5-4-8-14(15)20(27)24-10-16(13-6-2-1-3-7-13)22-11-17-19(26)18(25)12-23-17/h1-9,16-19,22-23,25-26H,10-12H2,(H,24,27)/t16-,17+,18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84474

(Aminocyclopentitetrol, 2g)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(cc2)C(=O)OCC=C)[C@H]1O |r| Show InChI InChI=1S/C16H21NO6/c1-2-7-23-16(22)10-5-3-9(4-6-10)8-17-11-12(18)14(20)15(21)13(11)19/h2-6,11-15,17-21H,1,7-8H2/t11-,12+,13-,14+,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84470

(Aminocyclopentitetrol, 2c)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(F)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16FNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402969
(CHEMBL2206821)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2ccc(F)cc2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25FN2O4/c21-15-6-8-16(9-7-15)27-13-26-12-18(14-4-2-1-3-5-14)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84468
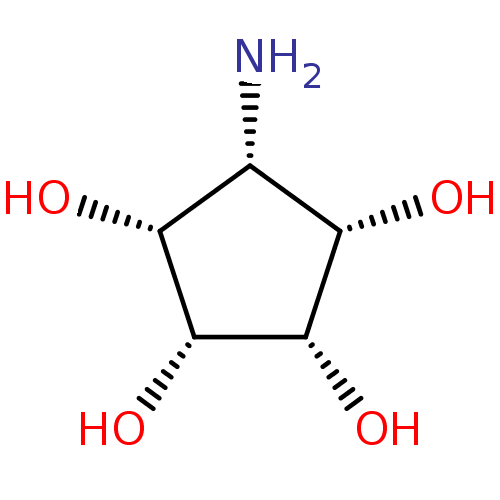
(Mannostatin analogue, 4b | Meso-aminocyclopentitet...)Show SMILES N[C@@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2/t1-,2+,3-,4+,5- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84473

(Aminocyclopentitetrol, 2f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19NO5/c1-19-8-4-2-7(3-5-8)6-14-9-10(15)12(17)13(18)11(9)16/h2-5,9-18H,6H2,1H3/t9-,10+,11-,12+,13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84472

(Aminocyclopentitetrol, 2e)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Br)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16BrNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50169002
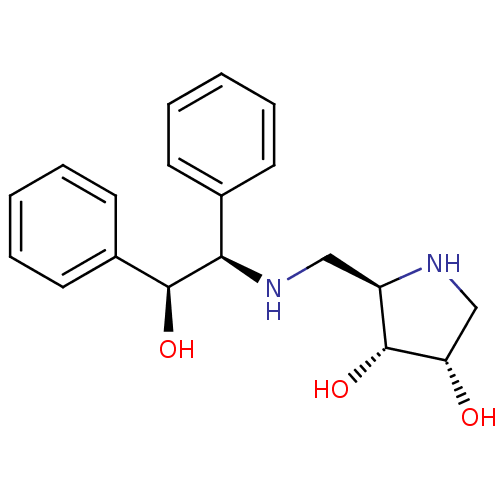
((2R,3R,4S)-2-[((S)-Phenyl-1-(R)-2-hydroxy-2-phenyl...)Show SMILES O[C@H]([C@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C19H24N2O3/c22-16-12-20-15(19(16)24)11-21-17(13-7-3-1-4-8-13)18(23)14-9-5-2-6-10-14/h1-10,15-24H,11-12H2/t15-,16+,17-,18+,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84471

(Aminocyclopentitetrol, 2d)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Cl)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16ClNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402971
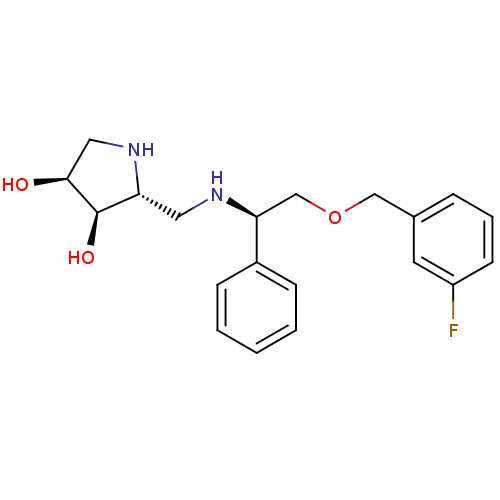
(CHEMBL2206819)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCc2cccc(F)c2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25FN2O3/c21-16-8-4-5-14(9-16)12-26-13-18(15-6-2-1-3-7-15)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84469

(Aminocyclopentitetrol, 2b)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C12H17NO4/c14-9-8(10(15)12(17)11(9)16)13-6-7-4-2-1-3-5-7/h1-5,8-17H,6H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+4 | -29.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
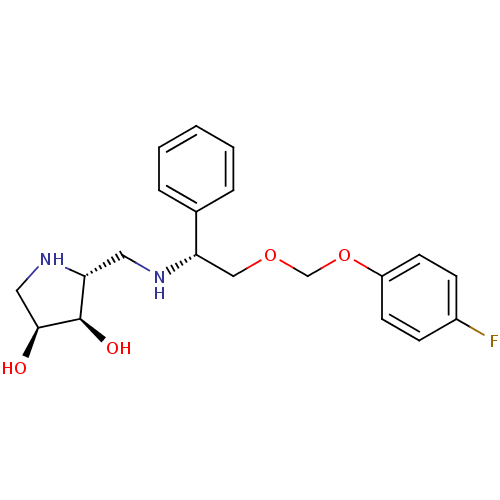
(Homo sapiens (Human)) | BDBM50402970
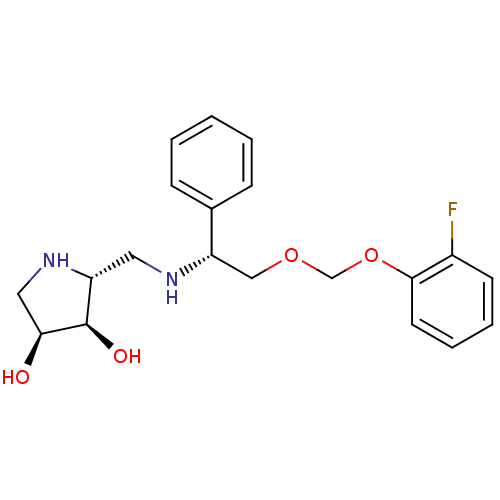
(CHEMBL2206820)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2ccccc2F)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25FN2O4/c21-15-8-4-5-9-19(15)27-13-26-12-17(14-6-2-1-3-7-14)22-10-16-20(25)18(24)11-23-16/h1-9,16-18,20,22-25H,10-13H2/t16-,17+,18+,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402974
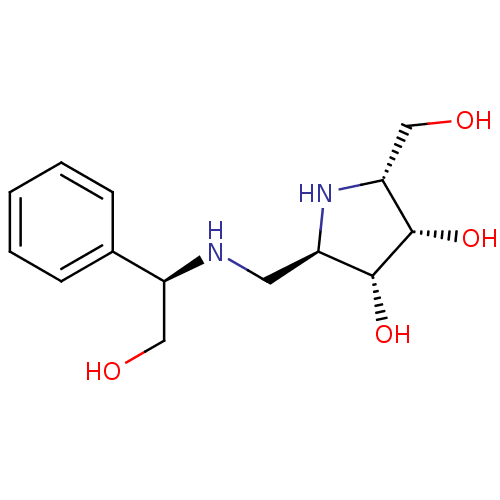
(CHEMBL2206816)Show SMILES OC[C@H](NC[C@H]1N[C@H](CO)[C@H](O)[C@@H]1O)c1ccccc1 |r| Show InChI InChI=1S/C14H22N2O4/c17-7-11(9-4-2-1-3-5-9)15-6-10-13(19)14(20)12(8-18)16-10/h1-5,10-20H,6-8H2/t10-,11+,12-,13-,14+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402968
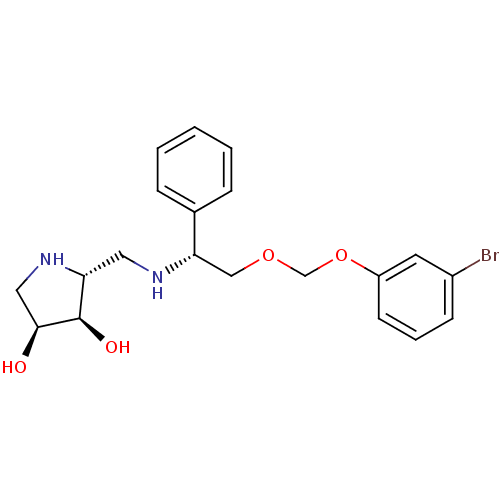
(CHEMBL2206822)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2cccc(Br)c2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25BrN2O4/c21-15-7-4-8-16(9-15)27-13-26-12-18(14-5-2-1-3-6-14)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402967
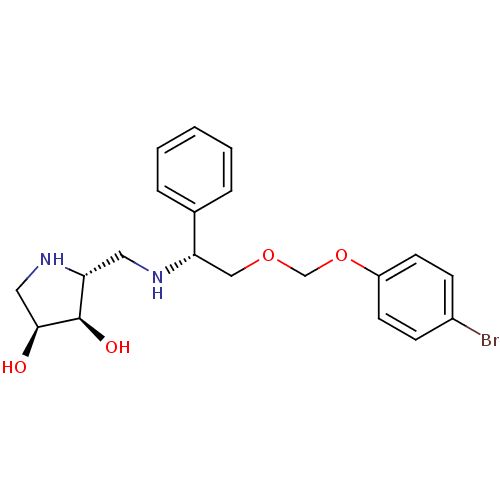
(CHEMBL2206823)Show SMILES O[C@H]1CN[C@H](CN[C@@H](COCOc2ccc(Br)cc2)c2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C20H25BrN2O4/c21-15-6-8-16(9-7-15)27-13-26-12-18(14-4-2-1-3-5-14)22-10-17-20(25)19(24)11-23-17/h1-9,17-20,22-25H,10-13H2/t17-,18+,19+,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50168990
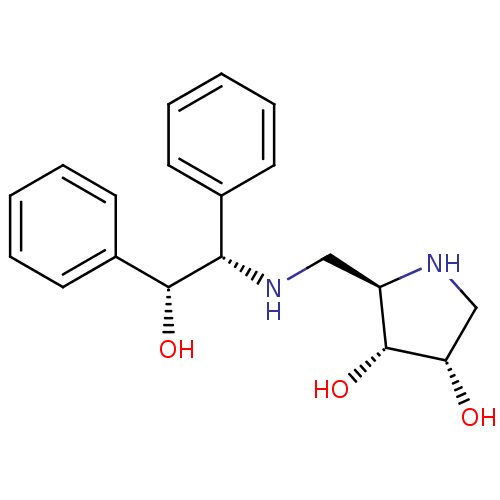
((2R,3R,4S)-2-[((R)-Phenyl-1-(S)-2-hydroxy-2-phenyl...)Show SMILES O[C@@H]([C@@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C19H24N2O3/c22-16-12-20-15(19(16)24)11-21-17(13-7-3-1-4-8-13)18(23)14-9-5-2-6-10-14/h1-10,15-24H,11-12H2/t15-,16+,17+,18-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50168988
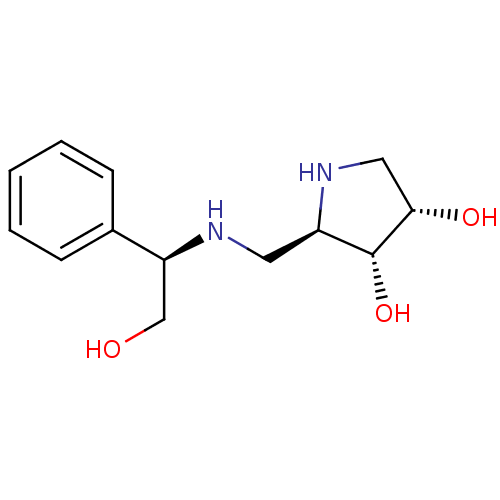
((2R,3R,4S)-2-({[(1R)-2-HYDROXY-1-PHENYLETHYL]AMINO...)Show SMILES OC[C@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1 |r| Show InChI InChI=1S/C13H20N2O3/c16-8-11(9-4-2-1-3-5-9)14-6-10-13(18)12(17)7-15-10/h1-5,10-18H,6-8H2/t10-,11+,12+,13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50169001
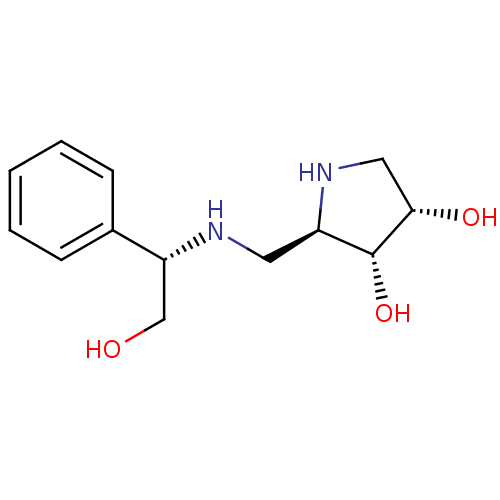
((2R,3R,4S)-2-({[(1S)-2-HYDROXY-1-PHENYLETHYL]AMINO...)Show SMILES OC[C@@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1 Show InChI InChI=1S/C13H20N2O3/c16-8-11(9-4-2-1-3-5-9)14-6-10-13(18)12(17)7-15-10/h1-5,10-18H,6-8H2/t10-,11-,12+,13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM50016703
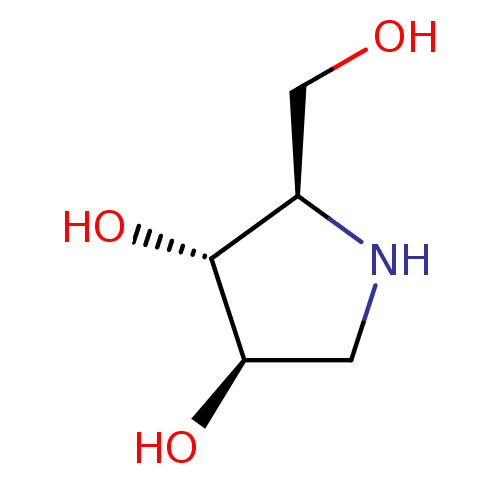
(2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402973
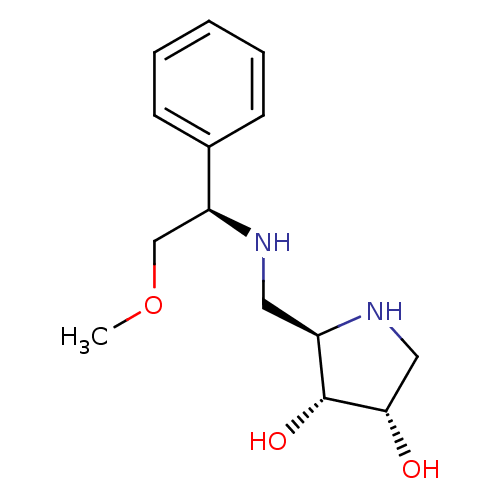
(CHEMBL2206817)Show SMILES COC[C@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1 |r| Show InChI InChI=1S/C14H22N2O3/c1-19-9-12(10-5-3-2-4-6-10)15-7-11-14(18)13(17)8-16-11/h2-6,11-18H,7-9H2,1H3/t11-,12+,13+,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50402985
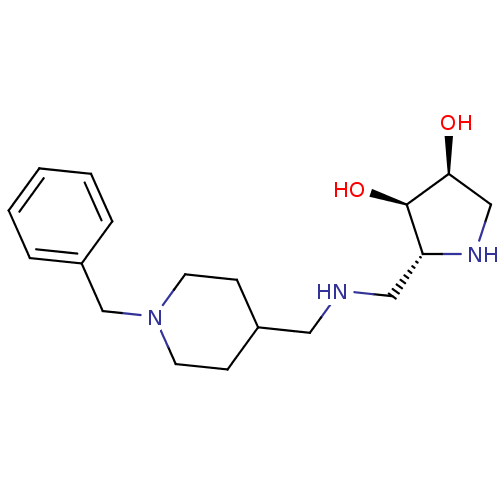
(CHEMBL2206829)Show SMILES O[C@H]1CN[C@H](CNCC2CCN(Cc3ccccc3)CC2)[C@H]1O |r| Show InChI InChI=1S/C18H29N3O2/c22-17-12-20-16(18(17)23)11-19-10-14-6-8-21(9-7-14)13-15-4-2-1-3-5-15/h1-5,14,16-20,22-23H,6-13H2/t16-,17+,18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687
Curated by ChEMBL
| Assay Description
Inhibition of human golgi alpha mannosidase 2 |
Bioorg Med Chem 20: 6945-59 (2012)
Article DOI: 10.1016/j.bmc.2012.10.011
BindingDB Entry DOI: 10.7270/Q26T0NTC |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data