

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089452 (CHEMBL3578201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456924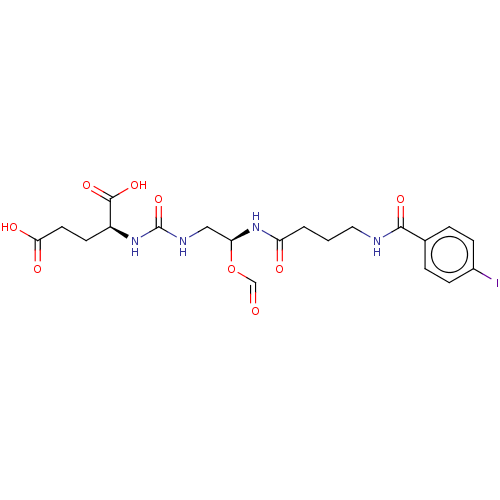 (US10736974, Compound YC-I-27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute Curated by ChEMBL | Assay Description Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G | Bioorg Med Chem Lett 20: 7222-5 (2010) Article DOI: 10.1016/j.bmcl.2010.10.109 BindingDB Entry DOI: 10.7270/Q2GB24B9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Binding affinity to NAALADase | J Med Chem 55: 9510-20 (2012) Article DOI: 10.1021/jm300710j BindingDB Entry DOI: 10.7270/Q28053R3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456928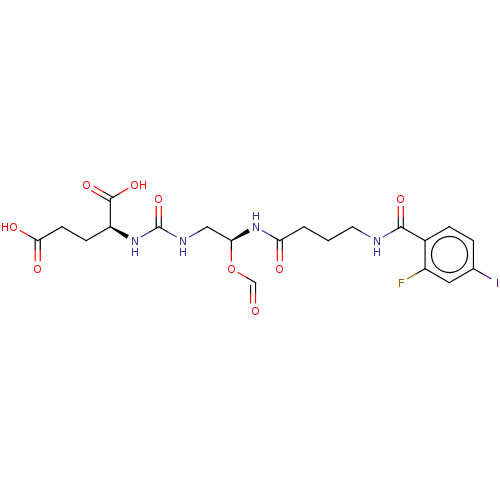 (US10736974, Compound XY-44) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089451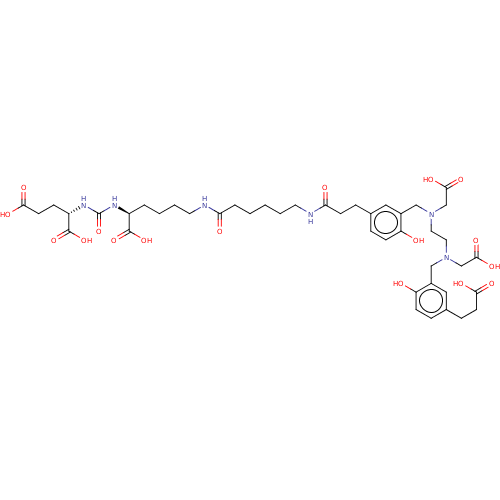 (CHEMBL3578202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456930 (US10736974, Compound XY-59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456923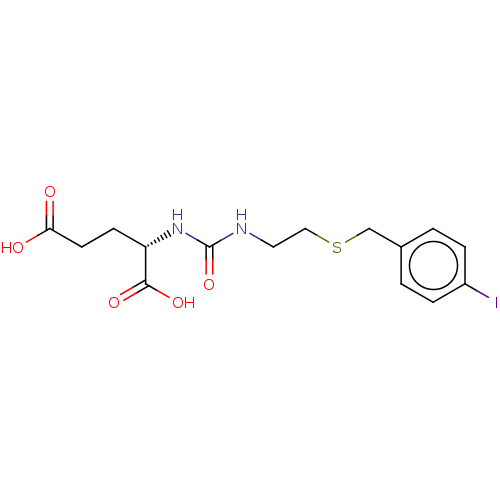 (US10736974, Compound DCIBC) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578399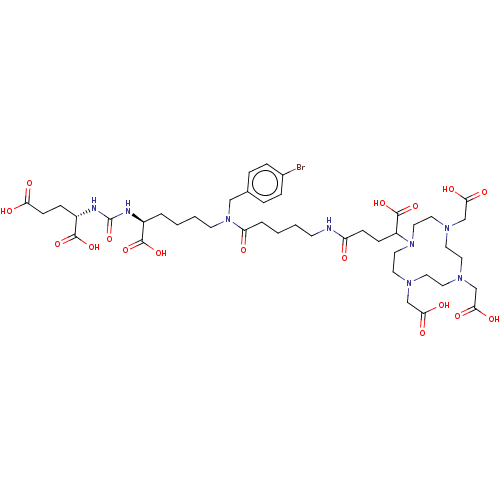 (US11478558, Compound L9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456931 (US10736974, Compound XY-58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046832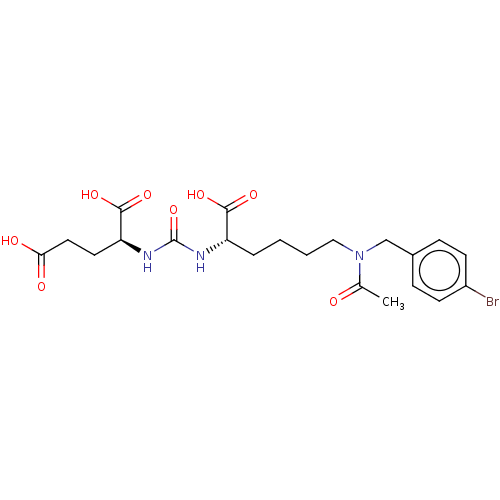 (CHEMBL3309678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046832 (CHEMBL3309678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089454 (CHEMBL3578199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479434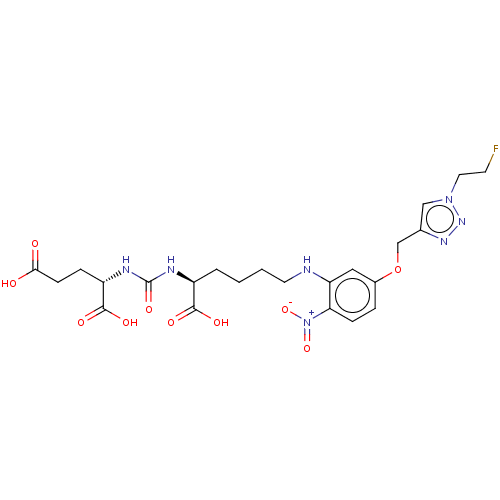 (US10894807, ID P242) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089455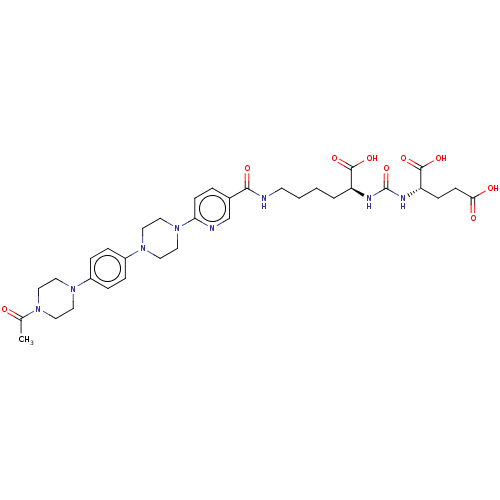 (CHEMBL3578197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM642537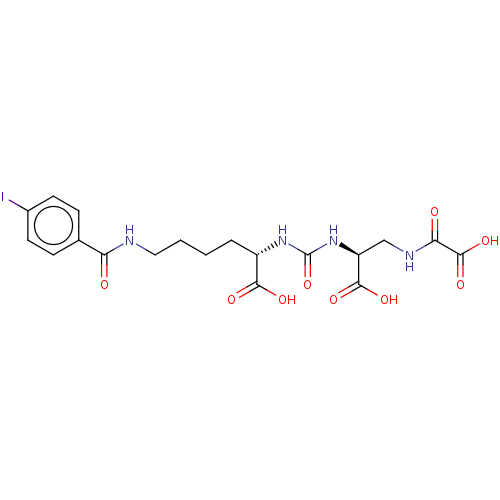 (US20230414794, Compound S2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17759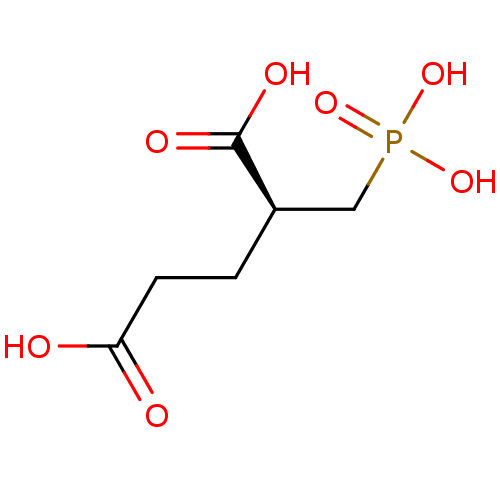 ((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Binding affinity to NAALADase | J Med Chem 55: 9510-20 (2012) Article DOI: 10.1021/jm300710j BindingDB Entry DOI: 10.7270/Q28053R3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456929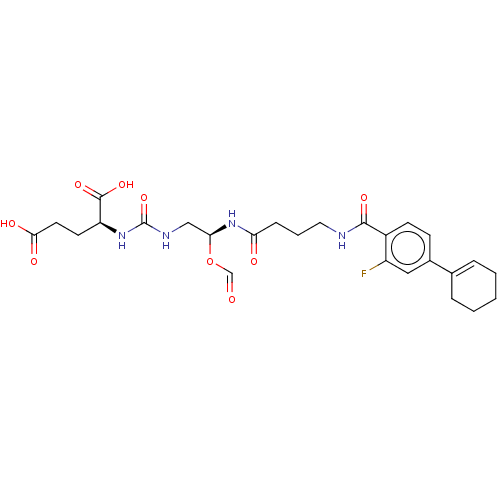 (US10736974, Compound XY-45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089453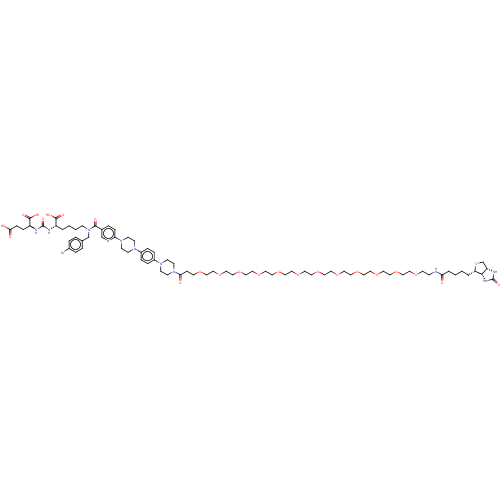 (CHEMBL3578200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578398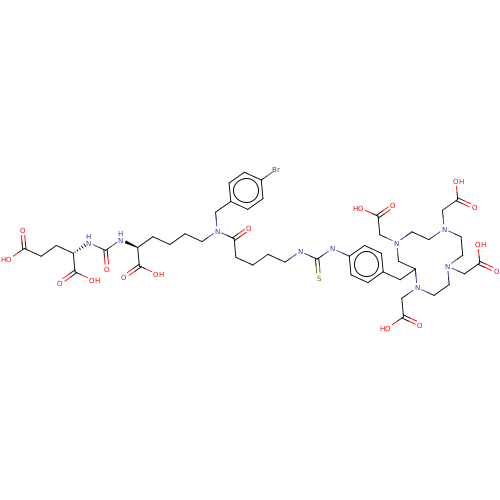 (US11478558, Compound L8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454862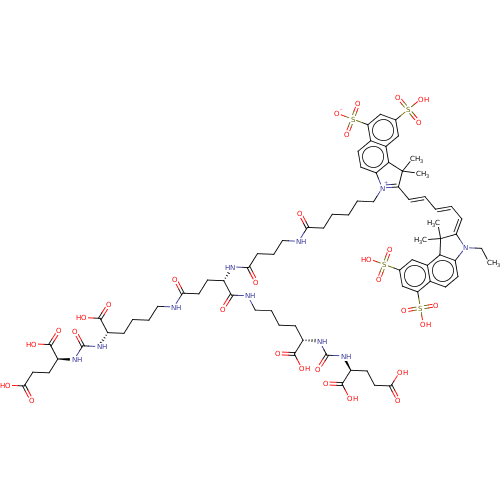 (CHEMBL4211875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479437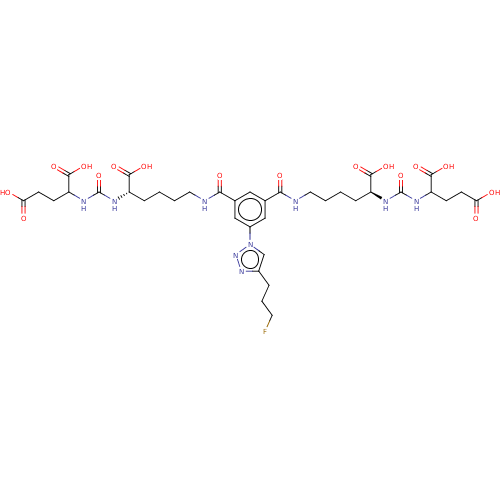 (US10894807, ID P246) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046850 (CHEMBL3311268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046850 (CHEMBL3311268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246934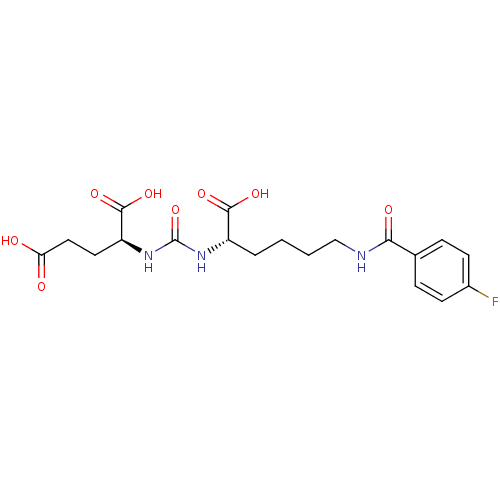 ((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by fluorescence-based assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479444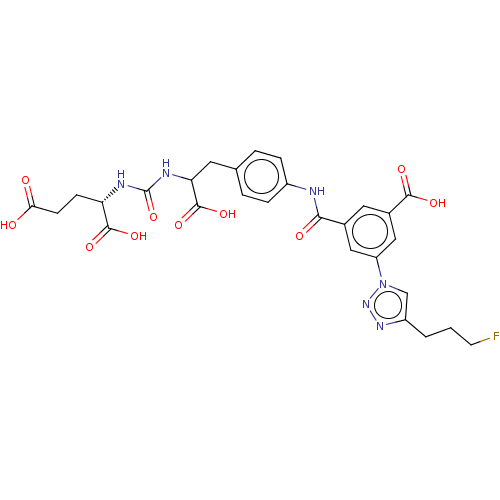 (US10894807, ID P253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479453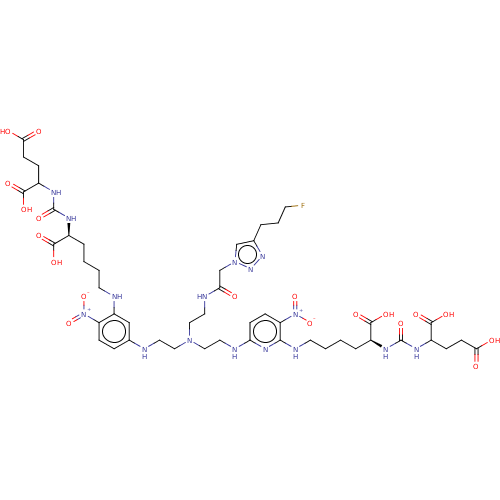 (US10894807, ID P273) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479458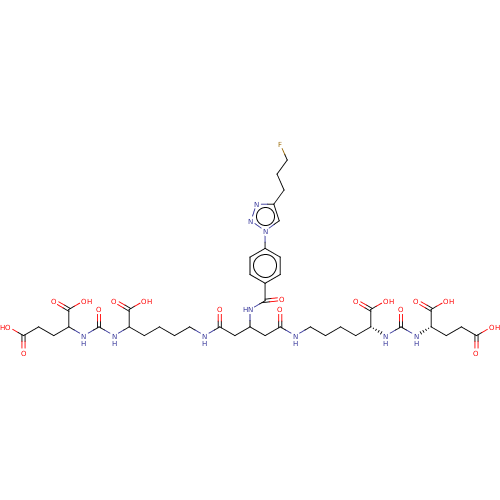 (US10894807, ID P278) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrate | J Med Chem 46: 1989-96 (2003) Article DOI: 10.1021/jm020515w BindingDB Entry DOI: 10.7270/Q2SQ8ZRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479450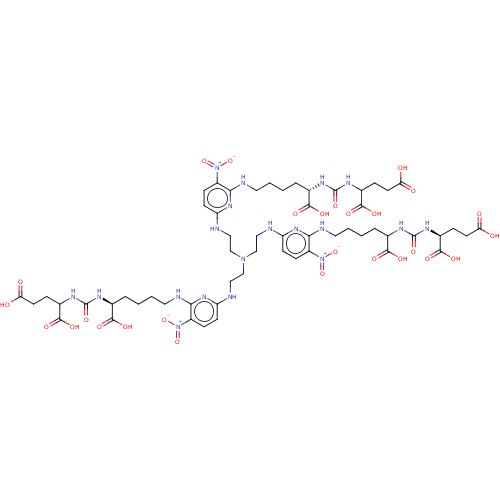 (US10894807, ID P270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046831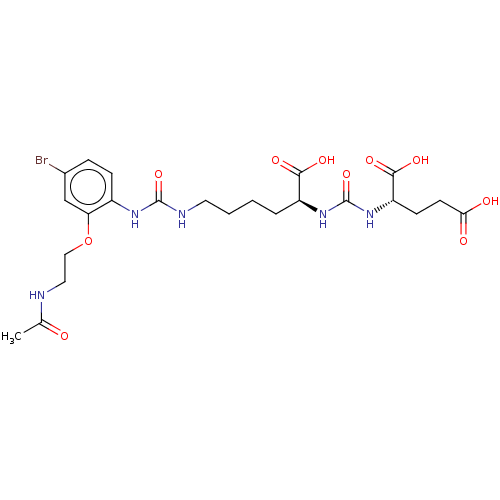 (CHEMBL3309680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246934 ((S)-2-(3-((S)-1-carboxy-5-(4-fluorobenzamido)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutions Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell extract using [3H]NAAG by radiometric assay | J Med Chem 51: 7933-43 (2008) Article DOI: 10.1021/jm801055h BindingDB Entry DOI: 10.7270/Q2RV0NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456920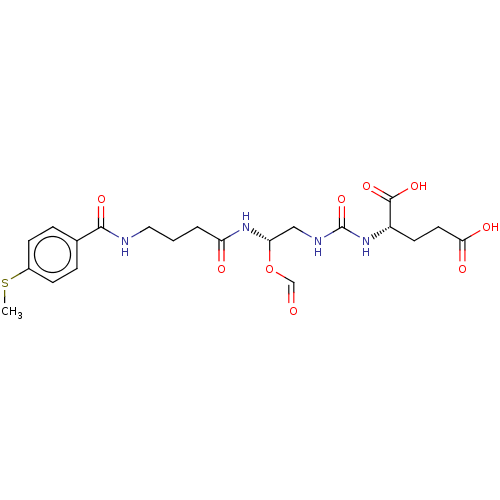 (US10736974, Entry 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578394 (US11478558, Compound L4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM578392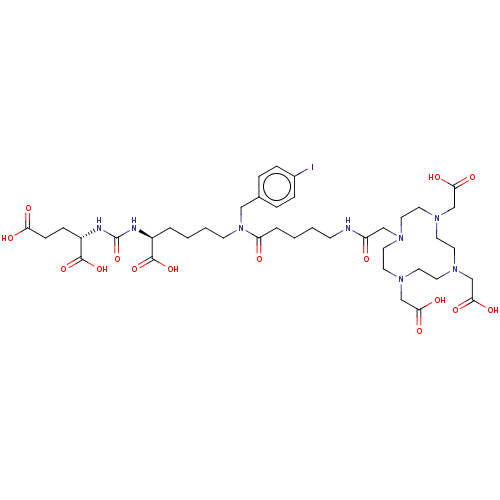 (US11478558, Compound L2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TBD | Citation and Details BindingDB Entry DOI: 10.7270/Q20P138Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50046835 (CHEMBL3309676) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Avi-tagged glutamate carboxypeptidase 2 extracellular domain (44 to 750 amino acids) using pteroyl-di-L-gl... | Bioorg Med Chem 22: 4099-108 (2014) Article DOI: 10.1016/j.bmc.2014.05.061 BindingDB Entry DOI: 10.7270/Q2NK3GP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for inhibition of the Folate hydrolase | J Med Chem 39: 619-22 (1996) Article DOI: 10.1021/jm950801q BindingDB Entry DOI: 10.7270/Q2FN16V8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479415 (US10894807, ID P200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479429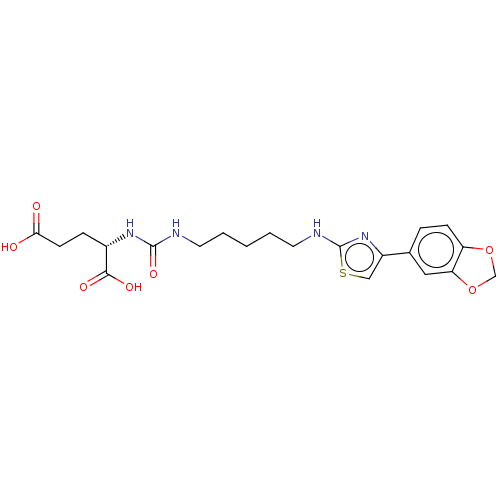 (US10894807, ID P235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479431 (US10894807, ID P238) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17759 ((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GCPII (unknown origin) NAALADase activity | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128044 BindingDB Entry DOI: 10.7270/Q2SQ9459 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM479449 (US10894807, ID P266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siemens Medical Solutions USA, Inc. US Patent | Assay Description The inhibitory activity of all compounds was determined using a fluorescent assay of human PSMA activity. The enzyme used in the assay was purchased ... | US Patent US10894807 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Binding affinity to NAALADase | J Med Chem 55: 9510-20 (2012) Article DOI: 10.1021/jm300710j BindingDB Entry DOI: 10.7270/Q28053R3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM456925 (US10736974, Compound YC-I-26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
THE JOHNS HOPKINS UNIVERSITY US Patent | Assay Description The PSMA inhibitory activity was determined using a modification of the fluorescence-based Amplex Red Glutamic Acid Assay. Briefly, lysates of LNCaP ... | US Patent US10736974 (2020) BindingDB Entry DOI: 10.7270/Q29C71GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 784 total ) | Next | Last >> |