

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-2 (Homo sapiens (Human)) | BDBM221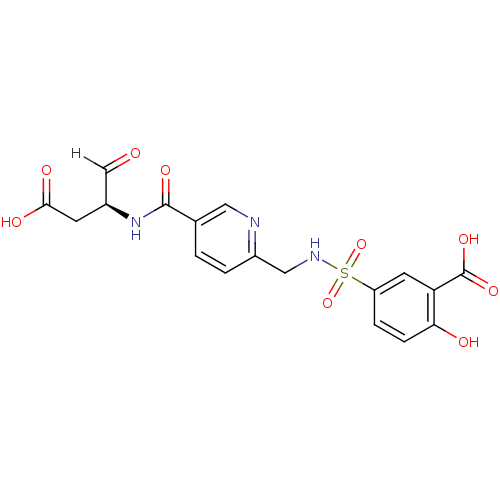 (5-{[(5-{[(2S)-1-carboxy-3-oxopropan-2-yl]carbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 960 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10323 ((S)-1-Benzyl-5-{1-[2-(phenoxymethyl)pyrrolidinyl]s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10318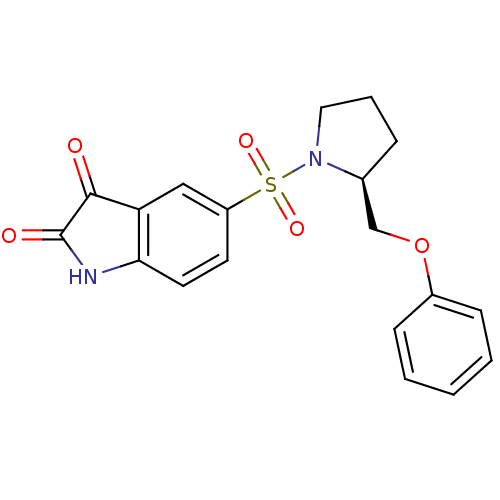 ((S)-5-{1-[2-(Phenoxymethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10315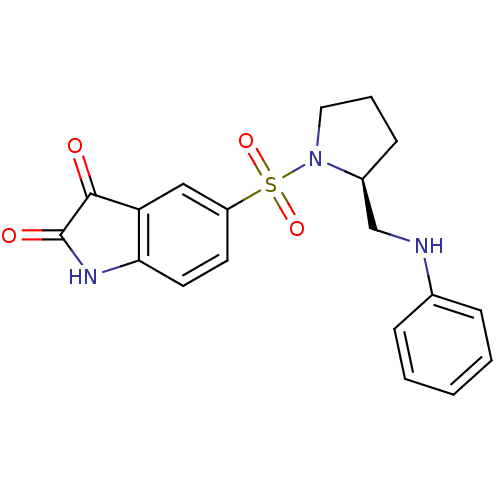 ((S)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM220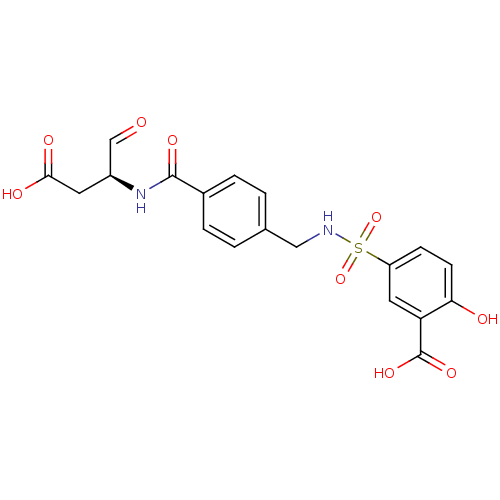 (5-{[(4-{[(2S)-1-carboxy-3-oxopropan-2-yl]carbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 9.00E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM222 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10316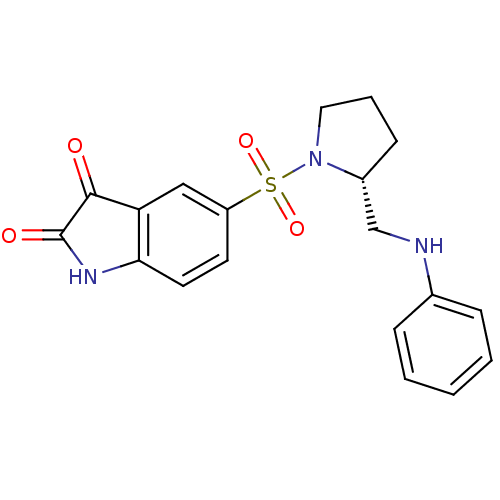 ((R)-5-{1-[2-(Anilinomethyl)pyrrolidinyl]sulfonyl}i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM224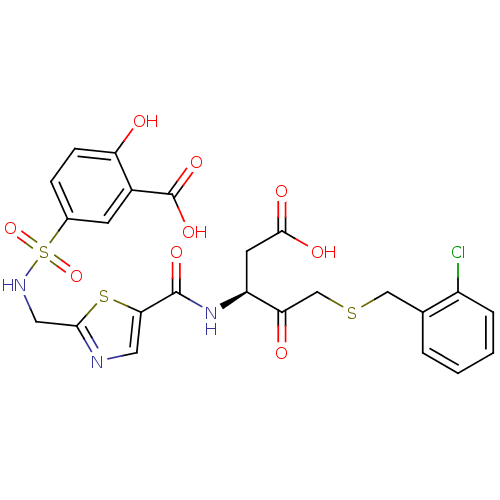 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM225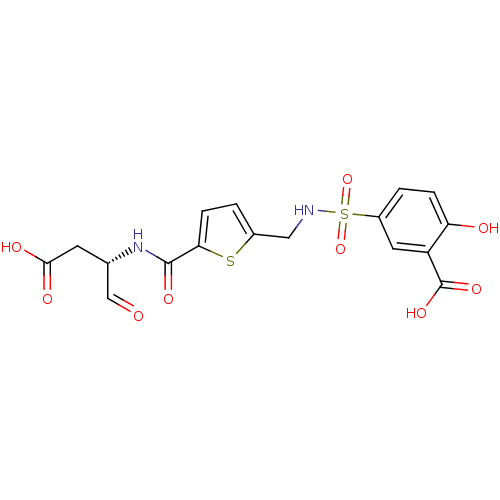 ((S)-5-5-(1-Carboxymethyl-2-oxo-ethylcarbamoyl)-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+4 | -26.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10305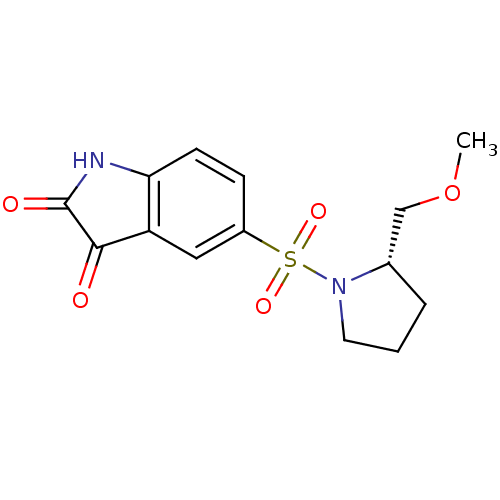 ((S)-5-[1-(2-Methoxymethyl)pyrrolidinylsulfonyl]isa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10298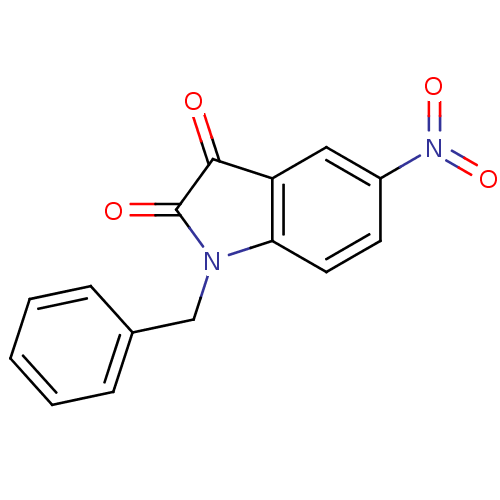 (1-benzyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50200932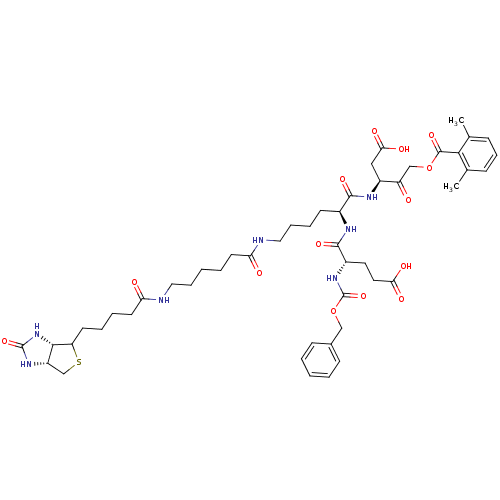 ((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh Curated by ChEMBL | Assay Description Inhibition of human caspase 2 | J Med Chem 49: 7636-45 (2006) Article DOI: 10.1021/jm060385h BindingDB Entry DOI: 10.7270/Q2319VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM223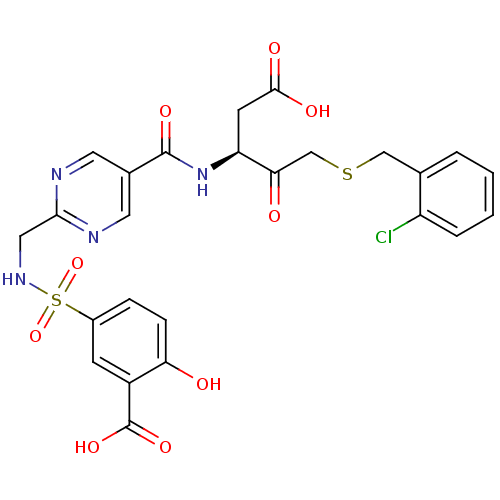 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM219 (5-({[(5-{[(2S)-1-carboxy-4-{[(2-chlorophenyl)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM227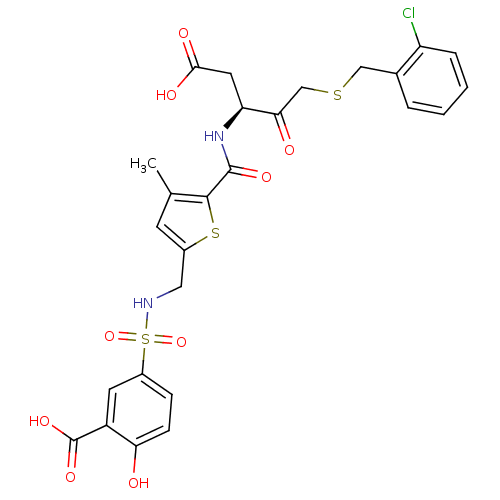 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | -25.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10309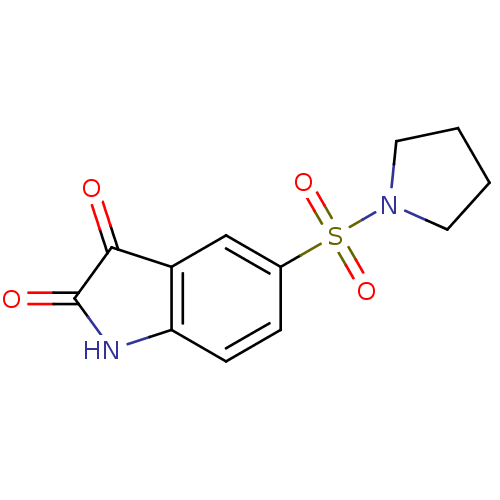 (5-(pyrrolidine-1-sulfonyl)-2,3-dihydro-1H-indole-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10297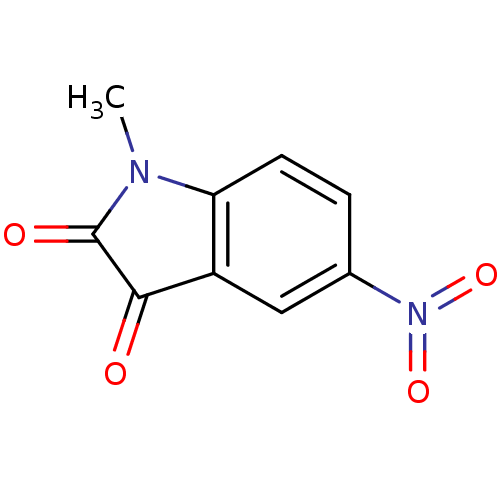 (1-methyl-5-nitro-2,3-dihydro-1H-indole-2,3-dione |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with... | J Med Chem 44: 2015-26 (2001) Article DOI: 10.1021/jm0100537 BindingDB Entry DOI: 10.7270/Q2ZW1J42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM226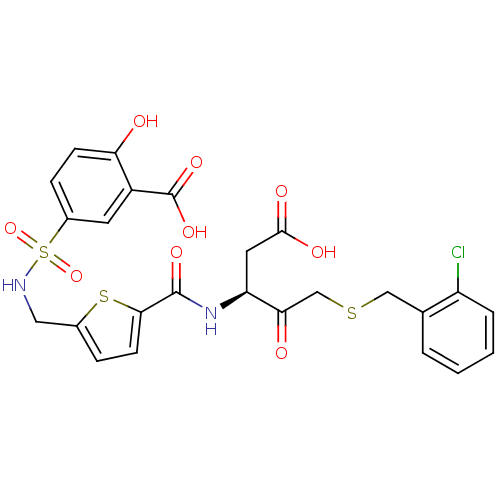 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50160957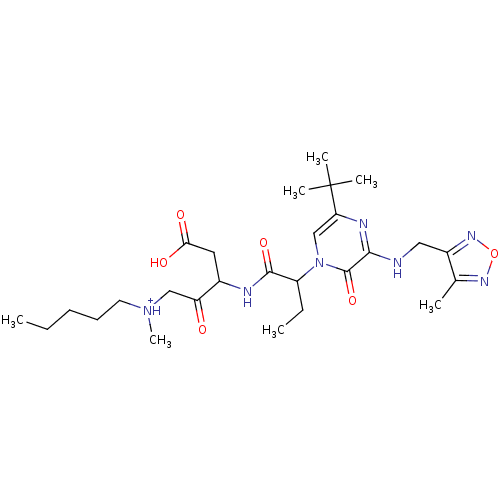 (CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against casp-2 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50160974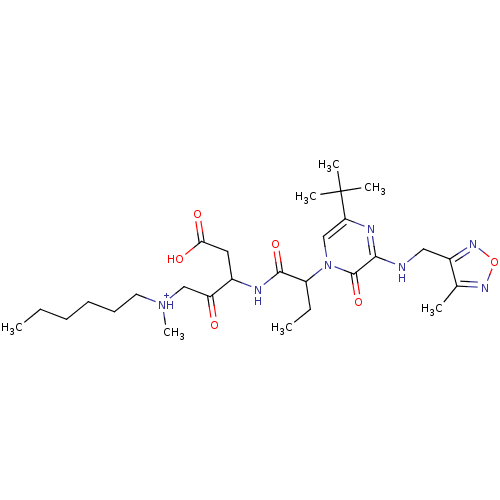 (CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against casp-2 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355101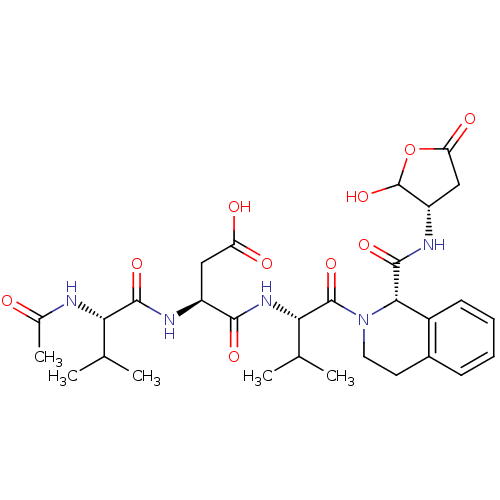 (CHEMBL1835324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355110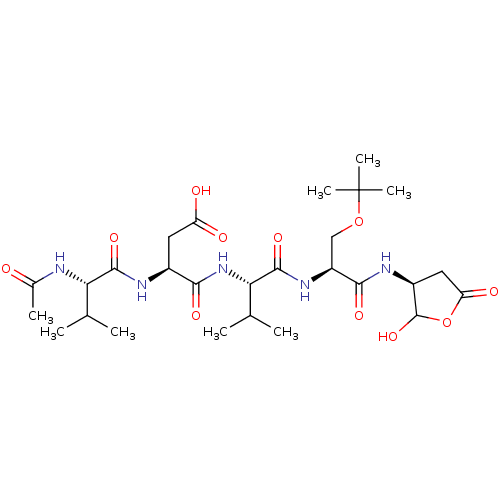 (CHEMBL1835208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355111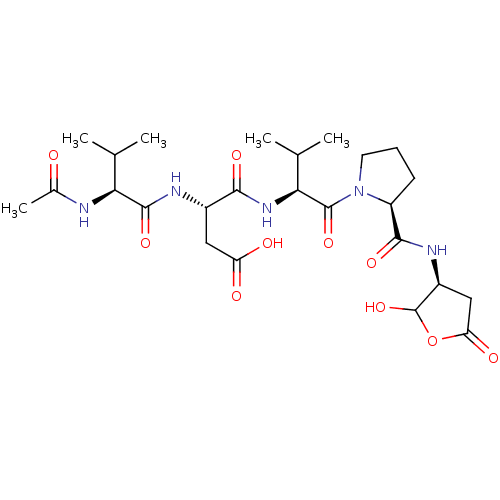 (CHEMBL1835210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355109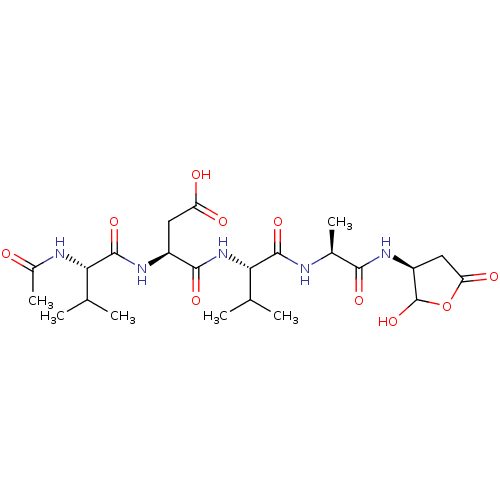 (CHEMBL1835209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355103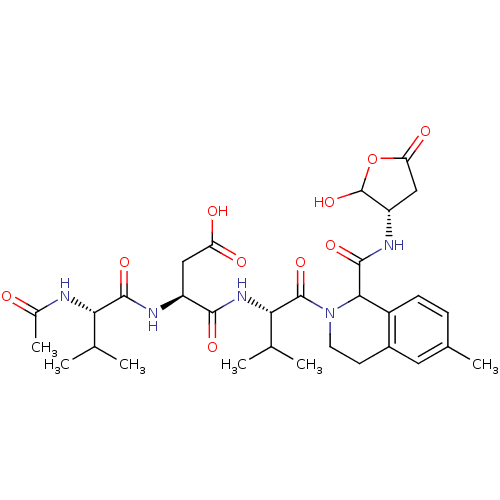 (CHEMBL1835399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355094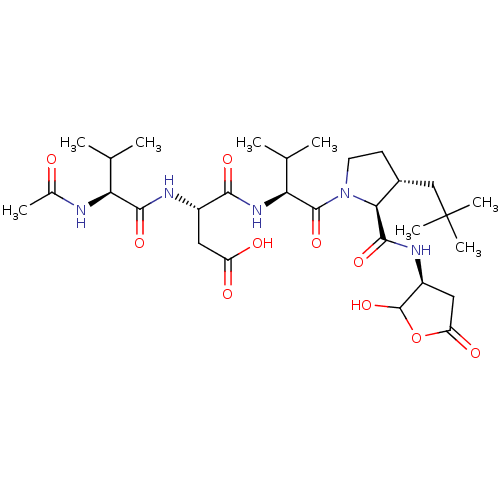 (CHEMBL1835317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355102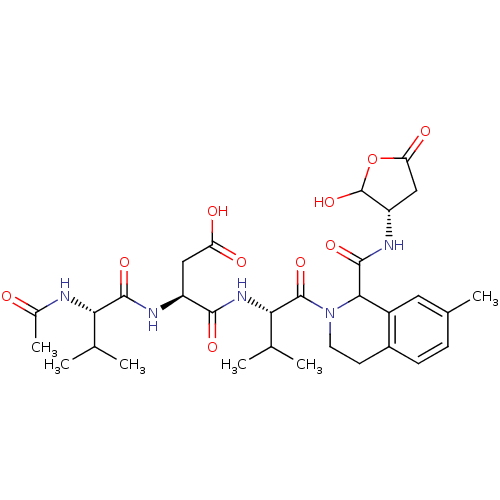 (CHEMBL1835325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355099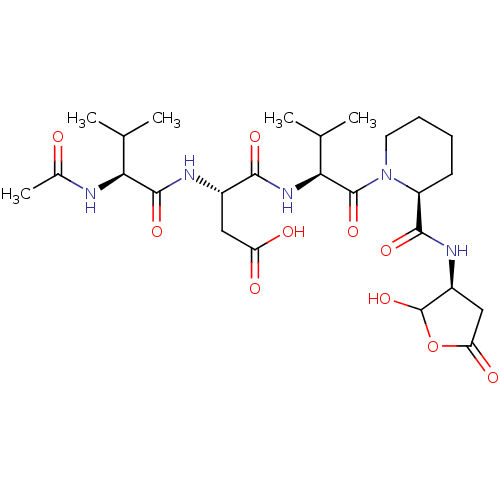 (CHEMBL1835322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355112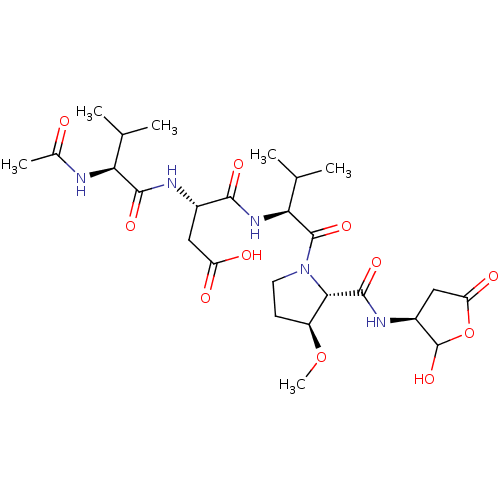 (CHEMBL1835211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355097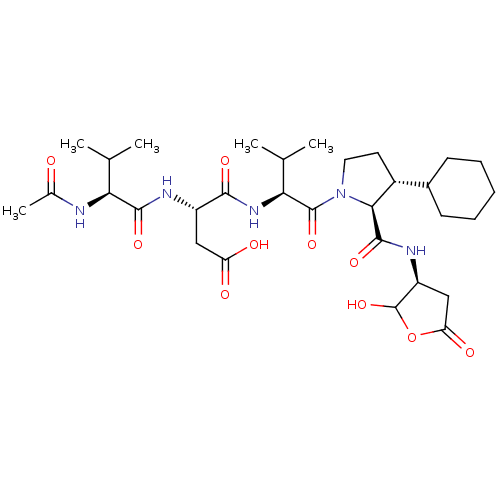 (CHEMBL1835320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355098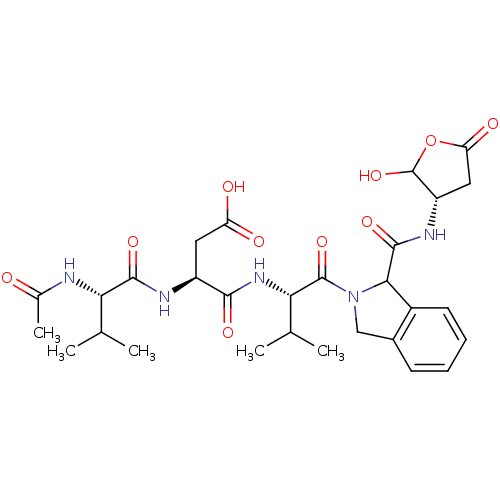 (CHEMBL1835321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355107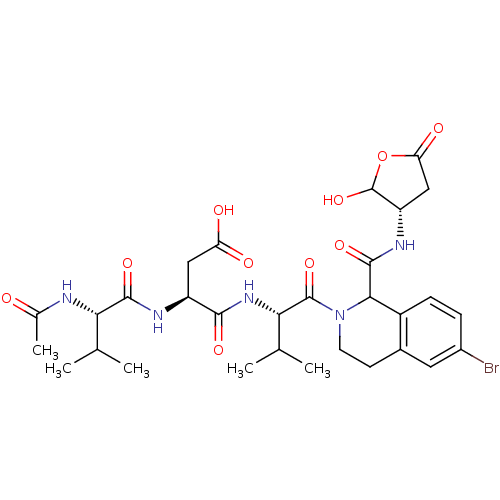 (CHEMBL1835403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355093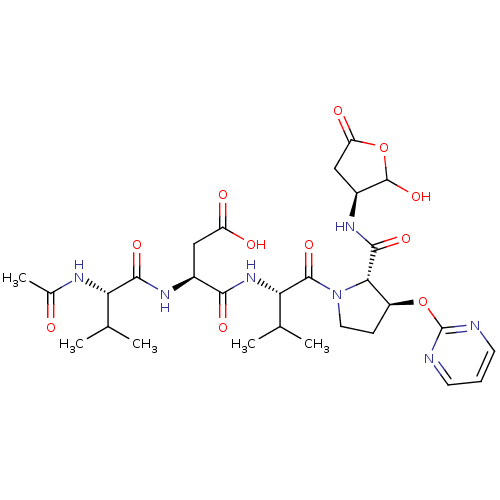 (CHEMBL1835316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10284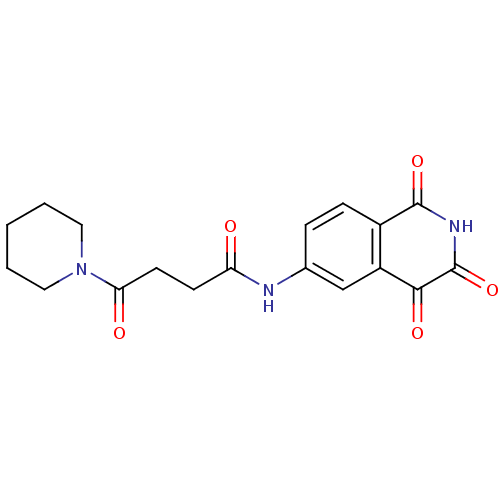 (4-Oxo-4-piperidin-1-yl-N-(1,3,4-trioxo-1,2,3,4-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10264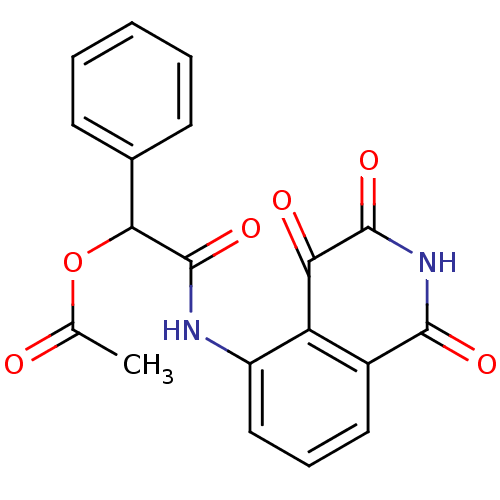 (2-oxo-1-phenyl-2-[(1,3,4-trioxo-1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355089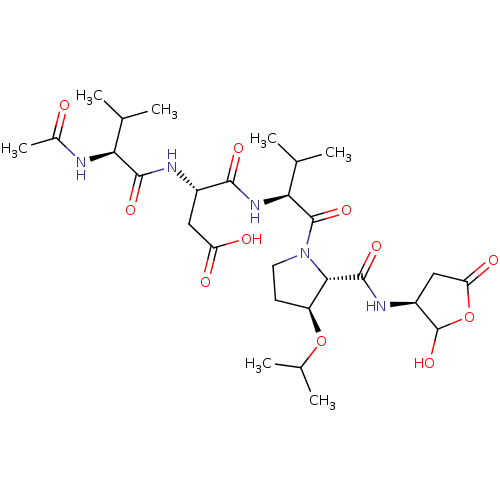 (CHEMBL1835212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355090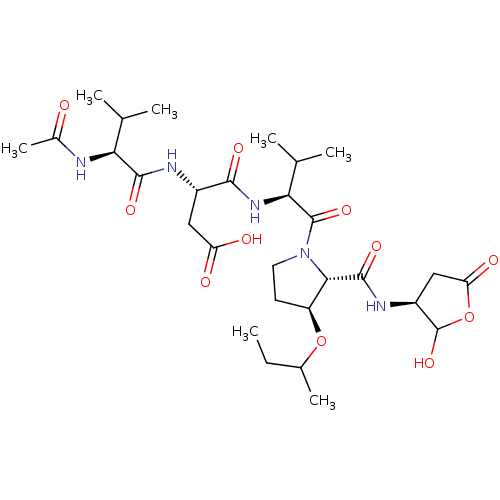 (CHEMBL1835313) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355095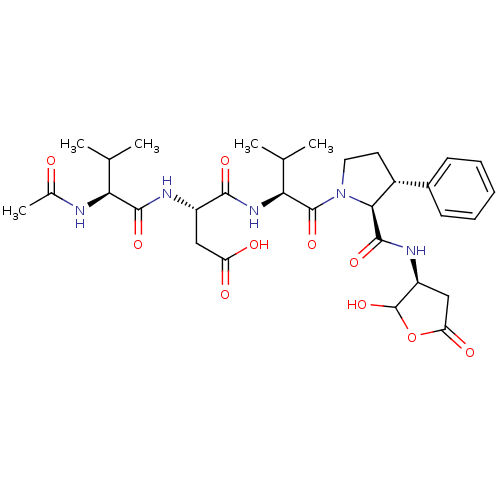 (CHEMBL1835318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355091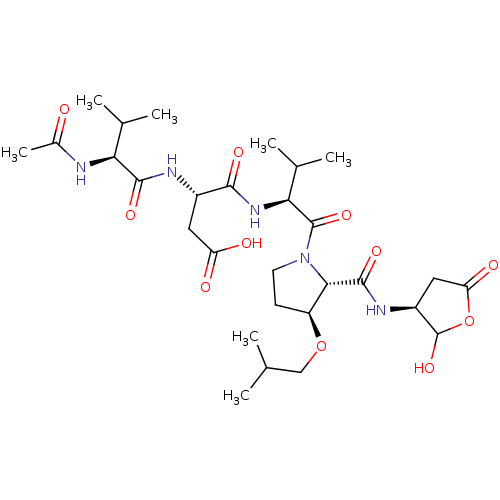 (CHEMBL1835314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 453 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355106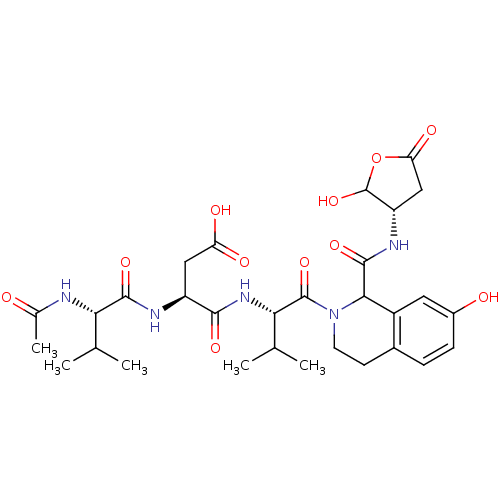 (CHEMBL1835402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10278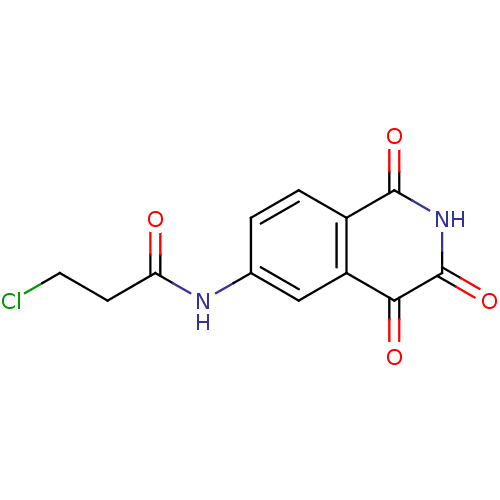 (3-Chloro-N-(1,2,3,4-tetrahydro-1,3,4-trioxoisoquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10287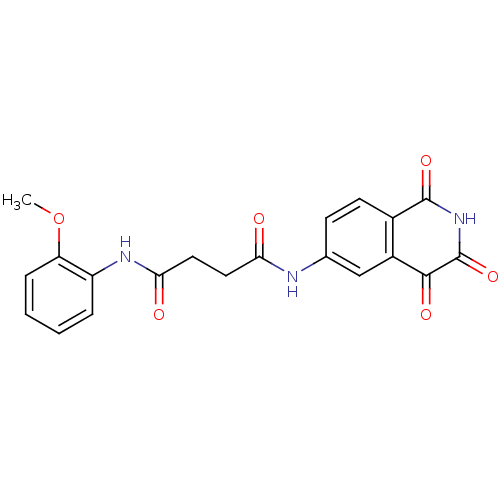 (Isoquinoline-1,3,4-trione 13f | N-(2-Methoxy-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355108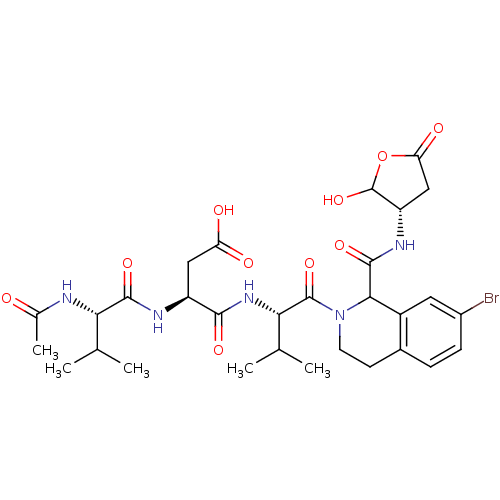 (CHEMBL1835404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 573 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355111 (CHEMBL1835210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355092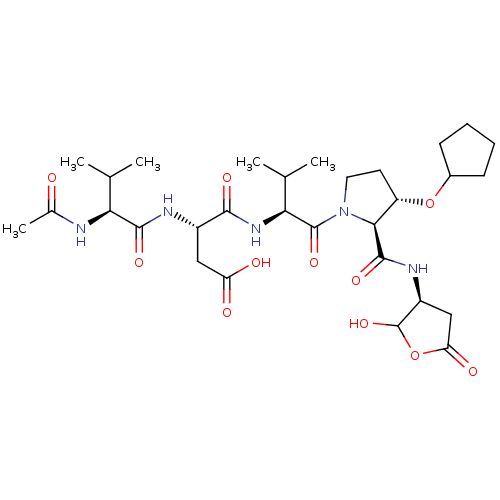 (CHEMBL1835315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 772 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355098 (CHEMBL1835321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10280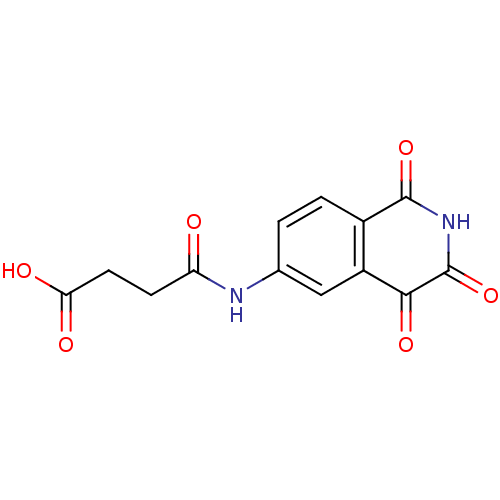 (3-[(1,3,4-trioxo-1,2,3,4-tetrahydroisoquinolin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 859 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355099 (CHEMBL1835322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355109 (CHEMBL1835209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10247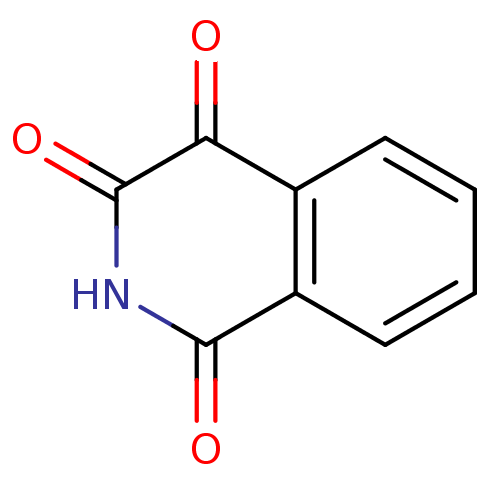 (1,2,3,4-tetrahydroisoquinoline-1,3,4-trione | Isoq...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |