

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemagglutinin (Influenza A virus (A/Shangdong/9/93(H3N2))) | BDBM4934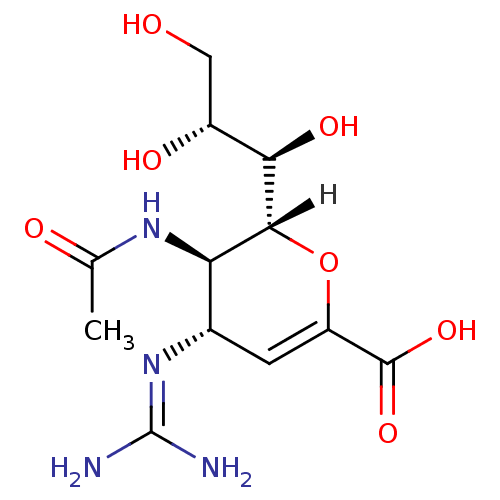 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 1 | -53.4 | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (A/Shangdong/9/93(H3N2))) | BDBM5210 (3-carbamimidamido-4-acetamido-5-[(1S,2R)-1,2,3-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | >1.00E+5 | >-23.7 | >1.00E+5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
Gilead Sciences Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 7: 1837-42 (1997) Article DOI: 10.1016/S0960-894X(97)00333-8 BindingDB Entry DOI: 10.7270/Q2FX77MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611021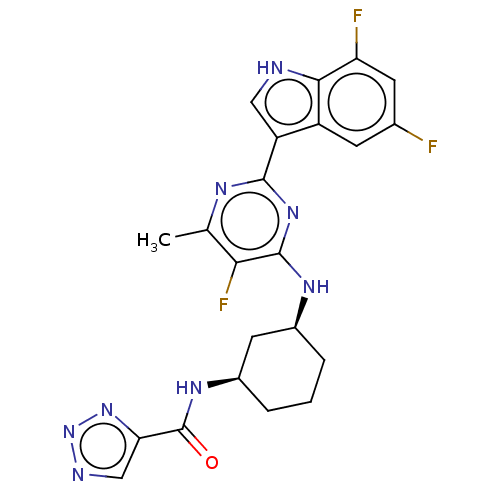 (US10626108, Compound 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611022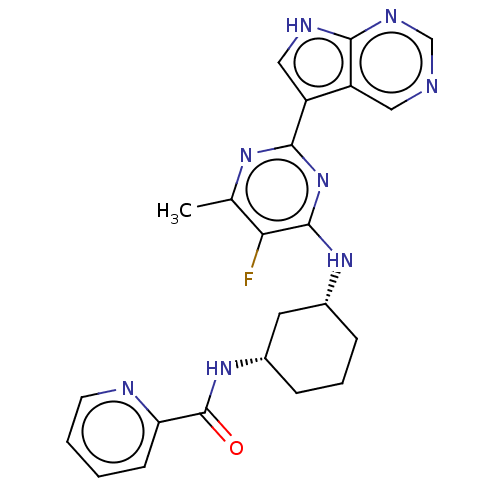 (US10626108, Compound 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611015 (US10626108, Compound 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611029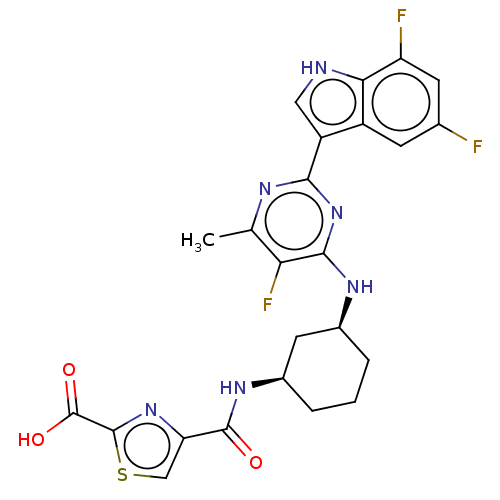 (US10626108, Compound 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611020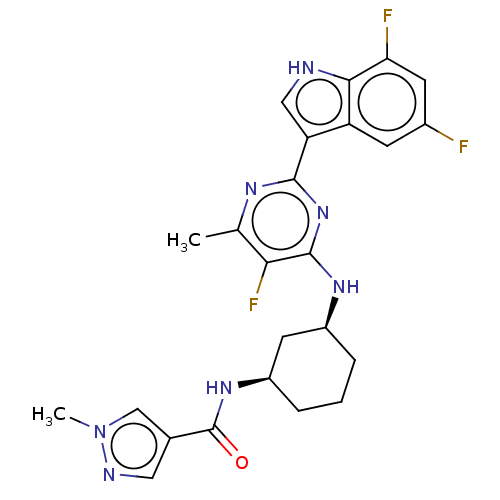 (US10626108, Compound 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5023 ((-)-(1R,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbuty...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5024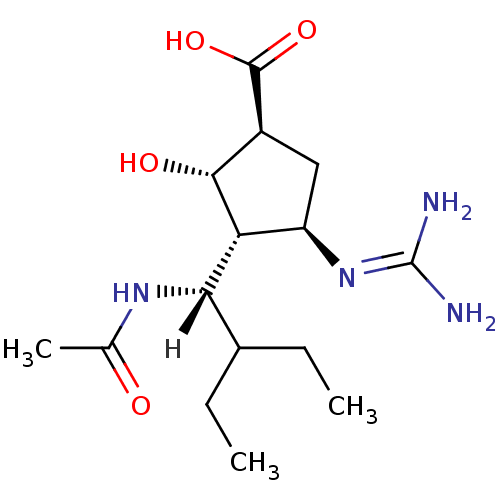 ((-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611056 (US10626108, Compound 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611028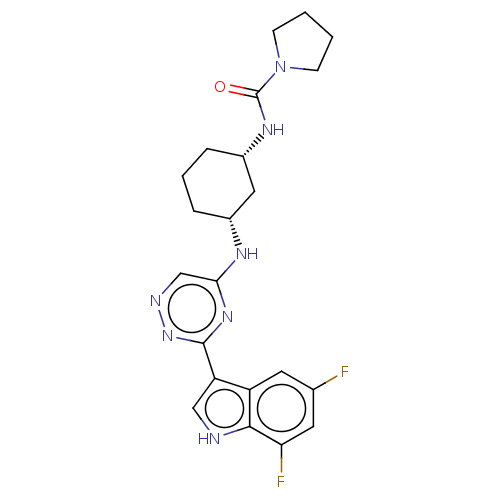 (US10626108, Compound 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM4934 ((2R,3R,4S)-4-carbamimidamido-3-acetamido-2-[(1R,2R...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611026 (US10626108, Compound 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50216627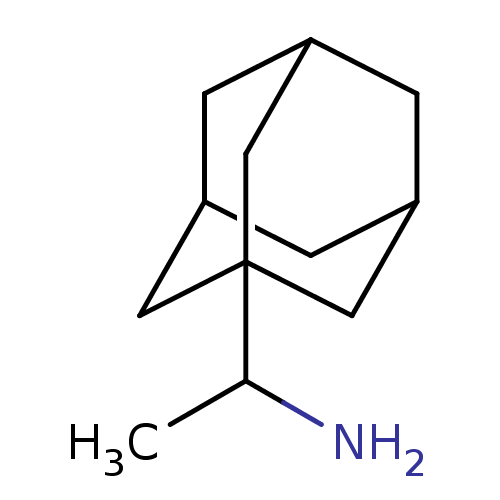 ((alpha-methyl-1-adamantyl)methylamine | 1-Adamanta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cytopathic effect (CPE) inhibition test is used for measuring the antiviral effect. Monolayer cells in 96-well plates are inoculated with 0.1 ml ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MW2MBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5019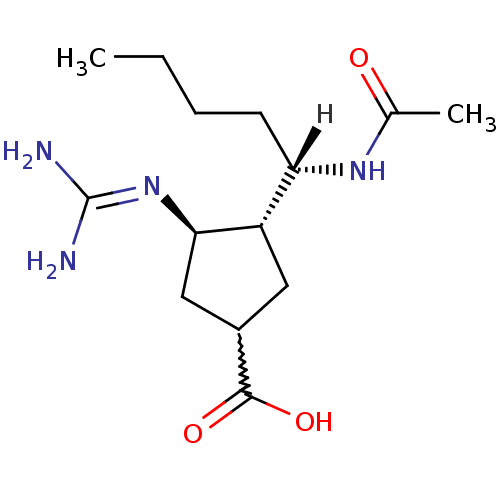 ((3R*,4R*)-3-[(1S*)-1-(Acetylamino)pentyl]-4-{[amin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin () | BDBM611014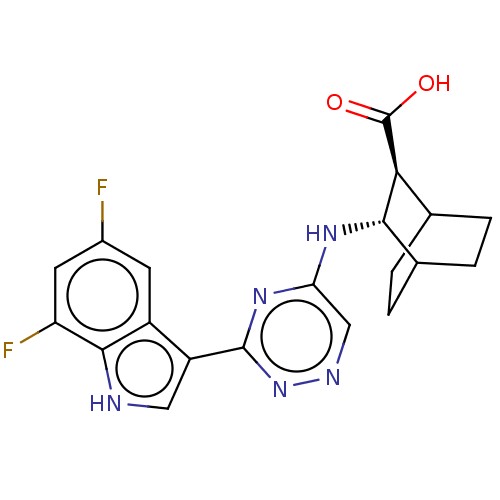 (US10626108, Compound 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3KH8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5021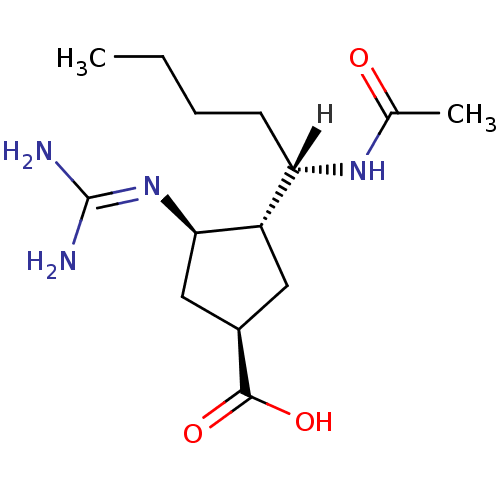 ((1R,3R,4R)-3-carbamimidamido-4-[(1S)-1-acetamidope...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247019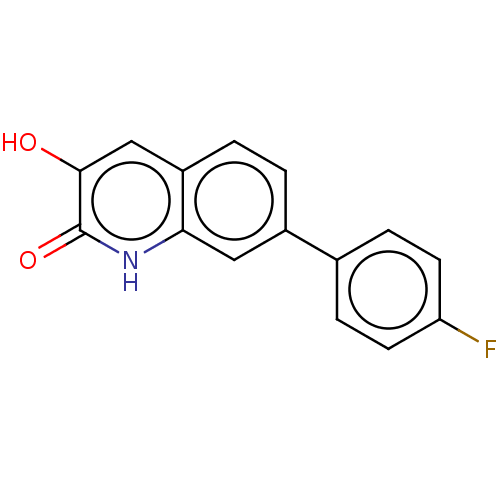 (US9701638, 10) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247018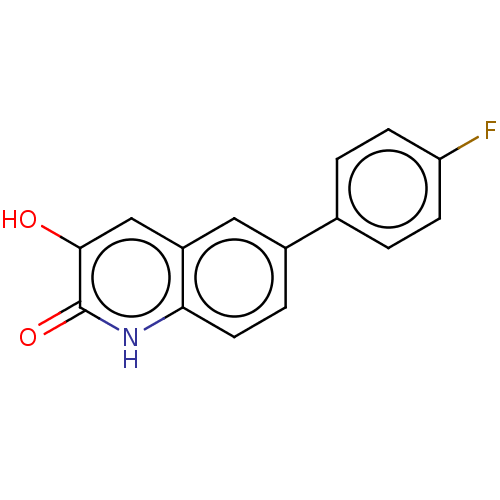 (US9701638, 9) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5018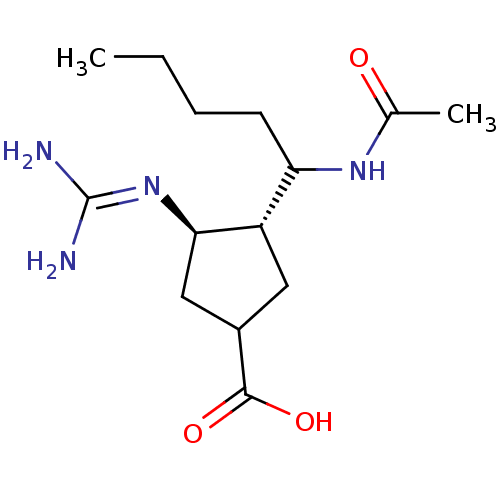 ((3R*,4R*)-3-[1-(Acetylamino)pentyl]-4-{[amino(imin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 893 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247021 (US9701638, 12) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50280403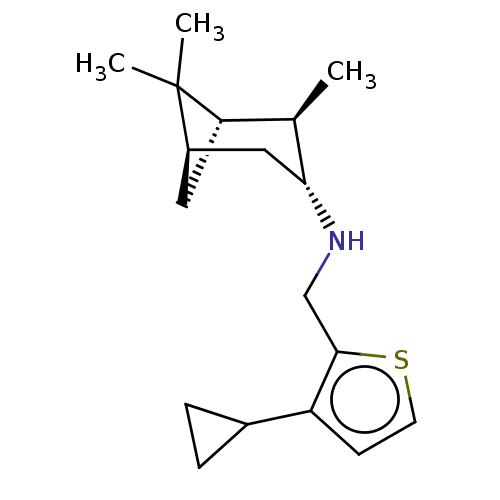 (CHEMBL4163615) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences & The Fifth Affiliated Hospital Curated by ChEMBL | Assay Description In vivo inhibition of leutenising hormone in rats. | J Med Chem 61: 5187-5198 (2018) Article DOI: 10.1021/acs.jmedchem.8b00042 BindingDB Entry DOI: 10.7270/Q2NV9MS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247024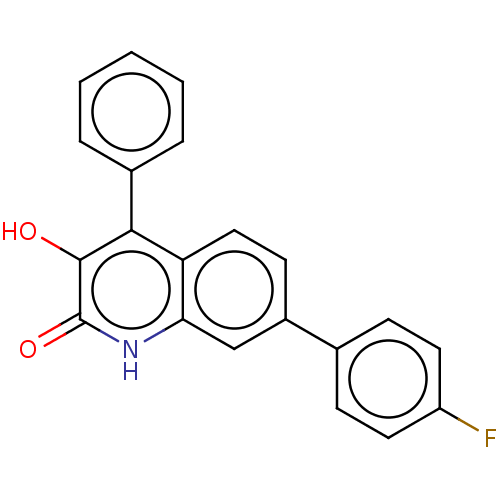 (US9701638, 15) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/X-31 H3N2)) | BDBM50402142 (CHEMBL2207987) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of hemagglutinin in Influenza A virus (A/X-31(H3N2)) infected in chicken red blood cells assessed as inhibition of hemolysis preincubated ... | Bioorg Med Chem 20: 7155-9 (2012) Article DOI: 10.1016/j.bmc.2012.09.064 BindingDB Entry DOI: 10.7270/Q28W3FFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247017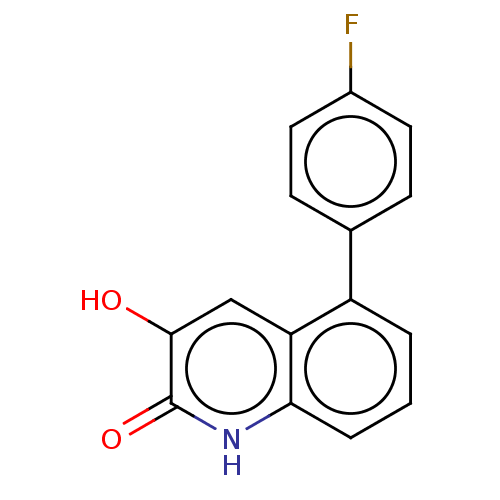 (US9701638, 8) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/X-31 H3N2)) | BDBM50402143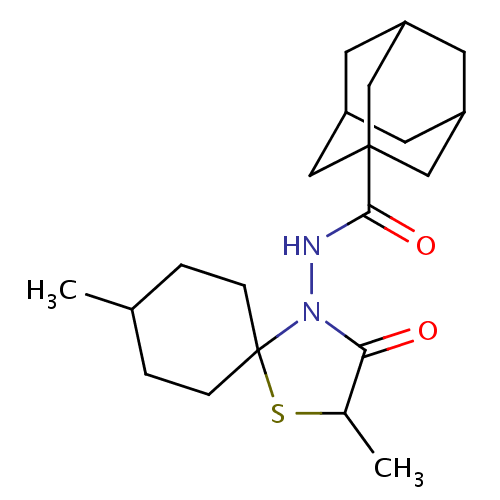 (CHEMBL2207978) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of hemagglutinin in Influenza A virus (A/X-31(H3N2)) infected in chicken red blood cells assessed as inhibition of hemolysis preincubated ... | Bioorg Med Chem 20: 7155-9 (2012) Article DOI: 10.1016/j.bmc.2012.09.064 BindingDB Entry DOI: 10.7270/Q28W3FFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360450 (CHEMBL1934618) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247020 (US9701638, 11) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5020 ((1S,3R,4R)-3-carbamimidamido-4-[(1S)-1-acetamidope...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.29E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM535848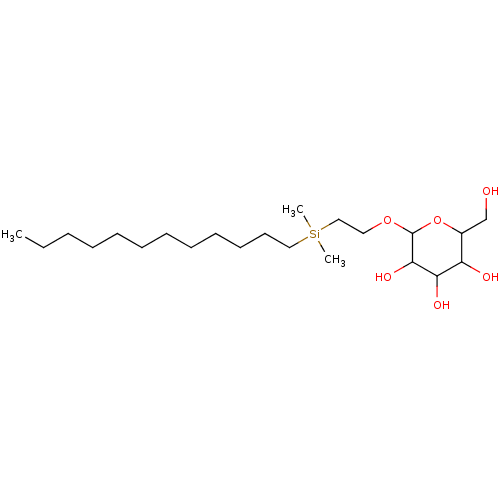 (US11241393, Sil 3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cytopathic effect (CPE) inhibition test is used for measuring the antiviral effect. Monolayer cells in 96-well plates are inoculated with 0.1 ml ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MW2MBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM535847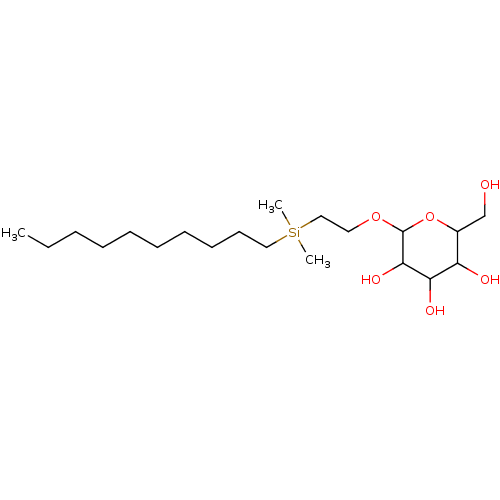 (US11241393, Sil 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cytopathic effect (CPE) inhibition test is used for measuring the antiviral effect. Monolayer cells in 96-well plates are inoculated with 0.1 ml ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MW2MBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247013 (US9701638, 4) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247014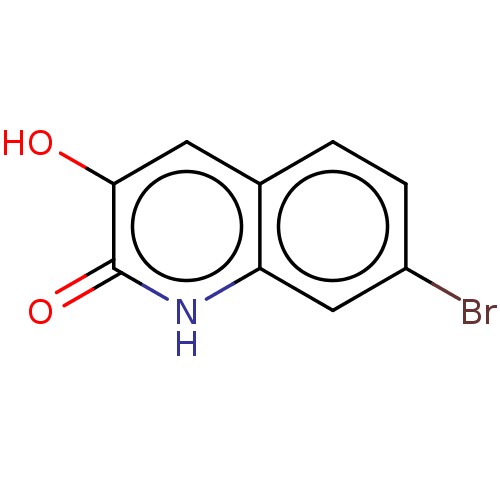 (US9701638, 5) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360450 (CHEMBL1934618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360452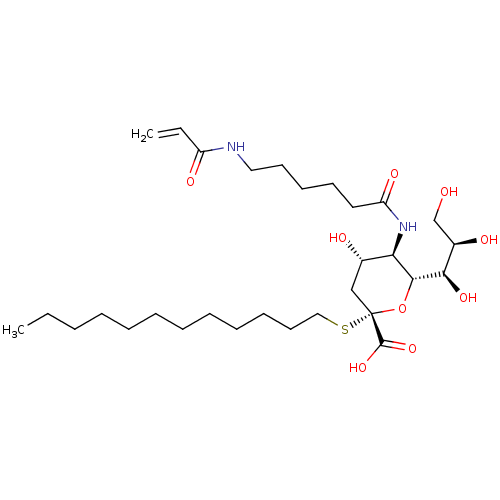 (CHEMBL1934620) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360452 (CHEMBL1934620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM535849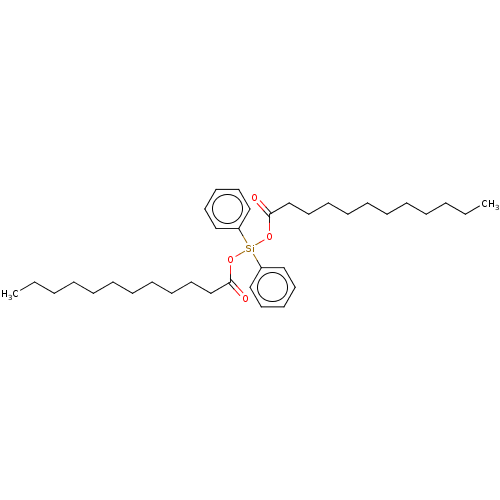 (US11241393, Sil 25) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cytopathic effect (CPE) inhibition test is used for measuring the antiviral effect. Monolayer cells in 96-well plates are inoculated with 0.1 ml ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2MW2MBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247015 (US9701638, 6) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247016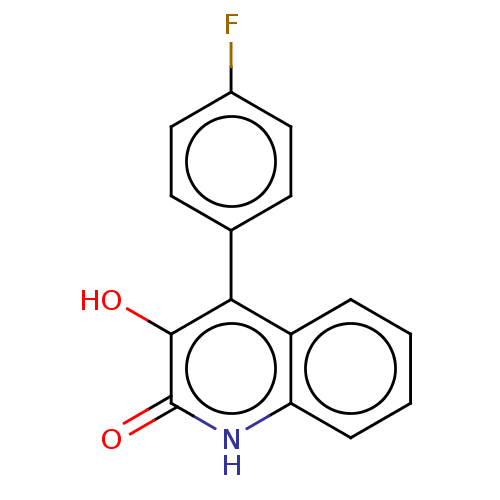 (US9701638, 7) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50551836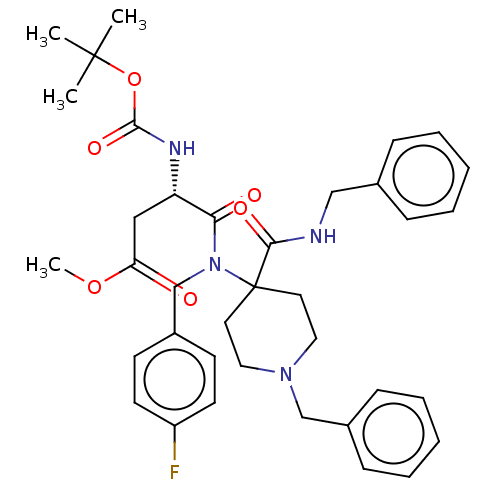 (CHEMBL3288937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Influenza A virus (A/PR 8/34 (H1N1)) hemagglutinin transfected in human HeLa cells assessed as reduction in low pH-indued polykaryon fo... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112223 BindingDB Entry DOI: 10.7270/Q28919GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247012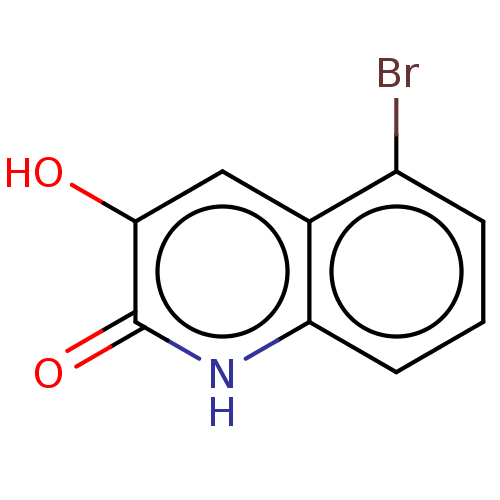 (US9701638, 3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247025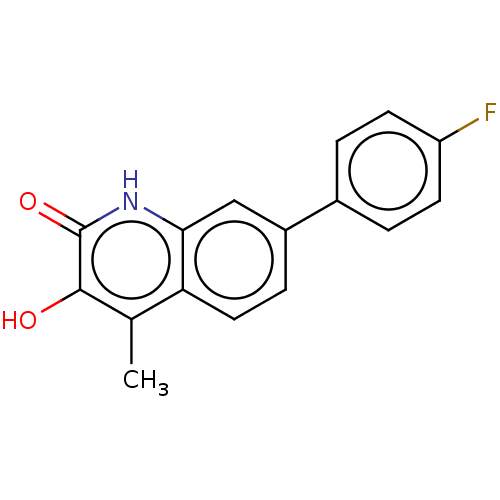 (US9701638, 16) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247022 (US9701638, 13) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM31148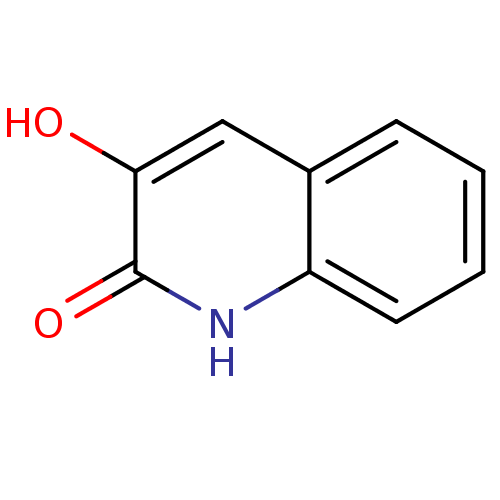 (3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza B virus (B/Victoria/70)) | BDBM5017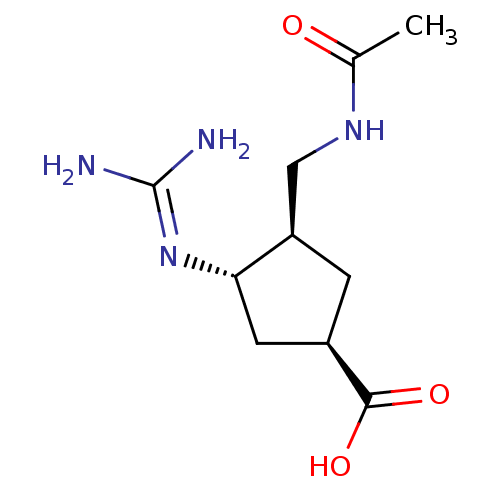 ((1R,3R,4S)-3-(Acetylamino-methyl)-4-guanidino-cycl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc. | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | J Med Chem 44: 4379-92 (2001) Article DOI: 10.1021/jm010277p BindingDB Entry DOI: 10.7270/Q2GX48RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM247011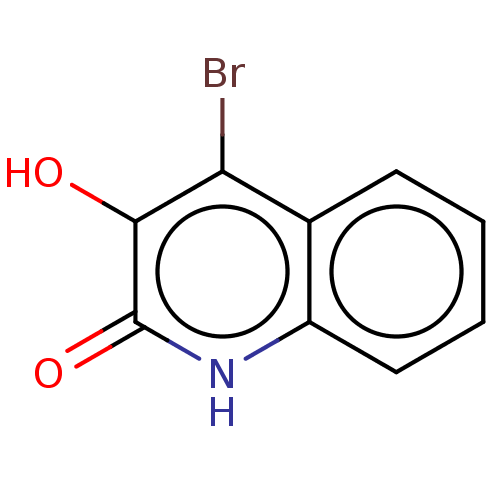 (US9701638, 2) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain swl A/California/04/2009...) | BDBM31169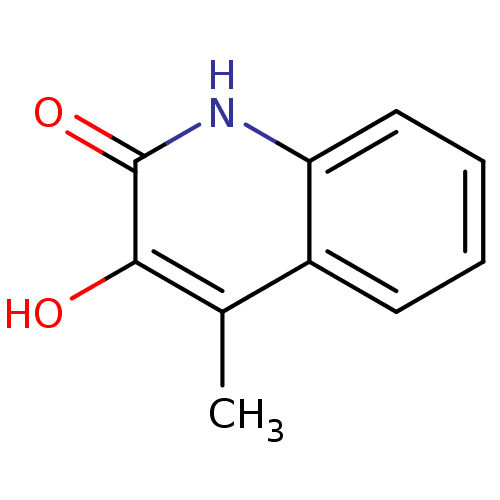 (3-hydroxyquinolin-2(1H)-one, 23 | US9701638, 14) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rutgers, The State University of New Jersey US Patent | Assay Description The PAN domain has been shown to cleave ssRNA as well as ssDNA. To demonstrate the inhibition of endonuclease cleavage by PAN, a high throughput assa... | US Patent US9701638 (2017) BindingDB Entry DOI: 10.7270/Q2XS5XDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50360451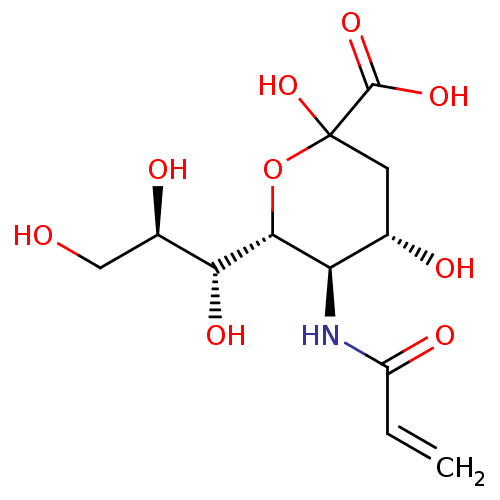 (CHEMBL1934619) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/Aichi/2/68 (H3N2) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hemagglutinin (Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50360451 (CHEMBL1934619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of Human influenza virus A/PR/8/34 (H1N1) hemagglutinin activity in chicken erythrocytes after 1 hr | Bioorg Med Chem 20: 446-54 (2011) Article DOI: 10.1016/j.bmc.2011.10.064 BindingDB Entry DOI: 10.7270/Q2SN09CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |