 Found 14168 hits Enz. Inhib. hit(s) with Target = 'Orexin/Hypocretin receptor type 1'
Found 14168 hits Enz. Inhib. hit(s) with Target = 'Orexin/Hypocretin receptor type 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108620
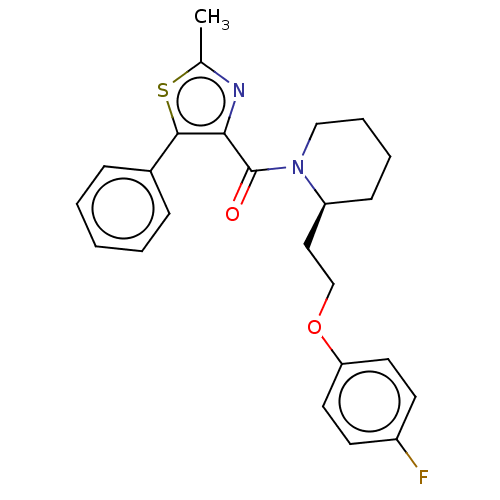
(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at orexin-1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 2875-87 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.012
BindingDB Entry DOI: 10.7270/Q2BZ67T9 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108620

(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM50417257
(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant rat OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419142
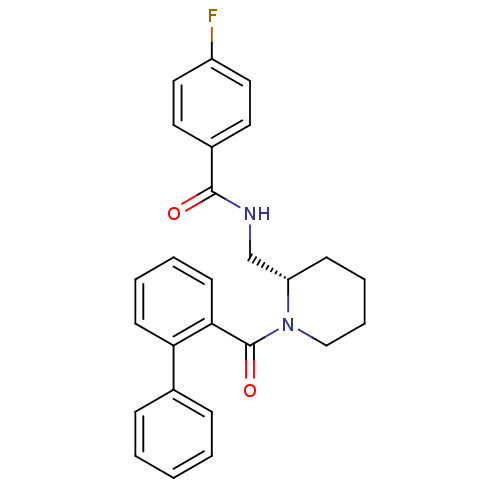
(CHEMBL1830963)Show SMILES Fc1ccc(cc1)C(=O)NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H25FN2O2/c27-21-15-13-20(14-16-21)25(30)28-18-22-10-6-7-17-29(22)26(31)24-12-5-4-11-23(24)19-8-2-1-3-9-19/h1-5,8-9,11-16,22H,6-7,10,17-18H2,(H,28,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419136
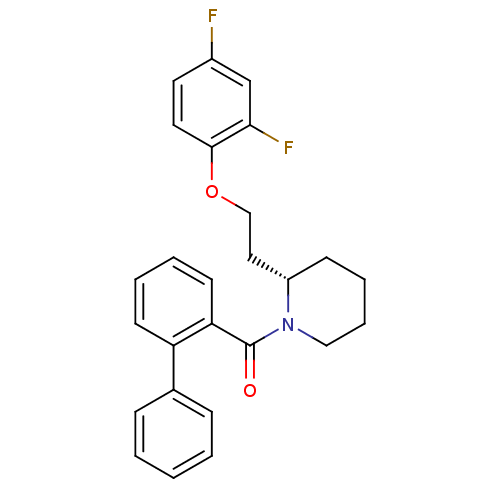
(CHEMBL1830961)Show SMILES Fc1ccc(OCC[C@@H]2CCCCN2C(=O)c2ccccc2-c2ccccc2)c(F)c1 |r| Show InChI InChI=1S/C26H25F2NO2/c27-20-13-14-25(24(28)18-20)31-17-15-21-10-6-7-16-29(21)26(30)23-12-5-4-11-22(23)19-8-2-1-3-9-19/h1-5,8-9,11-14,18,21H,6-7,10,15-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012606
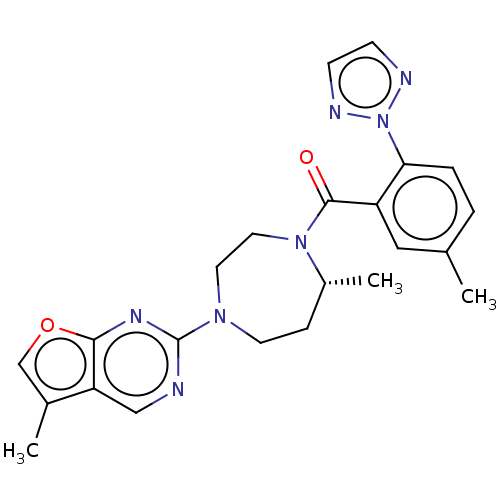
(CHEMBL3260826)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)coc2n1 |r| Show InChI InChI=1S/C23H25N7O2/c1-15-4-5-20(30-25-7-8-26-30)18(12-15)22(31)29-11-10-28(9-6-17(29)3)23-24-13-19-16(2)14-32-21(19)27-23/h4-5,7-8,12-14,17H,6,9-11H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148575
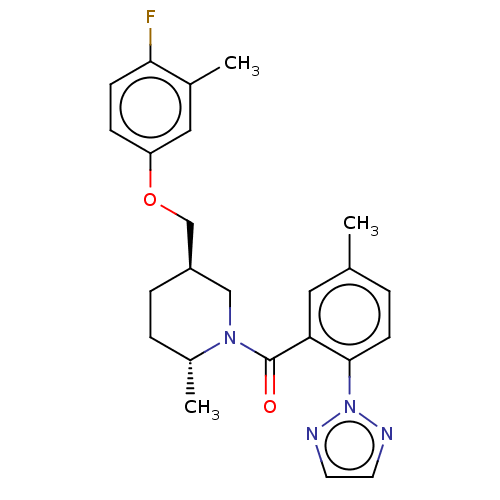
(CHEMBL3770503)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012602
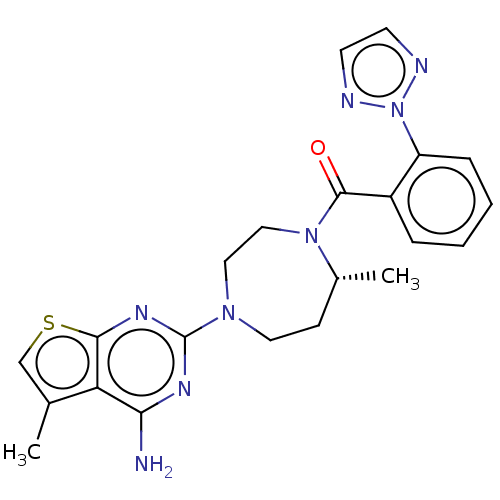
(CHEMBL3260836)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1nc(N)c2c(C)csc2n1 |r| Show InChI InChI=1S/C22H24N8OS/c1-14-13-32-20-18(14)19(23)26-22(27-20)28-10-7-15(2)29(12-11-28)21(31)16-5-3-4-6-17(16)30-24-8-9-25-30/h3-6,8-9,13,15H,7,10-12H2,1-2H3,(H2,23,26,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to orexin receptor 1 (unknown origin) |
Bioorg Med Chem Lett 23: 4761-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.057
BindingDB Entry DOI: 10.7270/Q25140NJ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012608
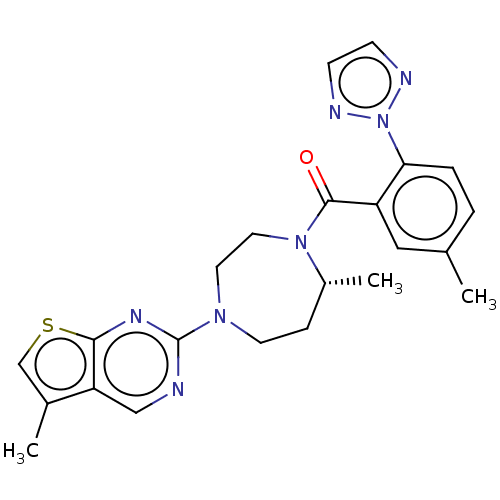
(CHEMBL3260828)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)csc2n1 |r| Show InChI InChI=1S/C23H25N7OS/c1-15-4-5-20(30-25-7-8-26-30)18(12-15)22(31)29-11-10-28(9-6-17(29)3)23-24-13-19-16(2)14-32-21(19)27-23/h4-5,7-8,12-14,17H,6,9-11H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012601
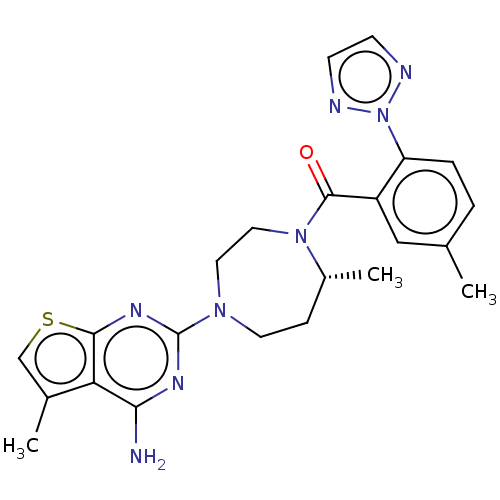
(CHEMBL3260835)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc(N)c2c(C)csc2n1 |r| Show InChI InChI=1S/C23H26N8OS/c1-14-4-5-18(31-25-7-8-26-31)17(12-14)22(32)30-11-10-29(9-6-16(30)3)23-27-20(24)19-15(2)13-33-21(19)28-23/h4-5,7-8,12-13,16H,6,9-11H2,1-3H3,(H2,24,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity against human orexin 1 receptor expressed in CHO cells assessed as effect in calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 20: 6405-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.090
BindingDB Entry DOI: 10.7270/Q2W37WKR |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695
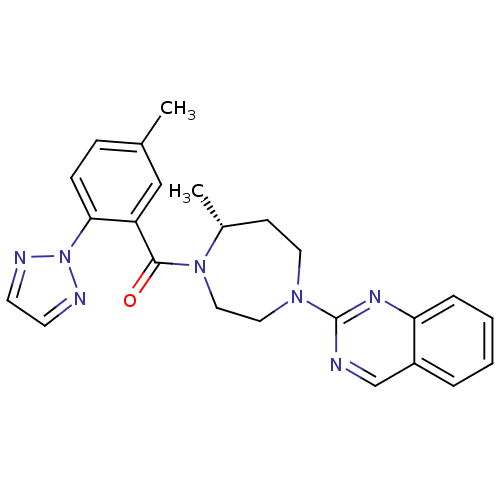
(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695

(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... |
J Med Chem 63: 1528-1543 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01787
BindingDB Entry DOI: 10.7270/Q2474F8R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012613
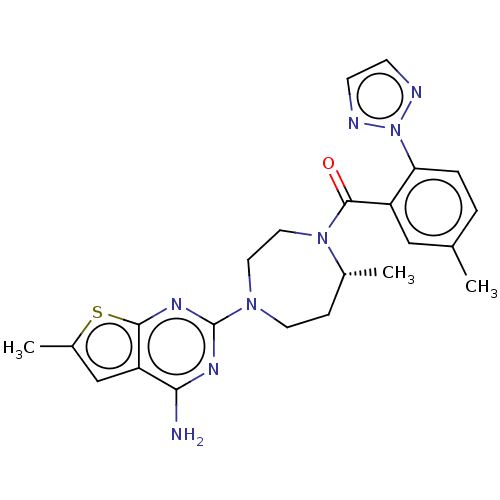
(CHEMBL3260833)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc(N)c2cc(C)sc2n1 |r| Show InChI InChI=1S/C23H26N8OS/c1-14-4-5-19(31-25-7-8-26-31)17(12-14)22(32)30-11-10-29(9-6-15(30)2)23-27-20(24)18-13-16(3)33-21(18)28-23/h4-5,7-8,12-13,15H,6,9-11H2,1-3H3,(H2,24,27,28)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321509
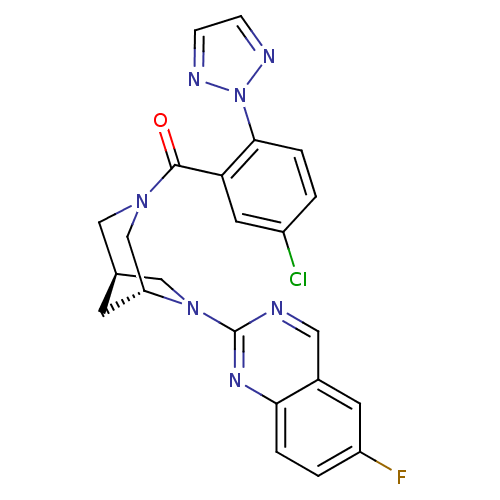
((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)((1R,5R)...)Show SMILES Fc1ccc2nc(ncc2c1)N1C[C@H]2C[C@@H]1CN(C2)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C23H19ClFN7O/c24-16-1-4-21(32-27-5-6-28-32)19(9-16)22(33)30-11-14-7-18(13-30)31(12-14)23-26-10-15-8-17(25)2-3-20(15)29-23/h1-6,8-10,14,18H,7,11-13H2/t14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50314681
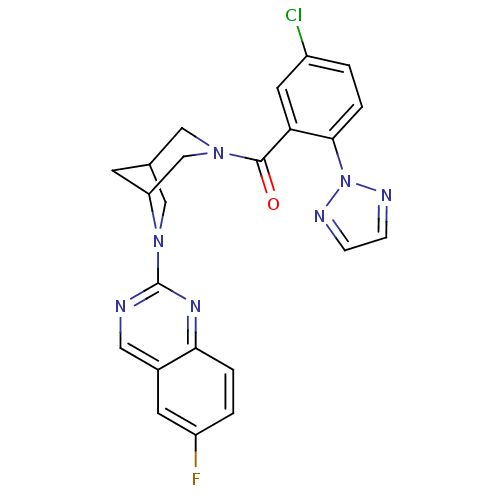
((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(6-fl...)Show SMILES Fc1ccc2nc(ncc2c1)N1CC2CC1CN(C2)C(=O)c1cc(Cl)ccc1-n1nccn1 Show InChI InChI=1S/C23H19ClFN7O/c24-16-1-4-21(32-27-5-6-28-32)19(9-16)22(33)30-11-14-7-18(13-30)31(12-14)23-26-10-15-8-17(25)2-3-20(15)29-23/h1-6,8-10,14,18H,7,11-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1 receptor by radioligand displacement assay |
Bioorg Med Chem Lett 20: 2311-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.138
BindingDB Entry DOI: 10.7270/Q2MK6D26 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060938
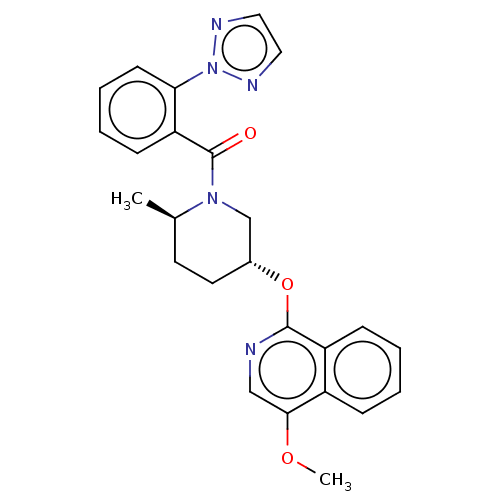
(CHEMBL3394847)Show SMILES COc1cnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c2ccccc12 |r| Show InChI InChI=1S/C25H25N5O3/c1-17-11-12-18(33-24-20-8-4-3-7-19(20)23(32-2)15-26-24)16-29(17)25(31)21-9-5-6-10-22(21)30-27-13-14-28-30/h3-10,13-15,17-18H,11-12,16H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604064

(US11660293, Example 171 | US11660293, Example 173)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;7.71,-2.82,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012611
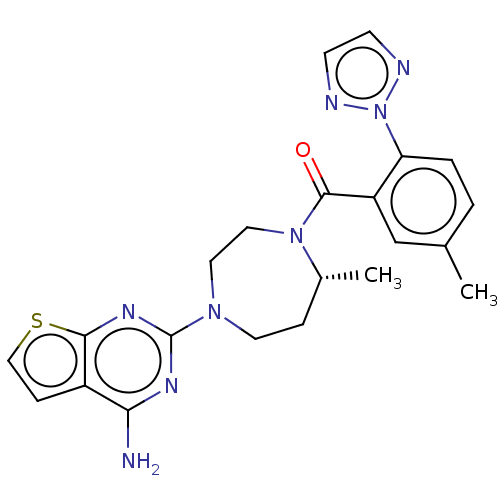
(CHEMBL3260831)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc(N)c2ccsc2n1 |r| Show InChI InChI=1S/C22H24N8OS/c1-14-3-4-18(30-24-7-8-25-30)17(13-14)21(31)29-11-10-28(9-5-15(29)2)22-26-19(23)16-6-12-32-20(16)27-22/h3-4,6-8,12-13,15H,5,9-11H2,1-2H3,(H2,23,26,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012607
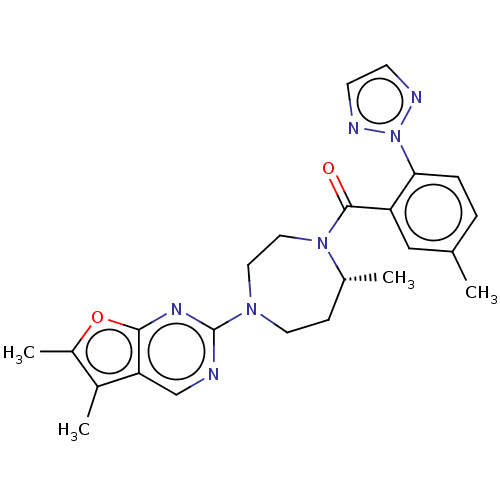
(CHEMBL3260827)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)c(C)oc2n1 |r| Show InChI InChI=1S/C24H27N7O2/c1-15-5-6-21(31-26-8-9-27-31)19(13-15)23(32)30-12-11-29(10-7-16(30)2)24-25-14-20-17(3)18(4)33-22(20)28-24/h5-6,8-9,13-14,16H,7,10-12H2,1-4H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50438824

(CHEMBL2413365)Show SMILES C[C@H]1[C@H](COc2nccc3ccccc23)CCCN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C26H27N5O2/c1-18-9-10-24(31-28-13-14-29-31)23(16-18)26(32)30-15-5-7-21(19(30)2)17-33-25-22-8-4-3-6-20(22)11-12-27-25/h3-4,6,8-14,16,19,21H,5,7,15,17H2,1-2H3/t19-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to orexin receptor 1 (unknown origin) |
Bioorg Med Chem Lett 23: 4761-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.057
BindingDB Entry DOI: 10.7270/Q25140NJ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50000984

(CHEMBL3235265)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1cc(C)ccc1-n1nccn1)C#Cc1cccc(CO)c1 |r| Show InChI InChI=1S/C25H26N4O2/c1-18-6-11-24(29-26-12-13-27-29)23(14-18)25(31)28-16-21(8-7-19(28)2)10-9-20-4-3-5-22(15-20)17-30/h3-6,11-15,19,21,30H,7-8,16-17H2,1-2H3/t19-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human OX1 receptor in cell membrane by in vitro radioligand binding assay |
Bioorg Med Chem Lett 24: 1784-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.026
BindingDB Entry DOI: 10.7270/Q2RV0Q6F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321516
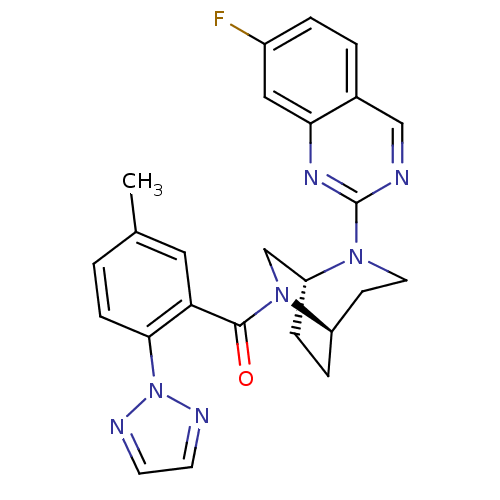
(((1R,5R)-2-(7-fluoroquinazolin-2-yl)-2,6-diazabicy...)Show SMILES Cc1ccc(c(c1)C(=O)N1C[C@H]2CC[C@@H]1CCN2c1ncc2ccc(F)cc2n1)-n1nccn1 |r,THB:7:9:17.15.16:13.12| Show InChI InChI=1S/C25H24FN7O/c1-16-2-7-23(33-28-9-10-29-33)21(12-16)24(34)32-15-20-6-5-19(32)8-11-31(20)25-27-14-17-3-4-18(26)13-22(17)30-25/h2-4,7,9-10,12-14,19-20H,5-6,8,11,15H2,1H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321510
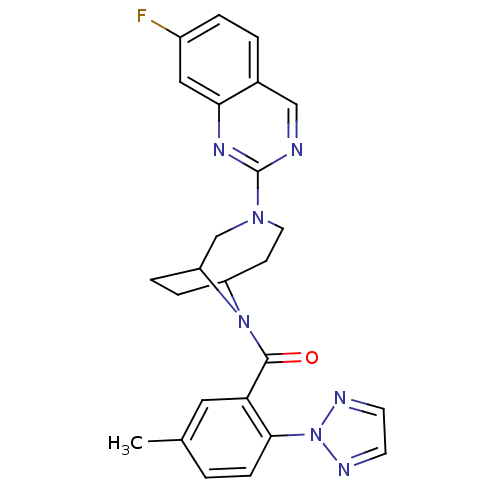
((3-(7-fluoroquinazolin-2-yl)-3,9-di azabicyclo[4.2...)Show SMILES Cc1ccc(c(c1)C(=O)N1C2CCC1CN(CC2)c1ncc2ccc(F)cc2n1)-n1nccn1 Show InChI InChI=1S/C25H24FN7O/c1-16-2-7-23(33-28-9-10-29-33)21(12-16)24(34)32-19-5-6-20(32)15-31(11-8-19)25-27-14-17-3-4-18(26)13-22(17)30-25/h2-4,7,9-10,12-14,19-20H,5-6,8,11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to orexin receptor 1 (unknown origin) |
Bioorg Med Chem Lett 23: 4761-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.057
BindingDB Entry DOI: 10.7270/Q25140NJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104692
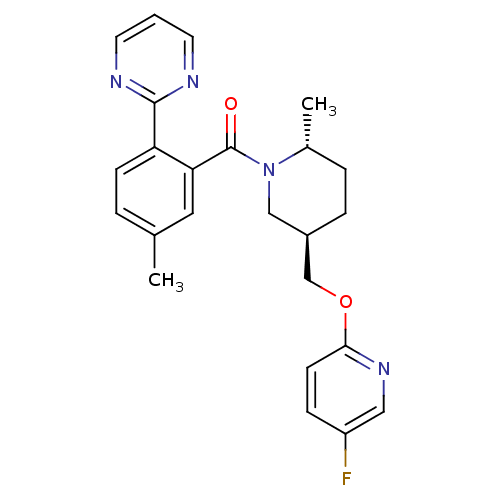
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-(2,6-Difluoro-4-methoxybenzyl)-2-(5,6-dimethoxypyridin-3-yl)-2H-1,2,4-benzothiadiazin-3(4H)-one 1,1-dioxide from human wild-typ... |
J Med Chem 63: 1528-1543 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01787
BindingDB Entry DOI: 10.7270/Q2474F8R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012600
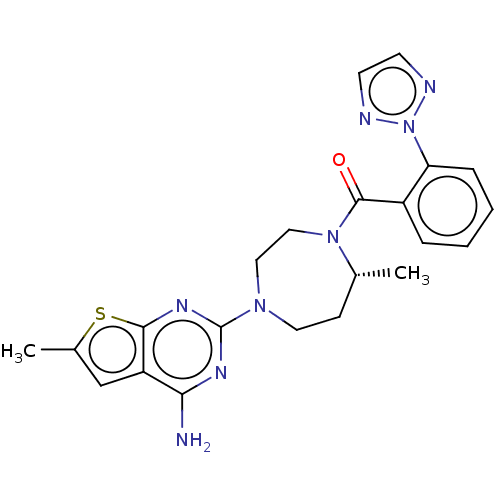
(CHEMBL3260834)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1nc(N)c2cc(C)sc2n1 |r| Show InChI InChI=1S/C22H24N8OS/c1-14-7-10-28(22-26-19(23)17-13-15(2)32-20(17)27-22)11-12-29(14)21(31)16-5-3-4-6-18(16)30-24-8-9-25-30/h3-6,8-9,13-14H,7,10-12H2,1-2H3,(H2,23,26,27)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50374443
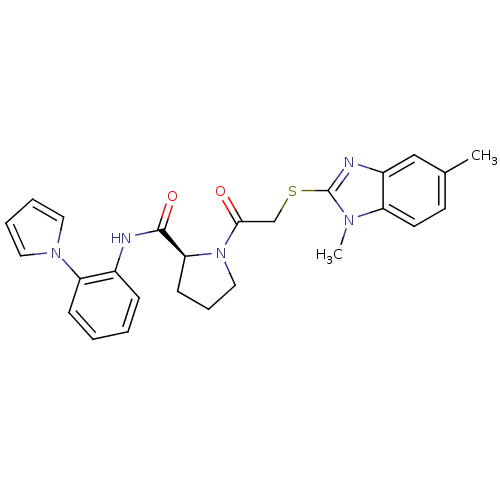
(CHEMBL272715)Show SMILES Cc1ccc2n(C)c(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)nc2c1 Show InChI InChI=1S/C26H27N5O2S/c1-18-11-12-21-20(16-18)28-26(29(21)2)34-17-24(32)31-15-7-10-23(31)25(33)27-19-8-3-4-9-22(19)30-13-5-6-14-30/h3-6,8-9,11-14,16,23H,7,10,15,17H2,1-2H3,(H,27,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-1-[2-(1-methyl-1H-benzoimidazol-2-ylsulfanyl)-acetyl]-pyrrolidine-2-carboxylic acid biphenyl-2-ylamide from human OX1R expres... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM104700
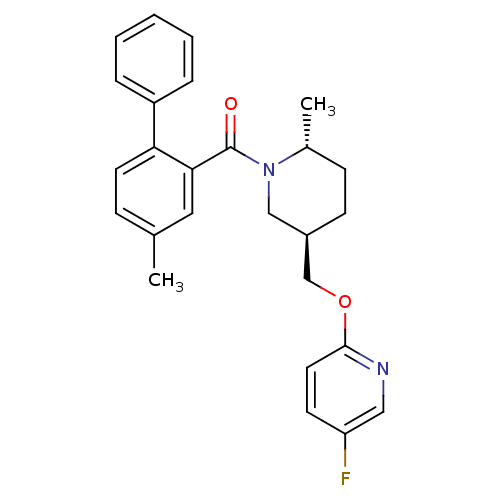
(US8569311, 1-10)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H27FN2O2/c1-18-8-12-23(21-6-4-3-5-7-21)24(14-18)26(30)29-16-20(10-9-19(29)2)17-31-25-13-11-22(27)15-28-25/h3-8,11-15,19-20H,9-10,16-17H2,1-2H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM603987

(US11660293, Example 61)Show SMILES Cc1nc(C(=O)N2C3CC(C3)CC2CNc2nc3ccc(F)cc3s2)c(s1)-c1ccccc1 |(-8.47,-.61,;-6.93,-.61,;-5.9,.53,;-4.49,-.1,;-3.16,.67,;-1.82,-.1,;-3.16,2.21,;-4.49,2.98,;-4.49,4.52,;-3.16,5.29,;-3.16,3.75,;-1.82,4.52,;-1.82,2.98,;-.49,2.21,;.85,2.98,;2.18,2.21,;2.34,.68,;3.85,.36,;4.62,-.97,;6.16,-.97,;6.93,.36,;8.47,.36,;6.16,1.7,;4.62,1.7,;3.59,2.84,;-4.65,-1.63,;-6.16,-1.95,;-3.56,-2.72,;-3.96,-4.2,;-2.87,-5.29,;-1.38,-4.89,;-.98,-3.41,;-2.07,-2.32,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50417257

(SB-649868)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CNC(=O)c2cccc3occc23)c(s1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H24FN3O3S/c1-16-29-23(24(34-16)17-8-10-18(27)11-9-17)26(32)30-13-3-2-5-19(30)15-28-25(31)21-6-4-7-22-20(21)12-14-33-22/h4,6-12,14,19H,2-3,5,13,15H2,1H3,(H,28,31)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]almorexant from recombinant human OX1R expressed in CHO cells |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318699
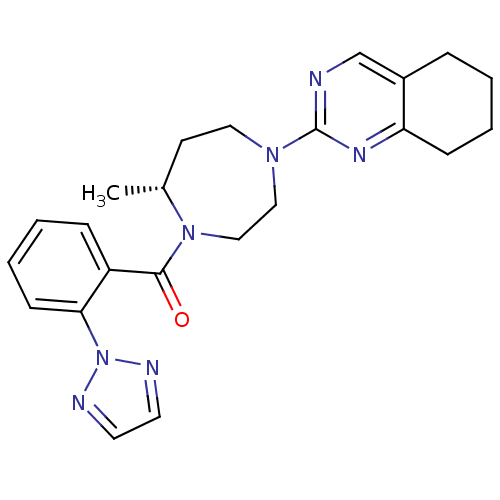
(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2CCCCc2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h3,5,7,9,11-12,16-17H,2,4,6,8,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318699

(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2CCCCc2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h3,5,7,9,11-12,16-17H,2,4,6,8,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012604
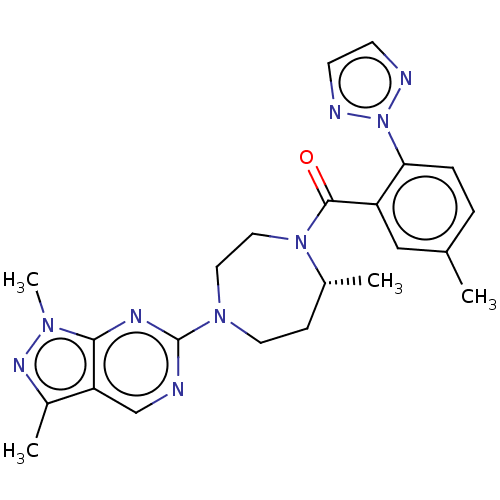
(CHEMBL3260824)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)nn(C)c2n1 |r| Show InChI InChI=1S/C23H27N9O/c1-15-5-6-20(32-25-8-9-26-32)18(13-15)22(33)31-12-11-30(10-7-16(31)2)23-24-14-19-17(3)28-29(4)21(19)27-23/h5-6,8-9,13-14,16H,7,10-12H2,1-4H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM158195

(US9029364, 1)Show SMILES C[C@@H]1CS[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 Show InChI InChI=1S/C21H22FN5O2S/c1-14-3-5-19(27-24-7-8-25-27)18(9-14)21(28)26-11-17(30-13-15(26)2)12-29-20-6-4-16(22)10-23-20/h3-10,15,17H,11-13H2,1-2H3/t15-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as orexin receptor OX1R and/or OX2R antagonists may be readily determined witho... |
US Patent US9029364 (2015)
BindingDB Entry DOI: 10.7270/Q2SF2TX1 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604048

(US11660293, Example 152 | US11660293, Example 154)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1nc(C)ccc1-n1nccn1 |r,wU:1.0,(-.48,4.78,;-1.81,4.01,;-3.14,4.78,;-4.48,4.01,;-4.48,2.47,;-3.14,3.24,;-3.14,1.7,;-1.81,2.47,;-.48,1.7,;.86,2.47,;2.19,1.7,;2.35,.16,;3.86,-.16,;4.63,-1.49,;6.17,-1.49,;6.94,-.16,;8.48,-.16,;6.17,1.18,;4.63,1.18,;3.6,2.32,;-3.14,.16,;-1.81,-.61,;-4.48,-.61,;-5.81,.16,;-7.14,-.61,;-8.48,.16,;-7.14,-2.15,;-5.81,-2.92,;-4.48,-2.15,;-3.14,-2.92,;-2.98,-4.46,;-1.48,-4.78,;-.71,-3.44,;-1.74,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012612
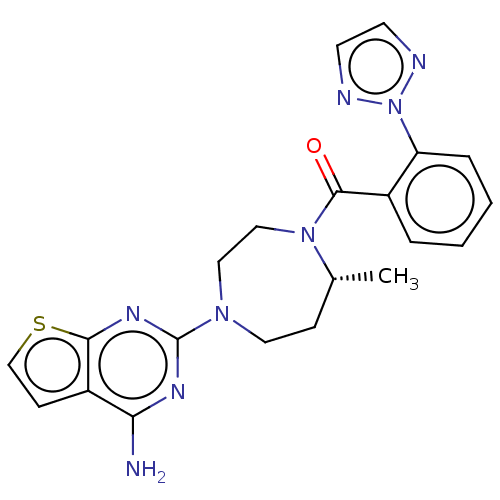
(CHEMBL3260832)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1nc(N)c2ccsc2n1 |r| Show InChI InChI=1S/C21H22N8OS/c1-14-6-10-27(21-25-18(22)16-7-13-31-19(16)26-21)11-12-28(14)20(30)15-4-2-3-5-17(15)29-23-8-9-24-29/h2-5,7-9,13-14H,6,10-12H2,1H3,(H2,22,25,26)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321511
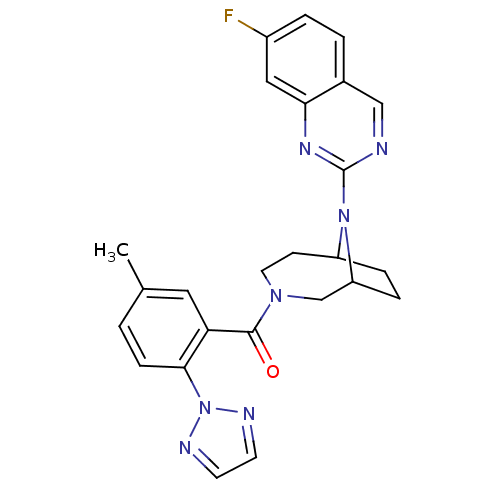
((+/-)-(9-(7-fluoroquinazolin-2-yl)-3,9-diazabicycl...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCC2CCC(C1)N2c1ncc2ccc(F)cc2n1)-n1nccn1 Show InChI InChI=1S/C25H24FN7O/c1-16-2-7-23(33-28-9-10-29-33)21(12-16)24(34)31-11-8-19-5-6-20(15-31)32(19)25-27-14-17-3-4-18(26)13-22(17)30-25/h2-4,7,9-10,12-14,19-20H,5-6,8,11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Rattus norvegicus (Rat)) | BDBM104689
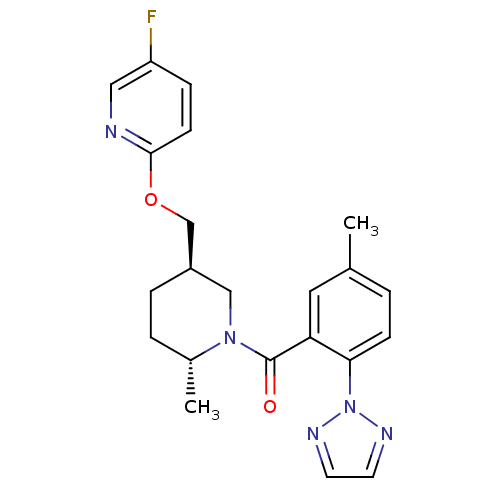
(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50314676
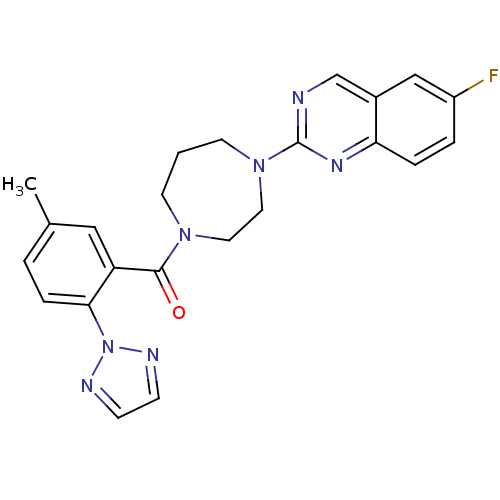
((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCN(CC1)c1ncc2cc(F)ccc2n1)-n1nccn1 Show InChI InChI=1S/C23H22FN7O/c1-16-3-6-21(31-26-7-8-27-31)19(13-16)22(32)29-9-2-10-30(12-11-29)23-25-15-17-14-18(24)4-5-20(17)28-23/h3-8,13-15H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1 receptor by radioligand displacement assay |
Bioorg Med Chem Lett 20: 2311-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.138
BindingDB Entry DOI: 10.7270/Q2MK6D26 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50419131
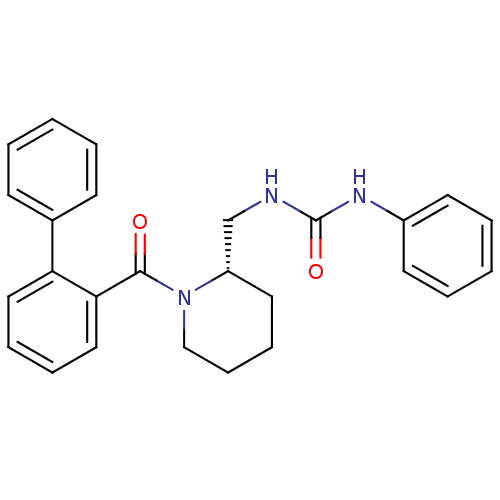
(CHEMBL1830968)Show SMILES O=C(NC[C@@H]1CCCCN1C(=O)c1ccccc1-c1ccccc1)Nc1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2/c30-25(24-17-8-7-16-23(24)20-11-3-1-4-12-20)29-18-10-9-15-22(29)19-27-26(31)28-21-13-5-2-6-14-21/h1-8,11-14,16-17,22H,9-10,15,18-19H2,(H2,27,28,31)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human OX1R expressed in CHO cells by FLIPR calcium based functional assay |
Bioorg Med Chem Lett 21: 5562-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.086
BindingDB Entry DOI: 10.7270/Q29K4CGM |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50440024
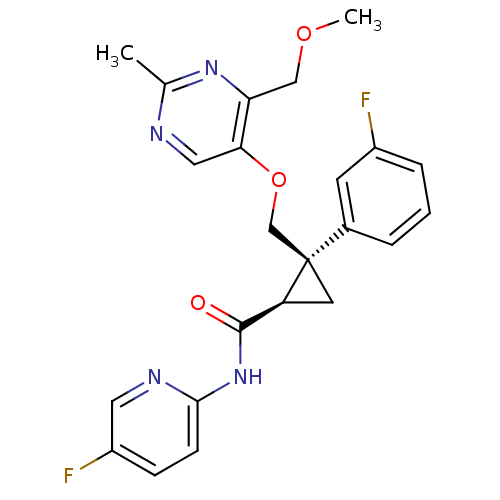
(CHEMBL2425785)Show SMILES COCc1nc(C)ncc1OC[C@]1(C[C@H]1C(=O)Nc1ccc(F)cn1)c1cccc(F)c1 |r| Show InChI InChI=1S/C23H22F2N4O3/c1-14-26-11-20(19(28-14)12-31-2)32-13-23(15-4-3-5-16(24)8-15)9-18(23)22(30)29-21-7-6-17(25)10-27-21/h3-8,10-11,18H,9,12-13H2,1-2H3,(H,27,29,30)/t18-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co. , Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-orexin-A from human OX1 receptor expressed in CHO cells after 30 mins by liquid scintillation counting analysis |
J Med Chem 56: 6371-85 (2013)
Article DOI: 10.1021/jm400772t
BindingDB Entry DOI: 10.7270/Q2QF8V91 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50438823
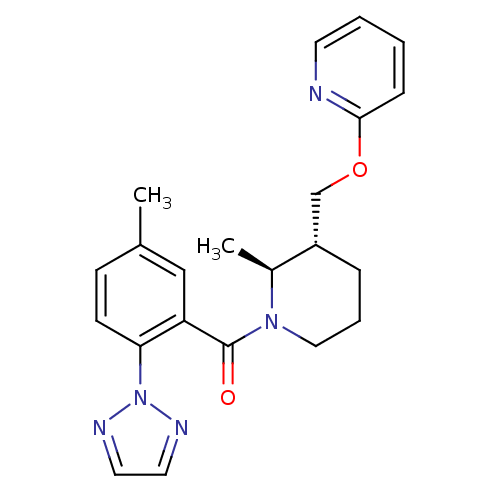
(CHEMBL2413366)Show SMILES C[C@H]1[C@H](COc2ccccn2)CCCN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N5O2/c1-16-8-9-20(27-24-11-12-25-27)19(14-16)22(28)26-13-5-6-18(17(26)2)15-29-21-7-3-4-10-23-21/h3-4,7-12,14,17-18H,5-6,13,15H2,1-2H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to orexin receptor 1 (unknown origin) |
Bioorg Med Chem Lett 23: 4761-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.057
BindingDB Entry DOI: 10.7270/Q25140NJ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104689

(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data