

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50366138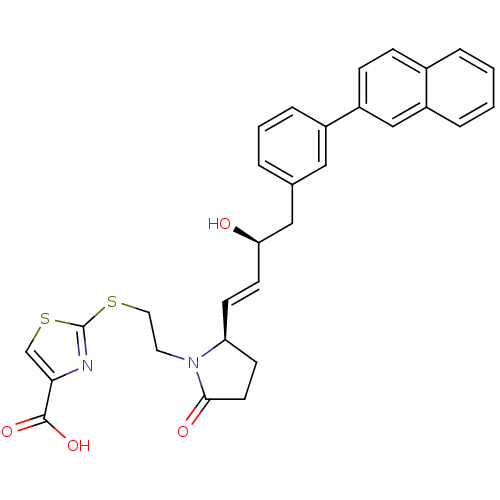 (CHEMBL1957437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50366138 (CHEMBL1957437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 2235-51 (2012) Article DOI: 10.1016/j.bmc.2012.02.018 BindingDB Entry DOI: 10.7270/Q2542P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373939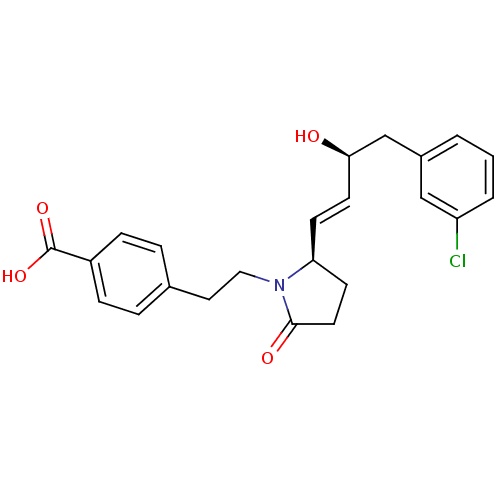 (CHEMBL258332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373942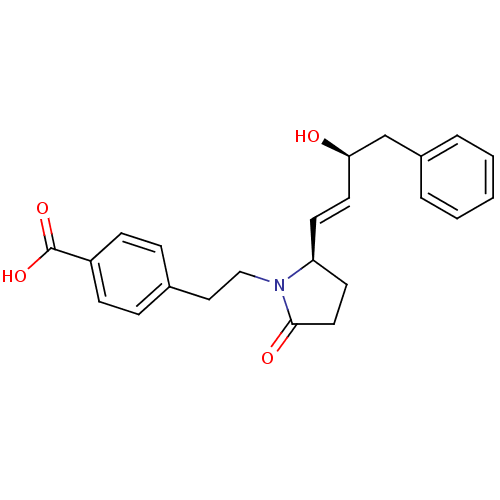 (CHEMBL272276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190273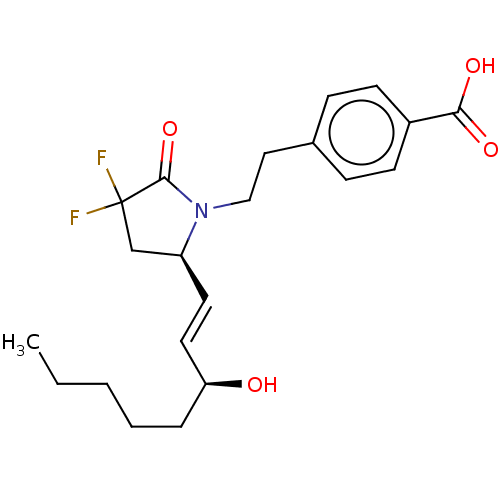 (US9180116, 21C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | 0.220 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190282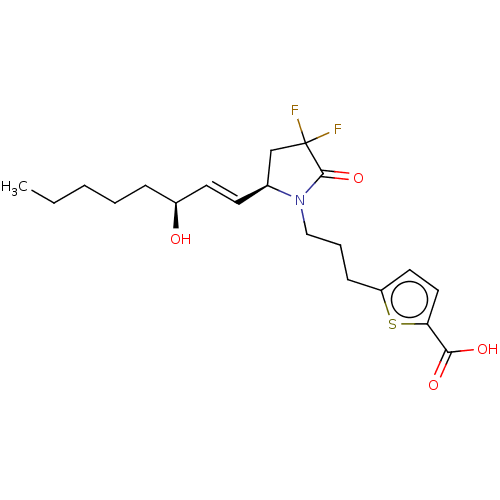 (US9180116, 33C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | 0.280 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385124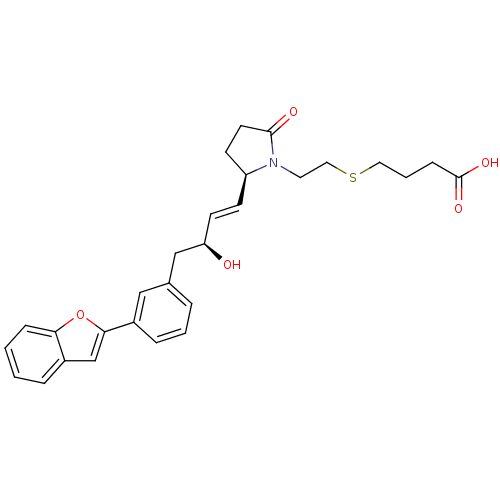 (CHEMBL2036314) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335985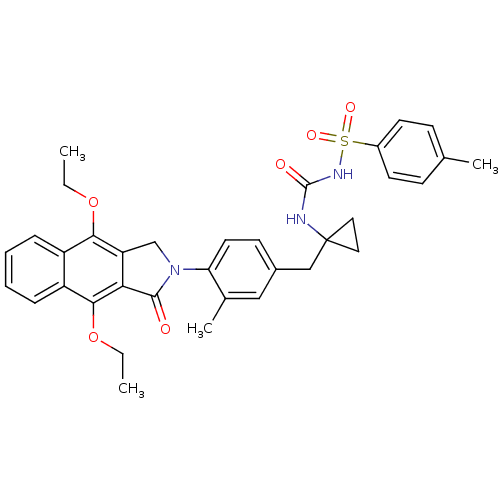 (CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190272 (US9180116, 12D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | 0.320 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385132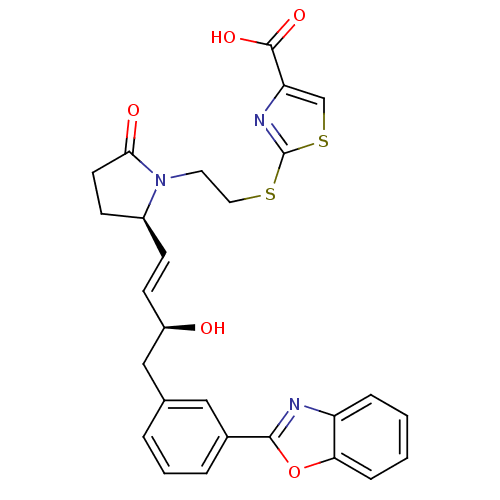 (CHEMBL2036323) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372064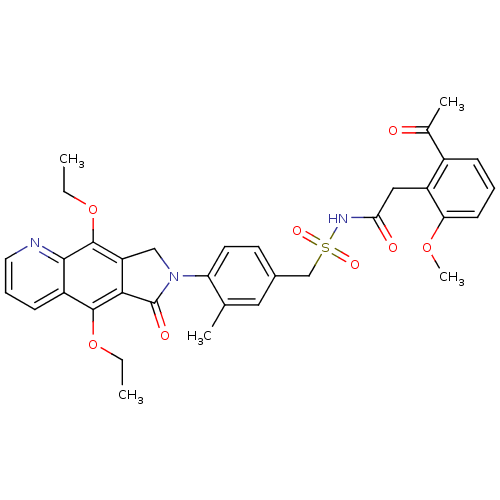 (CHEMBL256873) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385131 (CHEMBL2036322) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822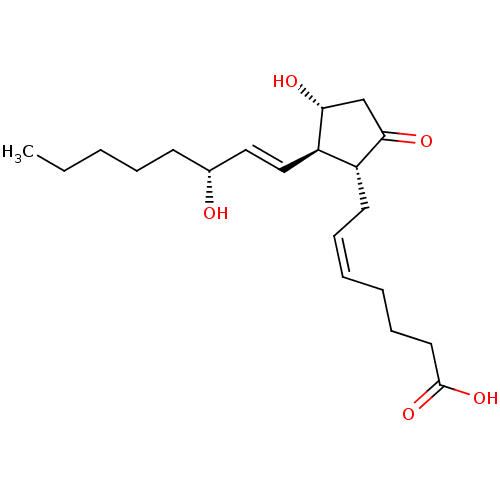 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385138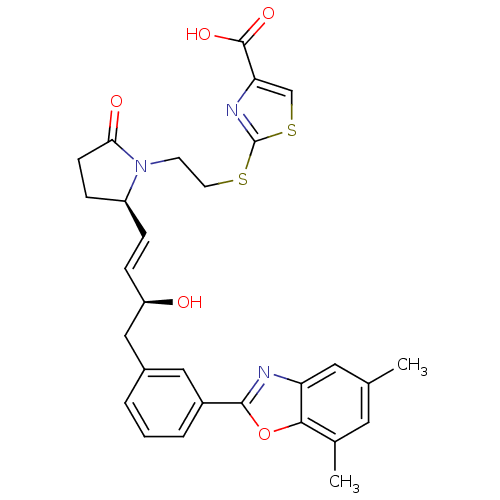 (CHEMBL2037291) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335986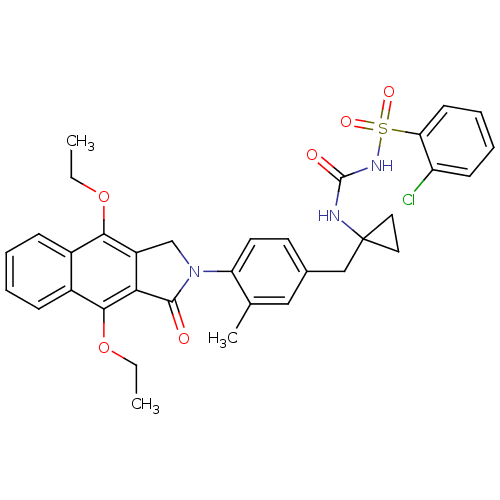 (2-chloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385136 (CHEMBL2037289) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335980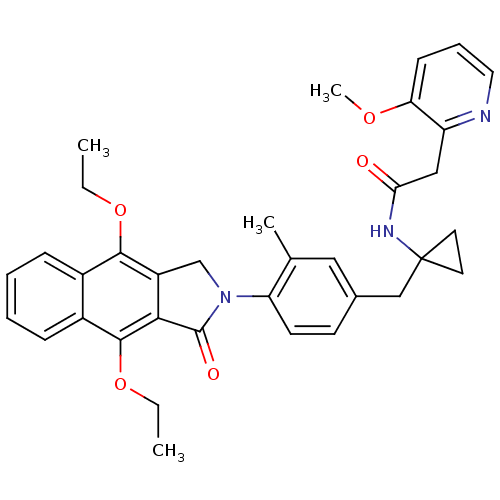 (CHEMBL1669017 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335989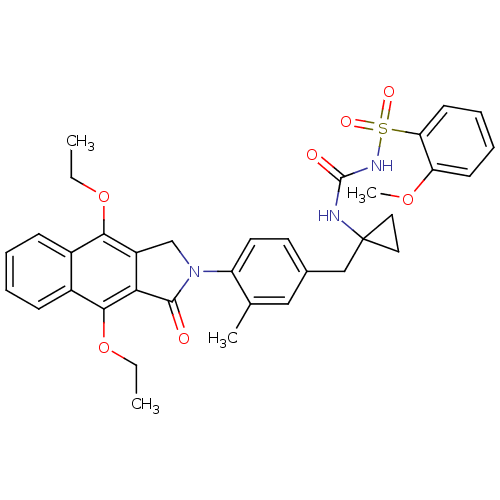 (CHEMBL1669009 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335981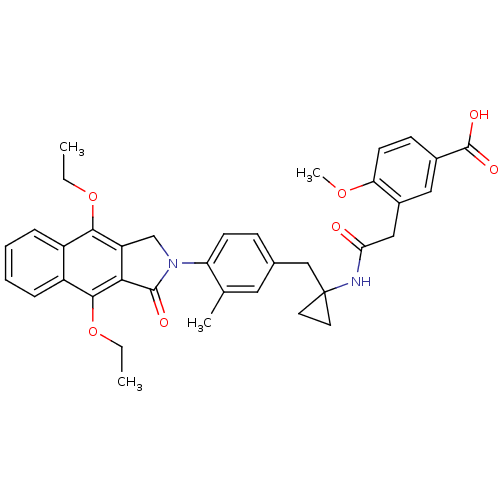 (3-(2-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]isoindol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385133 (CHEMBL2036324) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50333737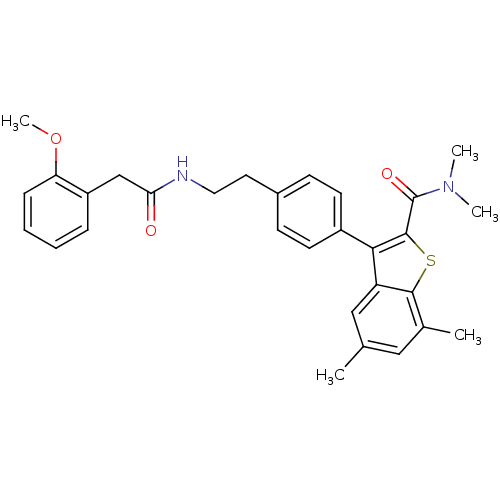 (3-(4-(2-(2-(2-methoxyphenyl)acetamido)ethyl)phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to EP4 receptor | Bioorg Med Chem Lett 21: 734-7 (2011) Article DOI: 10.1016/j.bmcl.2010.11.118 BindingDB Entry DOI: 10.7270/Q22N52JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335984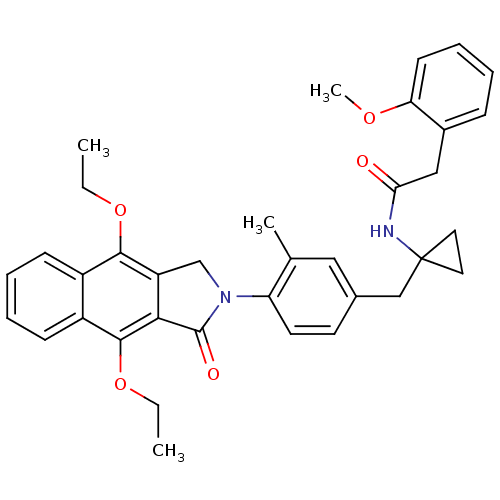 (CHEMBL1669018 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385139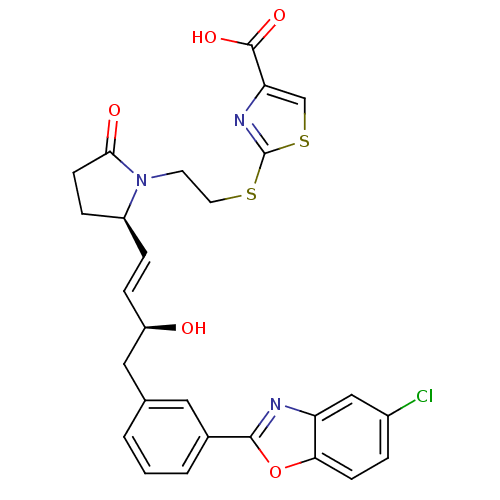 (CHEMBL2037292) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385122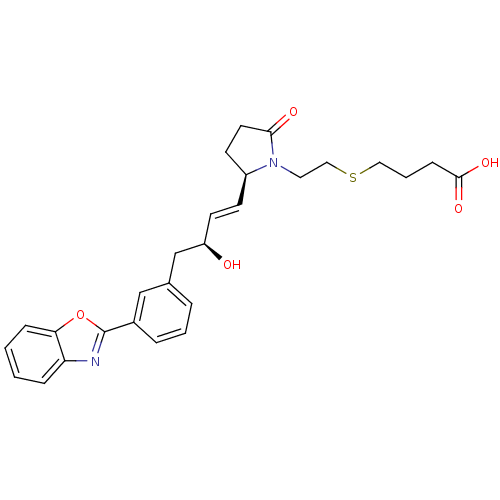 (CHEMBL2036312) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270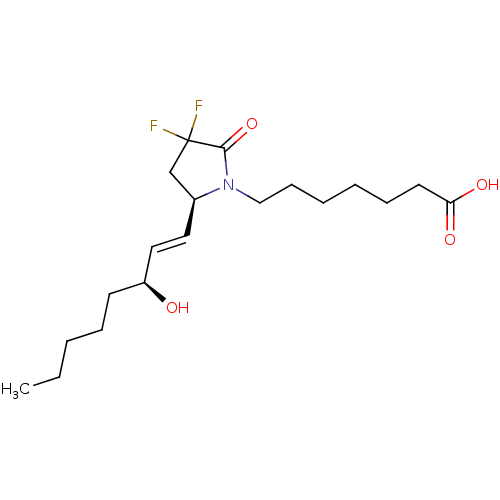 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | 0.570 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385135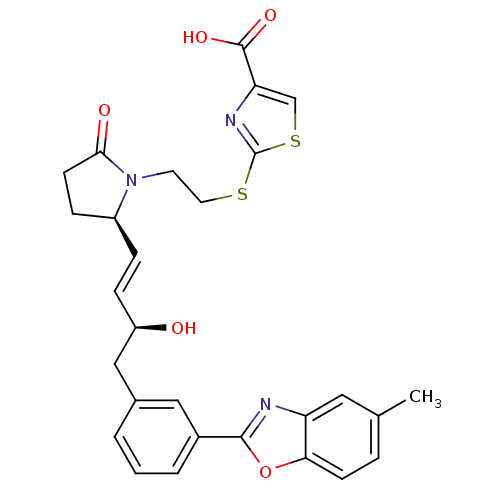 (CHEMBL2036326) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385137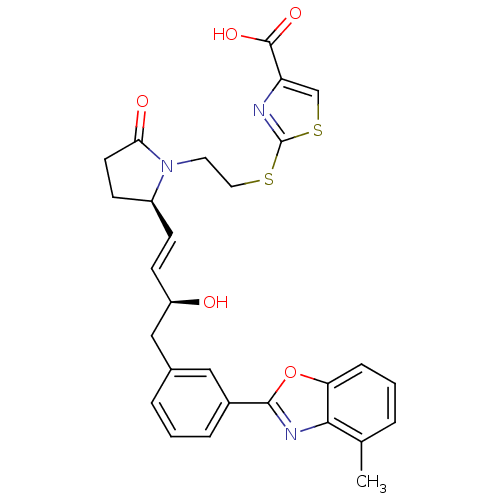 (CHEMBL2037290) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372051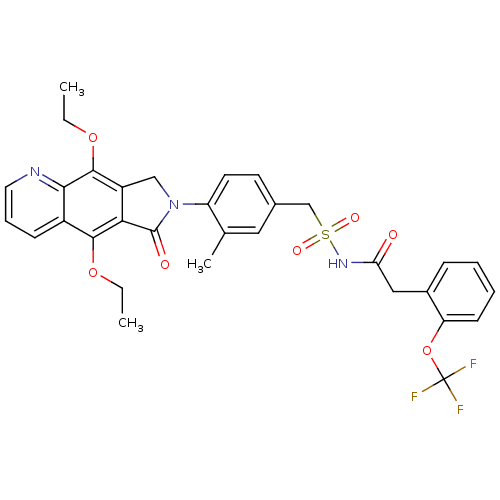 (CHEMBL269987) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335988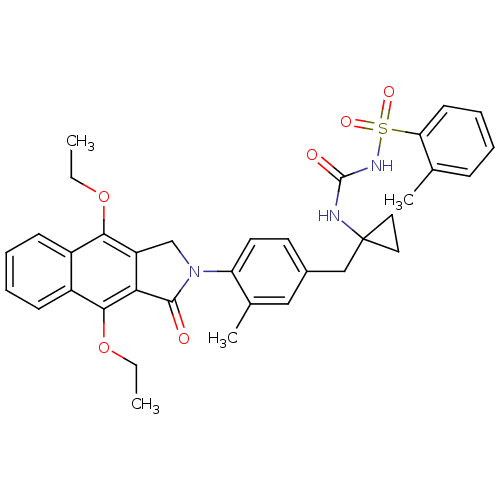 (CHEMBL1669010 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319837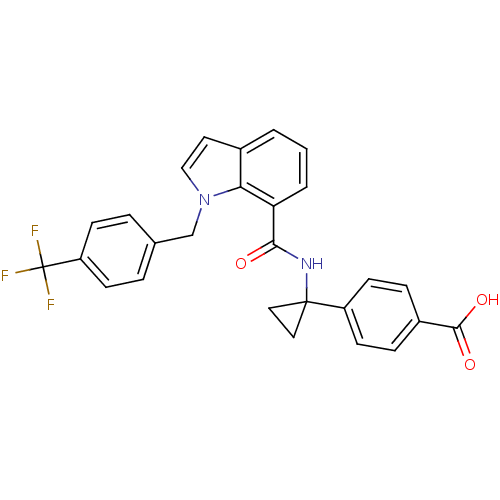 (4-(1-(1-(4-(trifluoromethyl)benzyl)-1H-indole-7-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385134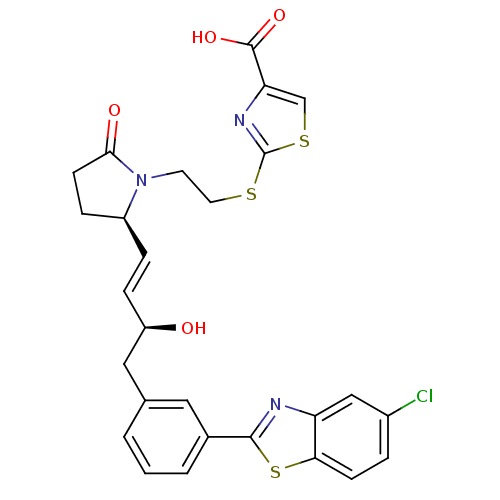 (CHEMBL2036325) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372054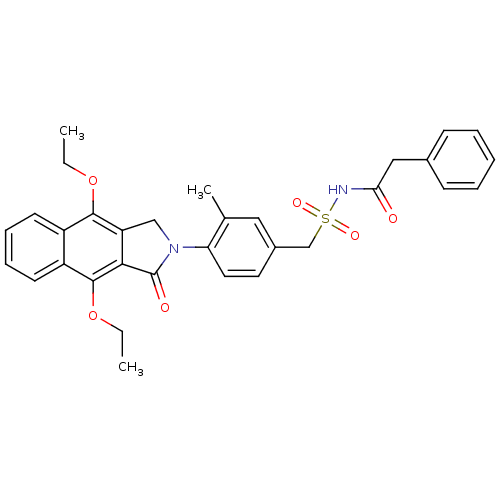 (CHEMBL255527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372052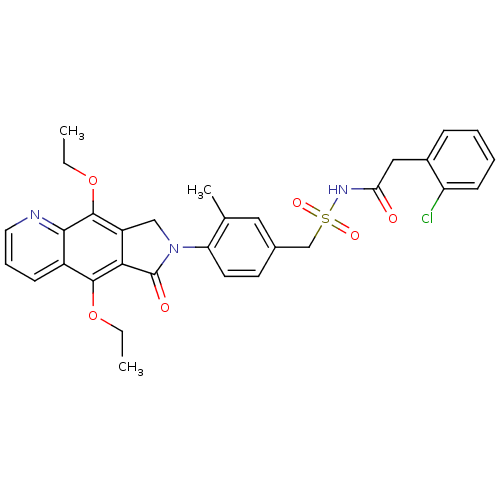 (CHEMBL218699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372050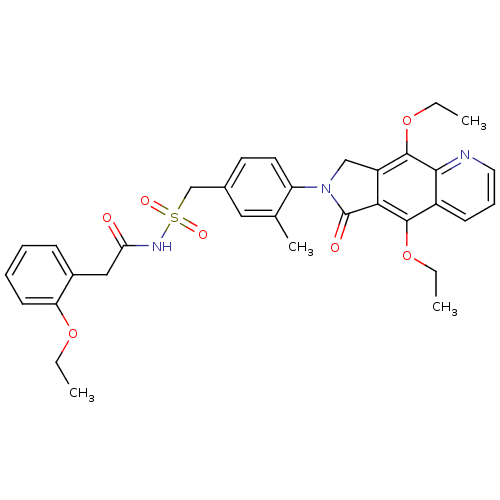 (CHEMBL255906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190275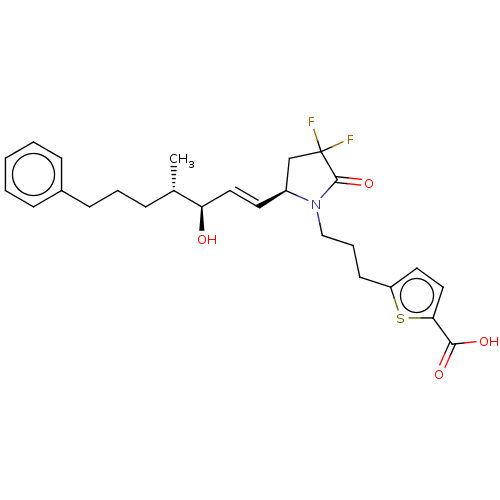 (US9180116, 28C | US9180116, 28H) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | 0.740 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372065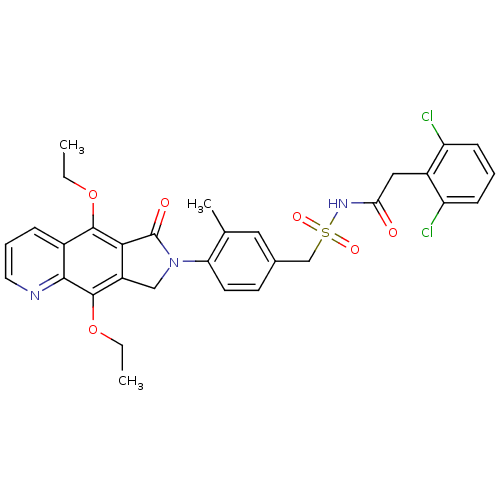 (CHEMBL272363) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50385121 (CHEMBL2036311) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 3502-22 (2012) Article DOI: 10.1016/j.bmc.2012.04.008 BindingDB Entry DOI: 10.7270/Q2D50P0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50334136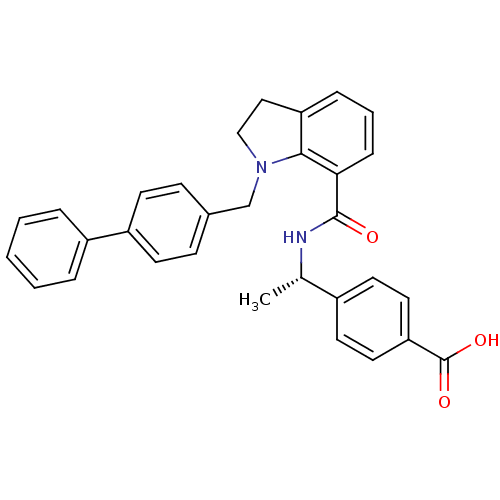 ((S)-4-(1-(1-(biphenyl-4-ylmethyl)indoline-7-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 21: 484-7 (2010) Article DOI: 10.1016/j.bmcl.2010.10.106 BindingDB Entry DOI: 10.7270/Q2BZ6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165949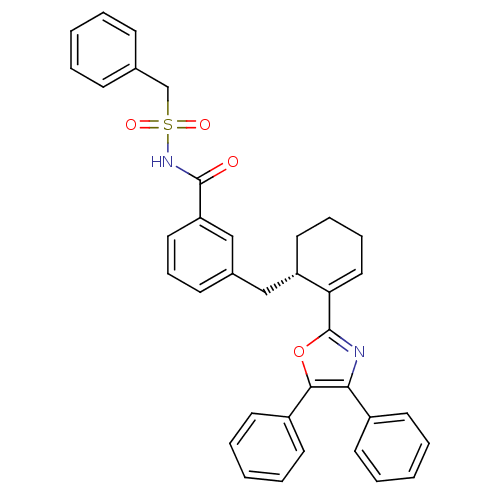 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM263346 (US9546162, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | 11 | n/a | n/a | 7.4 | n/a |
ALLERGAN, INC. US Patent | Assay Description Cells were seeded at a density of 5×104 cells per well in Biocoat® Poly-D-lysine-coated black-wall, clear-bottom 96-well plates (Becton-Dickinson) an... | US Patent US9546162 (2017) BindingDB Entry DOI: 10.7270/Q2P55QHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335990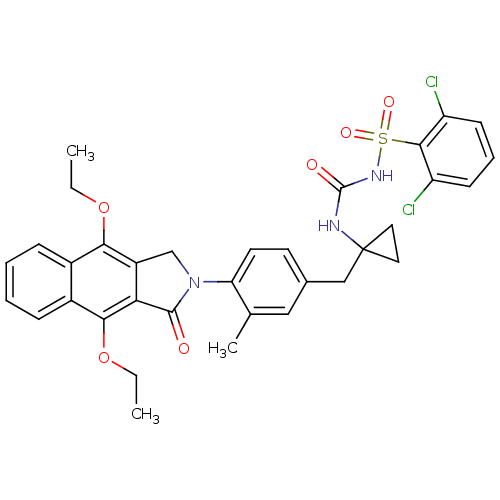 (2,6-dichloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012544 (CHEMBL3260768) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372056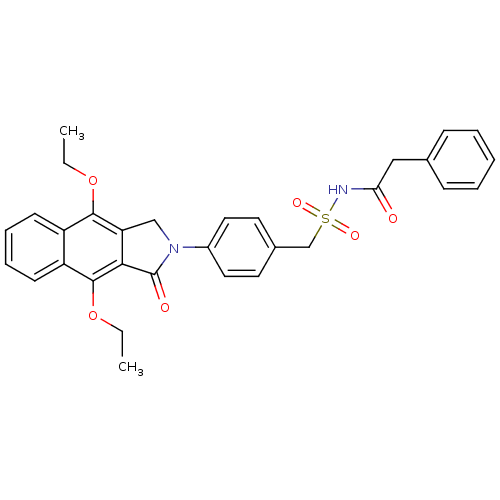 (CHEMBL255422) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319836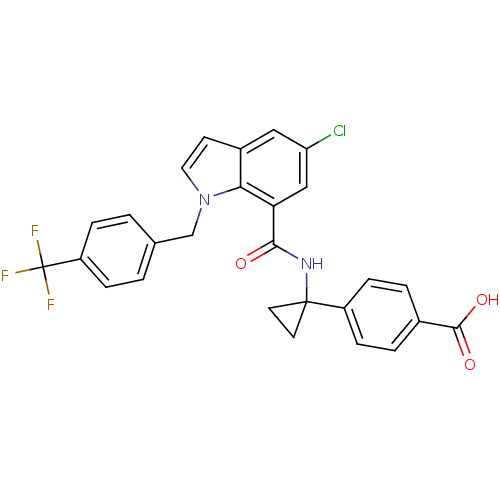 (4-(1-(5-chloro-1-(4-(trifluoromethyl)benzyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372049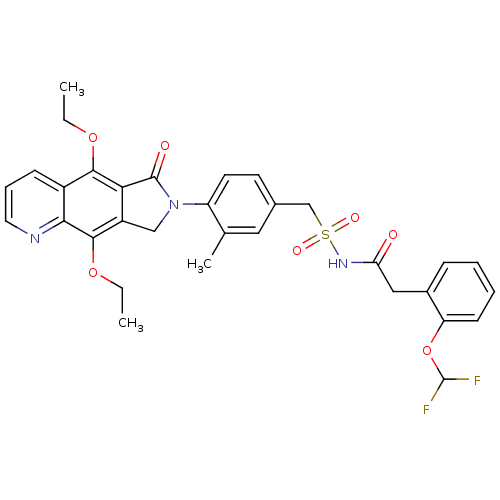 (CHEMBL257255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50336003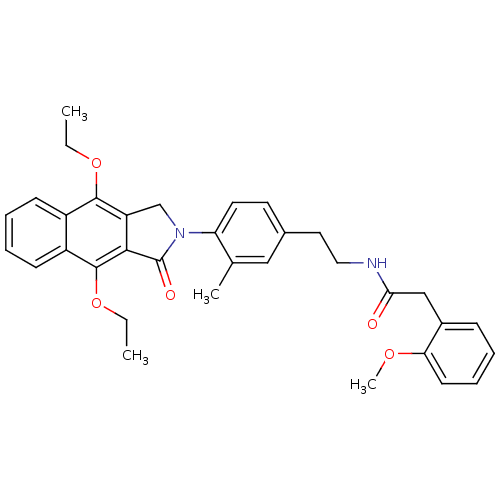 (CHEMBL1669023 | N-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335982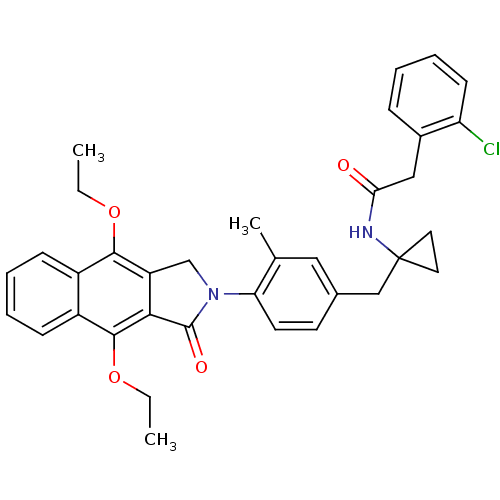 (2-(2-chlorophenyl)-N-(1-(4-(4,9-diethoxy-1-oxo-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50333727 (3-(4-((N-(1-(2-methoxyphenyl)cyclopropanecarbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to EP4 receptor | Bioorg Med Chem Lett 21: 734-7 (2011) Article DOI: 10.1016/j.bmcl.2010.11.118 BindingDB Entry DOI: 10.7270/Q22N52JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4185 total ) | Next | Last >> |