 Found 339 hits Enz. Inhib. hit(s) with Target = 'Phospho-N-acetylmuramoyl-pentapeptide-transferase'
Found 339 hits Enz. Inhib. hit(s) with Target = 'Phospho-N-acetylmuramoyl-pentapeptide-transferase' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343928
(CHEMBL1780216 | Muraymycin D2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17-,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Bacillus subtilis MraY using UDP-MurNAc-pentapeptide as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343928

(CHEMBL1780216 | Muraymycin D2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17-,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Bacillus subtilis MraY using radiolabeled UDP-GlcNAc as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343929
((2S,6S,9R,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17+,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Bacillus subtilis MraY using UDP-MurNAc-pentapeptide as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343929

((2S,6S,9R,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17+,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Bacillus subtilis MraY using radiolabeled UDP-GlcNAc as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343930
((2S,6S,9S,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CCCCCCCCCCCCCCC[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:34| Show InChI InChI=1S/C48H83N11O16/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28(54-41(66)32(27-19-23-53-46(50)55-27)58-47(71)57-31(26(2)3)43(67)68)40(65)52-22-17-21-51-33(44(69)70)38(75-45-37(64)34(61)29(25-49)73-45)39-35(62)36(63)42(74-39)59-24-20-30(60)56-48(59)72/h20,24,26-29,31-39,42,45,51,61-64H,4-19,21-23,25,49H2,1-3H3,(H,52,65)(H,54,66)(H,67,68)(H,69,70)(H3,50,53,55)(H,56,60,72)(H2,57,58,71)/t27-,28-,29+,31-,32-,33-,34+,35-,36+,37+,38-,39-,42+,45-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Bacillus subtilis MraY using radiolabeled UDP-GlcNAc as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343930

((2S,6S,9S,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CCCCCCCCCCCCCCC[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:34| Show InChI InChI=1S/C48H83N11O16/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28(54-41(66)32(27-19-23-53-46(50)55-27)58-47(71)57-31(26(2)3)43(67)68)40(65)52-22-17-21-51-33(44(69)70)38(75-45-37(64)34(61)29(25-49)73-45)39-35(62)36(63)42(74-39)59-24-20-30(60)56-48(59)72/h20,24,26-29,31-39,42,45,51,61-64H,4-19,21-23,25,49H2,1-3H3,(H,52,65)(H,54,66)(H,67,68)(H,69,70)(H3,50,53,55)(H,56,60,72)(H2,57,58,71)/t27-,28-,29+,31-,32-,33-,34+,35-,36+,37+,38-,39-,42+,45-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Bacillus subtilis MraY using UDP-MurNAc-pentapeptide as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343931
((2S,6S,9R,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CCCCCCCCCCCCCCC[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:34| Show InChI InChI=1S/C48H83N11O16/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28(54-41(66)32(27-19-23-53-46(50)55-27)58-47(71)57-31(26(2)3)43(67)68)40(65)52-22-17-21-51-33(44(69)70)38(75-45-37(64)34(61)29(25-49)73-45)39-35(62)36(63)42(74-39)59-24-20-30(60)56-48(59)72/h20,24,26-29,31-39,42,45,51,61-64H,4-19,21-23,25,49H2,1-3H3,(H,52,65)(H,54,66)(H,67,68)(H,69,70)(H3,50,53,55)(H,56,60,72)(H2,57,58,71)/t27-,28+,29+,31-,32-,33-,34+,35-,36+,37+,38-,39-,42+,45-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of Bacillus subtilis MraY using radiolabeled UDP-GlcNAc as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343931

((2S,6S,9R,16S)-16-((S)-((2S,3R,4S,5R)-5-(aminometh...)Show SMILES CCCCCCCCCCCCCCC[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:34| Show InChI InChI=1S/C48H83N11O16/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-18-28(54-41(66)32(27-19-23-53-46(50)55-27)58-47(71)57-31(26(2)3)43(67)68)40(65)52-22-17-21-51-33(44(69)70)38(75-45-37(64)34(61)29(25-49)73-45)39-35(62)36(63)42(74-39)59-24-20-30(60)56-48(59)72/h20,24,26-29,31-39,42,45,51,61-64H,4-19,21-23,25,49H2,1-3H3,(H,52,65)(H,54,66)(H,67,68)(H,69,70)(H3,50,53,55)(H,56,60,72)(H2,57,58,71)/t27-,28+,29+,31-,32-,33-,34+,35-,36+,37+,38-,39-,42+,45-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Competitive inhibition of Bacillus subtilis MraY using UDP-MurNAc-pentapeptide as substrate after 30 mins by Lineweaver-Burk plot |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465326

(CHEMBL4283345)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C54H93N11O18/c1-28(2)19-16-14-12-10-8-9-11-13-15-17-20-34(67)81-42(30(5)6)37(62-47(72)36(31-21-25-59-52(56)60-31)64-53(77)63-35(29(3)4)49(73)74)46(71)58-24-18-23-57-38(50(75)76)43(83-51-45(79-7)39(68)32(27-55)80-51)44-40(69)41(70)48(82-44)65-26-22-33(66)61-54(65)78/h22,26,28-32,35-45,48,51,57,68-70H,8-21,23-25,27,55H2,1-7H3,(H,58,71)(H,62,72)(H,73,74)(H,75,76)(H3,56,59,60)(H,61,66,78)(H2,63,64,77)/t31-,32+,35-,36-,37-,38-,39+,40-,41+,42-,43-,44-,45+,48+,51-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465326

(CHEMBL4283345)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C54H93N11O18/c1-28(2)19-16-14-12-10-8-9-11-13-15-17-20-34(67)81-42(30(5)6)37(62-47(72)36(31-21-25-59-52(56)60-31)64-53(77)63-35(29(3)4)49(73)74)46(71)58-24-18-23-57-38(50(75)76)43(83-51-45(79-7)39(68)32(27-55)80-51)44-40(69)41(70)48(82-44)65-26-22-33(66)61-54(65)78/h22,26,28-32,35-45,48,51,57,68-70H,8-21,23-25,27,55H2,1-7H3,(H,58,71)(H,62,72)(H,73,74)(H,75,76)(H3,56,59,60)(H,61,66,78)(H2,63,64,77)/t31-,32+,35-,36-,37-,38-,39+,40-,41+,42-,43-,44-,45+,48+,51-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465324
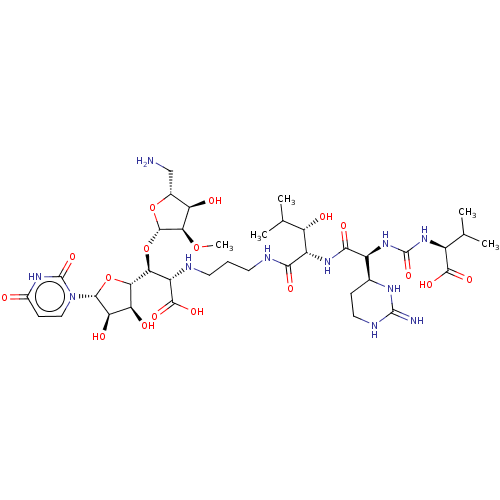
(CHEMBL4286766)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O17/c1-14(2)19(33(57)58)47-37(61)48-20(16-7-11-43-36(40)44-16)31(56)46-21(23(51)15(3)4)30(55)42-10-6-9-41-22(34(59)60)27(66-35-29(63-5)24(52)17(13-39)64-35)28-25(53)26(54)32(65-28)49-12-8-18(50)45-38(49)62/h8,12,14-17,19-29,32,35,41,51-54H,6-7,9-11,13,39H2,1-5H3,(H,42,55)(H,46,56)(H,57,58)(H,59,60)(H3,40,43,44)(H,45,50,62)(H2,47,48,61)/t16-,17+,19-,20-,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465327
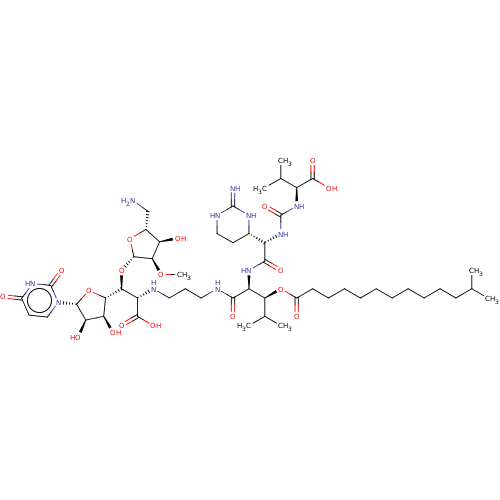
(CHEMBL4279378)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C52H89N11O18/c1-26(2)17-14-12-10-8-9-11-13-15-18-32(65)79-40(28(5)6)35(60-45(70)34(29-19-23-57-50(54)58-29)62-51(75)61-33(27(3)4)47(71)72)44(69)56-22-16-21-55-36(48(73)74)41(81-49-43(77-7)37(66)30(25-53)78-49)42-38(67)39(68)46(80-42)63-24-20-31(64)59-52(63)76/h20,24,26-30,33-43,46,49,55,66-68H,8-19,21-23,25,53H2,1-7H3,(H,56,69)(H,60,70)(H,71,72)(H,73,74)(H3,54,57,58)(H,59,64,76)(H2,61,62,75)/t29-,30+,33-,34-,35-,36-,37+,38-,39+,40-,41-,42-,43+,46+,49-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50465325

(CHEMBL4284923)Show SMILES [H][C@](O[C@@H]1O[C@H](CNC(C)=O)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C40H65N11O18/c1-15(2)21(35(60)61)49-39(64)50-22(18-8-12-44-38(41)46-18)33(59)48-23(25(54)16(3)4)32(58)43-11-7-10-42-24(36(62)63)29(69-37-31(66-6)26(55)19(67-37)14-45-17(5)52)30-27(56)28(57)34(68-30)51-13-9-20(53)47-40(51)65/h9,13,15-16,18-19,21-31,34,37,42,54-57H,7-8,10-12,14H2,1-6H3,(H,43,58)(H,45,52)(H,48,59)(H,60,61)(H,62,63)(H3,41,44,46)(H,47,53,65)(H2,49,50,64)/t18-,19+,21-,22-,23-,24-,25-,26+,27-,28+,29-,30-,31+,34+,37-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY expressed in Escherichia coli membrane using Park's nucleotide as substrate pretreated for 30 mins followed ... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465327

(CHEMBL4279378)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](OC(=O)CCCCCCCCCCC(C)C)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C52H89N11O18/c1-26(2)17-14-12-10-8-9-11-13-15-18-32(65)79-40(28(5)6)35(60-45(70)34(29-19-23-57-50(54)58-29)62-51(75)61-33(27(3)4)47(71)72)44(69)56-22-16-21-55-36(48(73)74)41(81-49-43(77-7)37(66)30(25-53)78-49)42-38(67)39(68)46(80-42)63-24-20-31(64)59-52(63)76/h20,24,26-30,33-43,46,49,55,66-68H,8-19,21-23,25,53H2,1-7H3,(H,56,69)(H,60,70)(H,71,72)(H,73,74)(H3,54,57,58)(H,59,64,76)(H2,61,62,75)/t29-,30+,33-,34-,35-,36-,37+,38-,39+,40-,41-,42-,43+,46+,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465324

(CHEMBL4286766)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O17/c1-14(2)19(33(57)58)47-37(61)48-20(16-7-11-43-36(40)44-16)31(56)46-21(23(51)15(3)4)30(55)42-10-6-9-41-22(34(59)60)27(66-35-29(63-5)24(52)17(13-39)64-35)28-25(53)26(54)32(65-28)49-12-8-18(50)45-38(49)62/h8,12,14-17,19-29,32,35,41,51-54H,6-7,9-11,13,39H2,1-5H3,(H,42,55)(H,46,56)(H,57,58)(H,59,60)(H3,40,43,44)(H,45,50,62)(H2,47,48,61)/t16-,17+,19-,20-,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Aquifex aeolicus (strain VF5)) | BDBM50465325

(CHEMBL4284923)Show SMILES [H][C@](O[C@@H]1O[C@H](CNC(C)=O)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)[C@@H](O)C(C)C)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C40H65N11O18/c1-15(2)21(35(60)61)49-39(64)50-22(18-8-12-44-38(41)46-18)33(59)48-23(25(54)16(3)4)32(58)43-11-7-10-42-24(36(62)63)29(69-37-31(66-6)26(55)19(67-37)14-45-17(5)52)30-27(56)28(57)34(68-30)51-13-9-20(53)47-40(51)65/h9,13,15-16,18-19,21-31,34,37,42,54-57H,7-8,10-12,14H2,1-6H3,(H,43,58)(H,45,52)(H,48,59)(H,60,61)(H,62,63)(H3,41,44,46)(H,47,53,65)(H2,49,50,64)/t18-,19+,21-,22-,23-,24-,25-,26+,27-,28+,29-,30-,31+,34+,37-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Aquifex aeolicus MraY expressed in Escherichia coli C41(DE3) using Park's nucleotide as substrate pretreated for 30 mins followed by su... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50526643

(CHEMBL4475677)Show SMILES [H][C@@]1(O[C@H]([C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@]2([H])NC[C@@H](CCCCCCCCCCCCCCCCC)[C@H](N(C)C2=O)C(O)=O)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C38H65N5O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23-22-40-26(34(49)42(2)27(23)36(50)51)32(55-37-31(48)28(45)24(21-39)53-37)33-29(46)30(47)35(54-33)43-20-19-25(44)41-38(43)52/h19-20,23-24,26-33,35,37,40,45-48H,3-18,21-22,39H2,1-2H3,(H,50,51)(H,41,44,52)/t23-,24-,26+,27+,28-,29+,30-,31-,32+,33+,35-,37+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of MraY in Staphylococcus aureus |
Eur J Med Chem 171: 462-474 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.071
BindingDB Entry DOI: 10.7270/Q2K35Z3R |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | CHEMBL5282738
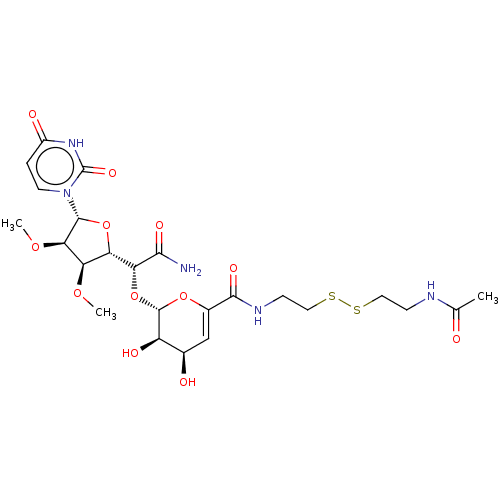
| PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343928

(CHEMBL1780216 | Muraymycin D2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17-,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of purified Bacillus subtilis MraY assessed as incorporation of MurNAc-[14C]pentapeptide into lipid 1 after 30 mins |
ACS Med Chem Lett 1: 258-262 (2010)
Article DOI: 10.1021/ml100057z
BindingDB Entry DOI: 10.7270/Q20Z73KS |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | BDBM50343928

(CHEMBL1780216 | Muraymycin D2)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@@H]1CCN=C(N)N1)C(=O)NCCCN[C@@H]([C@H](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)C(O)=O |r,t:23| Show InChI InChI=1S/C37H61N11O16/c1-14(2)12-17(43-30(55)21(16-6-10-42-35(39)44-16)47-36(60)46-20(15(3)4)32(56)57)29(54)41-9-5-8-40-22(33(58)59)27(64-34-26(53)23(50)18(13-38)62-34)28-24(51)25(52)31(63-28)48-11-7-19(49)45-37(48)61/h7,11,14-18,20-28,31,34,40,50-53H,5-6,8-10,12-13,38H2,1-4H3,(H,41,54)(H,43,55)(H,56,57)(H,58,59)(H3,39,42,44)(H,45,49,61)(H2,46,47,60)/t16-,17-,18+,20-,21-,22-,23+,24-,25+,26+,27-,28-,31+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus subtilis MraY using radiolabeled UDP-MurNAc-[14C]pentapeptide as substrate after 30 mins |
J Med Chem 54: 8421-39 (2011)
Article DOI: 10.1021/jm200906r
BindingDB Entry DOI: 10.7270/Q29P323M |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50465328

(CHEMBL4283943)Show SMILES [H][C@](O[C@@H]1O[C@H](CN)[C@@H](O)[C@H]1OC)([C@H](NCCCNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(O)=O)[C@]1([H])CCNC(=N)N1)C(O)=O)[C@@]1([H])O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C38H63N11O16/c1-15(2)13-18(44-31(55)22(17-7-11-43-36(40)45-17)48-37(60)47-21(16(3)4)33(56)57)30(54)42-10-6-9-41-23(34(58)59)27(65-35-29(62-5)24(51)19(14-39)63-35)28-25(52)26(53)32(64-28)49-12-8-20(50)46-38(49)61/h8,12,15-19,21-29,32,35,41,51-53H,6-7,9-11,13-14,39H2,1-5H3,(H,42,54)(H,44,55)(H,56,57)(H,58,59)(H3,40,43,45)(H,46,50,61)(H2,47,48,60)/t17-,18-,19+,21-,22-,23-,24+,25-,26+,27-,28-,29+,32+,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37Rv MraY expressed in Mycobacterium smegmatis using Park's nucleotide-N-epsilon-C6-dansyl as substrate mea... |
J Nat Prod 81: 942-948 (2018)
Article DOI: 10.1021/acs.jnatprod.7b01054
BindingDB Entry DOI: 10.7270/Q2XS5Z1V |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221211

(CHEMBL97077)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccccc1)C(N)=O |c:23| Show InChI InChI=1S/C23H26N4O11/c1-35-16-15(31)21(27-8-7-13(29)26-23(27)34)37-17(16)18(19(24)32)38-22-14(30)11(28)9-12(36-22)20(33)25-10-5-3-2-4-6-10/h2-9,11,14-18,21-22,28,30-31H,1H3,(H2,24,32)(H,25,33)(H,26,29,34)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221211

(CHEMBL97077)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccccc1)C(N)=O |c:23| Show InChI InChI=1S/C23H26N4O11/c1-35-16-15(31)21(27-8-7-13(29)26-23(27)34)37-17(16)18(19(24)32)38-22-14(30)11(28)9-12(36-22)20(33)25-10-5-3-2-4-6-10/h2-9,11,14-18,21-22,28,30-31H,1H3,(H2,24,32)(H,25,33)(H,26,29,34)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against Translocase I |
Bioorg Med Chem Lett 13: 2833-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0JP8 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221473
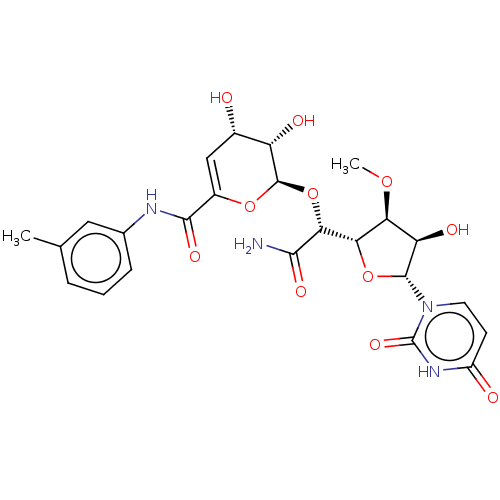
(CHEMBL94424)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1cccc(C)c1)C(N)=O |c:23| Show InChI InChI=1S/C24H28N4O11/c1-10-4-3-5-11(8-10)26-21(34)13-9-12(29)15(31)23(37-13)39-19(20(25)33)18-17(36-2)16(32)22(38-18)28-7-6-14(30)27-24(28)35/h3-9,12,15-19,22-23,29,31-32H,1-2H3,(H2,25,33)(H,26,34)(H,27,30,35)/t12-,15-,16+,17-,18-,19+,22+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221201
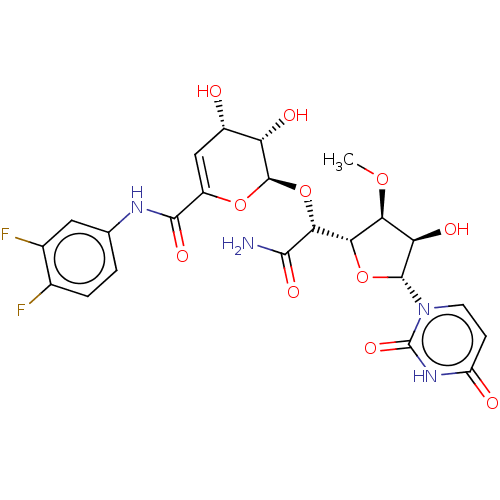
(CHEMBL97712)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccc(F)c(F)c1)C(N)=O |c:23| Show InChI InChI=1S/C23H24F2N4O11/c1-37-16-15(33)21(29-5-4-13(31)28-23(29)36)39-17(16)18(19(26)34)40-22-14(32)11(30)7-12(38-22)20(35)27-8-2-3-9(24)10(25)6-8/h2-7,11,14-18,21-22,30,32-33H,1H3,(H2,26,34)(H,27,35)(H,28,31,36)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135598

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCC[C@@H](C)NC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C24H33N5O12/c1-9-4-3-5-10(20(35)26-9)27-21(36)12-8-11(30)14(32)23(39-12)41-18(19(25)34)17-16(38-2)15(33)22(40-17)29-7-6-13(31)28-24(29)37/h6-11,14-18,22-23,30,32-33H,3-5H2,1-2H3,(H2,25,34)(H,26,35)(H,27,36)(H,28,31,37)/t9-,10+,11+,14+,15-,16+,17+,18-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135598

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCC[C@@H](C)NC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C24H33N5O12/c1-9-4-3-5-10(20(35)26-9)27-21(36)12-8-11(30)14(32)23(39-12)41-18(19(25)34)17-16(38-2)15(33)22(40-17)29-7-6-13(31)28-24(29)37/h6-11,14-18,22-23,30,32-33H,3-5H2,1-2H3,(H2,25,34)(H,26,35)(H,27,36)(H,28,31,37)/t9-,10+,11+,14+,15-,16+,17+,18-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | CHEMBL5265940

| KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135598

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCC[C@@H](C)NC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C24H33N5O12/c1-9-4-3-5-10(20(35)26-9)27-21(36)12-8-11(30)14(32)23(39-12)41-18(19(25)34)17-16(38-2)15(33)22(40-17)29-7-6-13(31)28-24(29)37/h6-11,14-18,22-23,30,32-33H,3-5H2,1-2H3,(H2,25,34)(H,26,35)(H,27,36)(H,28,31,37)/t9-,10+,11+,14+,15-,16+,17+,18-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against translocase-I |
Bioorg Med Chem Lett 13: 2833-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0JP8 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135599

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCCCNC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C23H31N5O12/c1-37-15-14(32)21(28-7-5-12(30)27-23(28)36)39-16(15)17(18(24)33)40-22-13(31)10(29)8-11(38-22)20(35)26-9-4-2-3-6-25-19(9)34/h5,7-10,13-17,21-22,29,31-32H,2-4,6H2,1H3,(H2,24,33)(H,25,34)(H,26,35)(H,27,30,36)/t9-,10-,13-,14+,15-,16-,17+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against translocase-I |
Bioorg Med Chem Lett 13: 2833-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0JP8 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135599

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCCCNC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C23H31N5O12/c1-37-15-14(32)21(28-7-5-12(30)27-23(28)36)39-16(15)17(18(24)33)40-22-13(31)10(29)8-11(38-22)20(35)26-9-4-2-3-6-25-19(9)34/h5,7-10,13-17,21-22,29,31-32H,2-4,6H2,1H3,(H2,24,33)(H,25,34)(H,26,35)(H,27,30,36)/t9-,10-,13-,14+,15-,16-,17+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50135599

((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...)Show SMILES CO[C@H]1[C@@H](O)[C@@H](O[C@@H]1[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCCCNC1=O)C(N)=O)n1ccc(=O)[nH]c1=O |c:13| Show InChI InChI=1S/C23H31N5O12/c1-37-15-14(32)21(28-7-5-12(30)27-23(28)36)39-16(15)17(18(24)33)40-22-13(31)10(29)8-11(38-22)20(35)26-9-4-2-3-6-25-19(9)34/h5,7-10,13-17,21-22,29,31-32H,2-4,6H2,1H3,(H2,24,33)(H,25,34)(H,26,35)(H,27,30,36)/t9-,10-,13-,14+,15-,16-,17+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221409
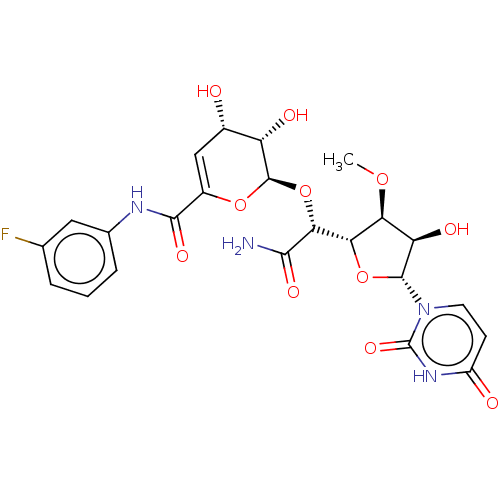
(CHEMBL96162)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1cccc(F)c1)C(N)=O |c:23| Show InChI InChI=1S/C23H25FN4O11/c1-36-16-15(32)21(28-6-5-13(30)27-23(28)35)38-17(16)18(19(25)33)39-22-14(31)11(29)8-12(37-22)20(34)26-10-4-2-3-9(24)7-10/h2-8,11,14-18,21-22,29,31-32H,1H3,(H2,25,33)(H,26,34)(H,27,30,35)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Bacillus subtilis) | CHEMBL5272467

| KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50386963
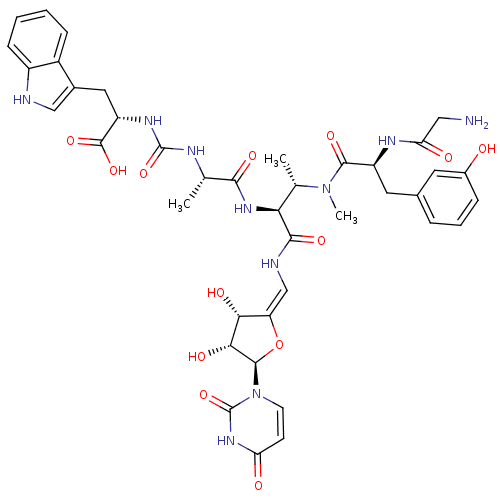
(CHEMBL2048828)Show SMILES C[C@H](NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)N[C@@H]([C@H](C)N(C)C(=O)[C@H](Cc1cccc(O)c1)NC(=O)CN)C(=O)N\C=C1/O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C40H48N10O13/c1-19(44-39(61)46-27(38(59)60)15-22-17-42-25-10-5-4-9-24(22)25)34(56)48-31(20(2)49(3)36(58)26(45-30(53)16-41)14-21-7-6-8-23(51)13-21)35(57)43-18-28-32(54)33(55)37(63-28)50-12-11-29(52)47-40(50)62/h4-13,17-20,26-27,31-33,37,42,51,54-55H,14-16,41H2,1-3H3,(H,43,57)(H,45,53)(H,48,56)(H,59,60)(H2,44,46,61)(H,47,52,62)/b28-18-/t19-,20-,26-,27-,31-,32+,33+,37+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY using UDP-MurNAc-dansylpentapeptide substrate assessed as formation of dansylated lipid I incubated for 3 to... |
Bioorg Med Chem Lett 22: 4810-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.050
BindingDB Entry DOI: 10.7270/Q2J967FP |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50386961

(CHEMBL2048825)Show SMILES C[C@H](N)C(=O)N(C)[C@@H](C)[C@H](NC(=O)[C@H](C)NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)N\C=C1\C[C@@H](O)[C@@H](O1)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C32H41N9O10/c1-15(33)28(46)40(4)17(3)25(27(45)35-14-19-12-23(42)29(51-19)41-10-9-24(43)38-32(41)50)39-26(44)16(2)36-31(49)37-22(30(47)48)11-18-13-34-21-8-6-5-7-20(18)21/h5-10,13-17,22-23,25,29,34,42H,11-12,33H2,1-4H3,(H,35,45)(H,39,44)(H,47,48)(H2,36,37,49)(H,38,43,50)/b19-14-/t15-,16-,17-,22-,23+,25-,29+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus MraY using UDP-MurNAc-dansylpentapeptide substrate assessed as formation of dansylated lipid I incubated for 3 to... |
Bioorg Med Chem Lett 22: 4810-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.050
BindingDB Entry DOI: 10.7270/Q2J967FP |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221204

(CHEMBL327358)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1cccc(F)c1F)C(N)=O |c:23| Show InChI InChI=1S/C23H24F2N4O11/c1-37-16-15(33)21(29-6-5-12(31)28-23(29)36)39-17(16)18(19(26)34)40-22-14(32)10(30)7-11(38-22)20(35)27-9-4-2-3-8(24)13(9)25/h2-7,10,14-18,21-22,30,32-33H,1H3,(H2,26,34)(H,27,35)(H,28,31,36)/t10-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221205
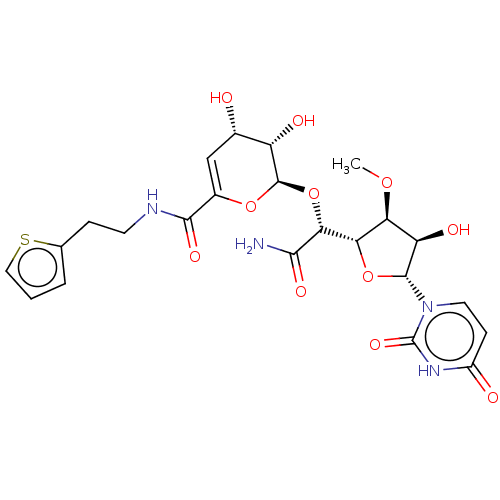
(CHEMBL317088)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)NCCc1cccs1)C(N)=O |c:23| Show InChI InChI=1S/C23H28N4O11S/c1-35-16-15(31)21(27-7-5-13(29)26-23(27)34)37-17(16)18(19(24)32)38-22-14(30)11(28)9-12(36-22)20(33)25-6-4-10-3-2-8-39-10/h2-3,5,7-9,11,14-18,21-22,28,30-31H,4,6H2,1H3,(H2,24,32)(H,25,33)(H,26,29,34)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221054

(CHEMBL97606)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccc(C)cc1)C(N)=O |c:23| Show InChI InChI=1S/C24H28N4O11/c1-10-3-5-11(6-4-10)26-21(34)13-9-12(29)15(31)23(37-13)39-19(20(25)33)18-17(36-2)16(32)22(38-18)28-8-7-14(30)27-24(28)35/h3-9,12,15-19,22-23,29,31-32H,1-2H3,(H2,25,33)(H,26,34)(H,27,30,35)/t12-,15-,16+,17-,18-,19+,22+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50526632

(CHEMBL4473600)Show SMILES [H][C@@]1(O[C@H]([C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@]2([H])C[C@H](CCN2)NC(=O)CCCCCCCCCCCCCCC)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C35H61N5O10/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(41)38-22-16-18-37-23(20-22)31(50-34-30(46)27(43)24(21-36)48-34)32-28(44)29(45)33(49-32)40-19-17-26(42)39-35(40)47/h17,19,22-24,27-34,37,43-46H,2-16,18,20-21,36H2,1H3,(H,38,41)(H,39,42,47)/t22-,23-,24+,27+,28-,29+,30+,31-,32-,33+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of MraY in Staphylococcus aureus |
Eur J Med Chem 171: 462-474 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.071
BindingDB Entry DOI: 10.7270/Q2K35Z3R |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | BDBM50526632

(CHEMBL4473600)Show SMILES [H][C@@]1(O[C@H]([C@@]2([H])O[C@H]([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@]2([H])C[C@H](CCN2)NC(=O)CCCCCCCCCCCCCCC)O[C@H](CN)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C35H61N5O10/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(41)38-22-16-18-37-23(20-22)31(50-34-30(46)27(43)24(21-36)48-34)32-28(44)29(45)33(49-32)40-19-17-26(42)39-35(40)47/h17,19,22-24,27-34,37,43-46H,2-16,18,20-21,36H2,1H3,(H,38,41)(H,39,42,47)/t22-,23-,24+,27+,28-,29+,30+,31-,32-,33+,34-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of MraY in Staphylococcus aureus |
Eur J Med Chem 171: 462-474 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.071
BindingDB Entry DOI: 10.7270/Q2K35Z3R |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221475
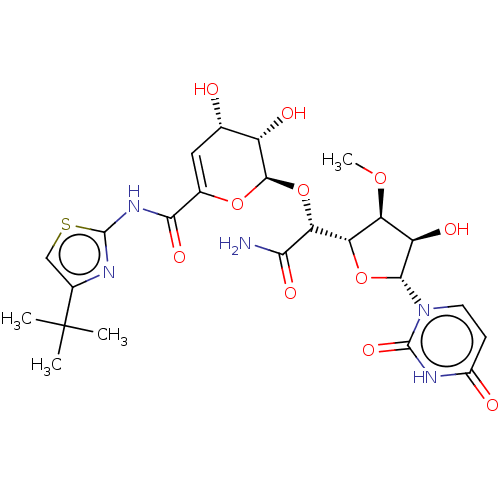
(CHEMBL94839)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1nc(cs1)C(C)(C)C)C(N)=O |c:23| Show InChI InChI=1S/C24H31N5O11S/c1-24(2,3)11-8-41-22(26-11)28-19(35)10-7-9(30)13(32)21(38-10)40-17(18(25)34)16-15(37-4)14(33)20(39-16)29-6-5-12(31)27-23(29)36/h5-9,13-17,20-21,30,32-33H,1-4H3,(H2,25,34)(H,26,28,35)(H,27,31,36)/t9-,13-,14+,15-,16-,17+,20+,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221469
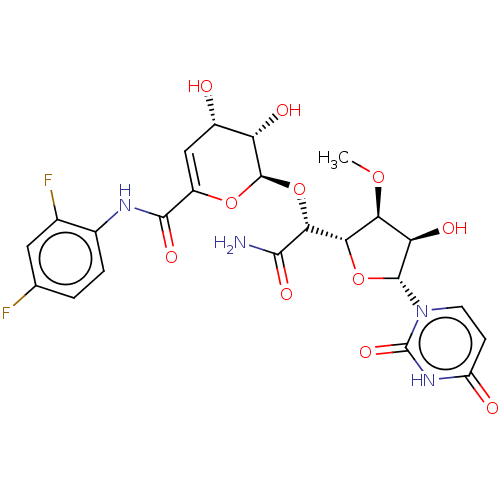
(CHEMBL329448)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccc(F)cc1F)C(N)=O |c:23| Show InChI InChI=1S/C23H24F2N4O11/c1-37-16-15(33)21(29-5-4-13(31)28-23(29)36)39-17(16)18(19(26)34)40-22-14(32)11(30)7-12(38-22)20(35)27-10-3-2-8(24)6-9(10)25/h2-7,11,14-18,21-22,30,32-33H,1H3,(H2,26,34)(H,27,35)(H,28,31,36)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Staphylococcus aureus (strain MRSA252)) | CHEMBL5277590
| KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221051
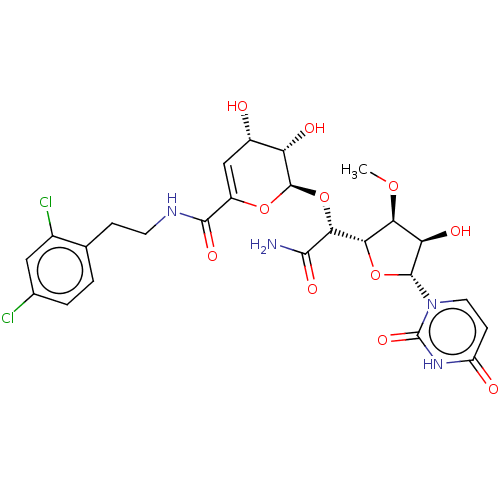
(CHEMBL319731)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)NCCc1ccc(Cl)cc1Cl)C(N)=O |c:23| Show InChI InChI=1S/C25H28Cl2N4O11/c1-39-18-17(35)23(31-7-5-15(33)30-25(31)38)41-19(18)20(21(28)36)42-24-16(34)13(32)9-14(40-24)22(37)29-6-4-10-2-3-11(26)8-12(10)27/h2-3,5,7-9,13,16-20,23-24,32,34-35H,4,6H2,1H3,(H2,28,36)(H,29,37)(H,30,33,38)/t13-,16-,17+,18-,19-,20+,23+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221457

(CHEMBL94400)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccc(Cl)cc1)C(N)=O |c:23| Show InChI InChI=1S/C23H25ClN4O11/c1-36-16-15(32)21(28-7-6-13(30)27-23(28)35)38-17(16)18(19(25)33)39-22-14(31)11(29)8-12(37-22)20(34)26-10-4-2-9(24)3-5-10/h2-8,11,14-18,21-22,29,31-32H,1H3,(H2,25,33)(H,26,34)(H,27,30,35)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221331
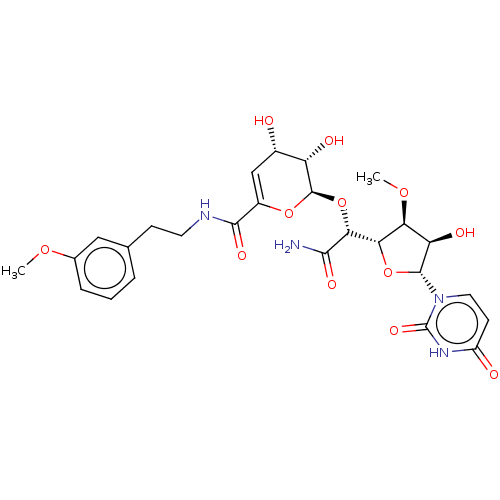
(CHEMBL430672)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)NCCc1cccc(OC)c1)C(N)=O |c:23| Show InChI InChI=1S/C26H32N4O12/c1-38-13-5-3-4-12(10-13)6-8-28-23(36)15-11-14(31)17(33)25(40-15)42-21(22(27)35)20-19(39-2)18(34)24(41-20)30-9-7-16(32)29-26(30)37/h3-5,7,9-11,14,17-21,24-25,31,33-34H,6,8H2,1-2H3,(H2,27,35)(H,28,36)(H,29,32,37)/t14-,17-,18+,19-,20-,21+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221407
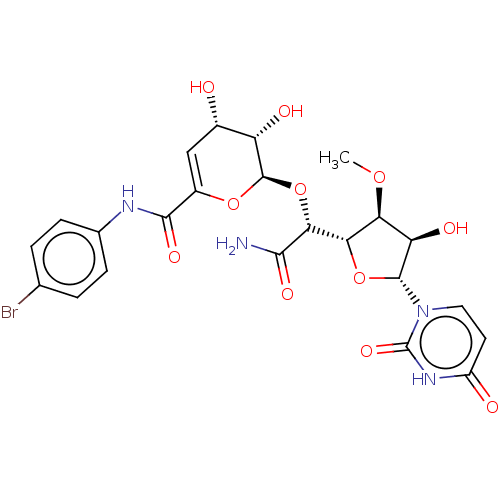
(CHEMBL418960)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1ccc(Br)cc1)C(N)=O |c:23| Show InChI InChI=1S/C23H25BrN4O11/c1-36-16-15(32)21(28-7-6-13(30)27-23(28)35)38-17(16)18(19(25)33)39-22-14(31)11(29)8-12(37-22)20(34)26-10-4-2-9(24)3-5-10/h2-8,11,14-18,21-22,29,31-32H,1H3,(H2,25,33)(H,26,34)(H,27,30,35)/t11-,14-,15+,16-,17-,18+,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221408
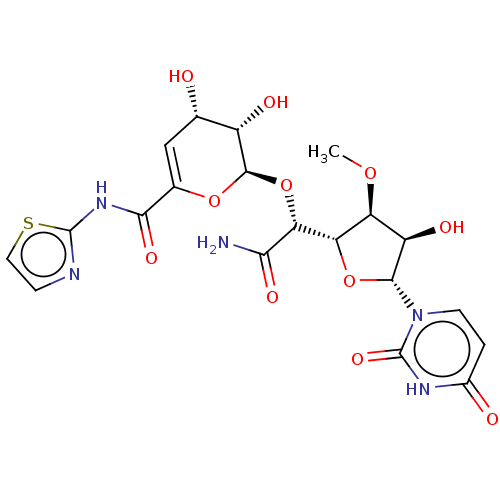
(CHEMBL97276)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)Nc1nccs1)C(N)=O |c:23| Show InChI InChI=1S/C20H23N5O11S/c1-33-12-11(29)17(25-4-2-9(27)23-20(25)32)35-13(12)14(15(21)30)36-18-10(28)7(26)6-8(34-18)16(31)24-19-22-3-5-37-19/h2-7,10-14,17-18,26,28-29H,1H3,(H2,21,30)(H,22,24,31)(H,23,27,32)/t7-,10-,11+,12-,13-,14+,17+,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |
Phospho-N-acetylmuramoyl-pentapeptide-transferase
(Escherichia coli (strain K12)) | BDBM50221330
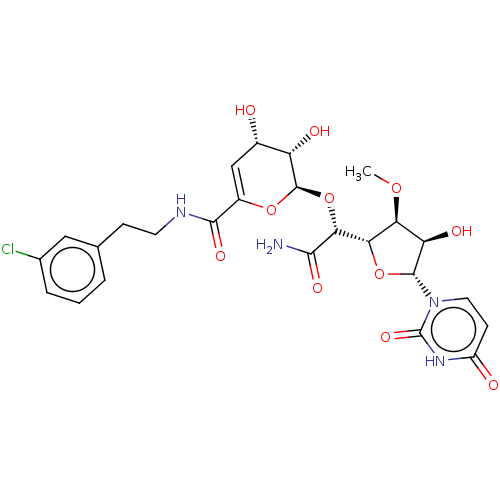
(CHEMBL96561)Show SMILES [H][C@@]1(O[C@H]([C@H](O)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)NCCc1cccc(Cl)c1)C(N)=O |c:23| Show InChI InChI=1S/C25H29ClN4O11/c1-38-18-17(34)23(30-8-6-15(32)29-25(30)37)40-19(18)20(21(27)35)41-24-16(33)13(31)10-14(39-24)22(36)28-7-5-11-3-2-4-12(26)9-11/h2-4,6,8-10,13,16-20,23-24,31,33-34H,5,7H2,1H3,(H2,27,35)(H,28,36)(H,29,32,37)/t13-,16-,17+,18-,19-,20+,23+,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against translocase I |
Bioorg Med Chem Lett 13: 2829-32 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data