 Found 128 hits Enz. Inhib. hit(s) with Target = 'REST corepressor 1'
Found 128 hits Enz. Inhib. hit(s) with Target = 'REST corepressor 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
REST corepressor 1
(Homo sapiens (Human)) | BDBM50262048
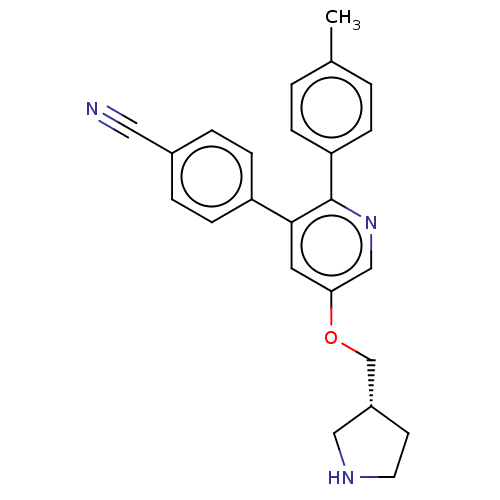
(CHEMBL3134377)Show SMILES Cc1ccc(cc1)-c1ncc(OC[C@@H]2CCNC2)cc1-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H23N3O/c1-17-2-6-21(7-3-17)24-23(20-8-4-18(13-25)5-9-20)12-22(15-27-24)28-16-19-10-11-26-14-19/h2-9,12,15,19,26H,10-11,14,16H2,1H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50458057

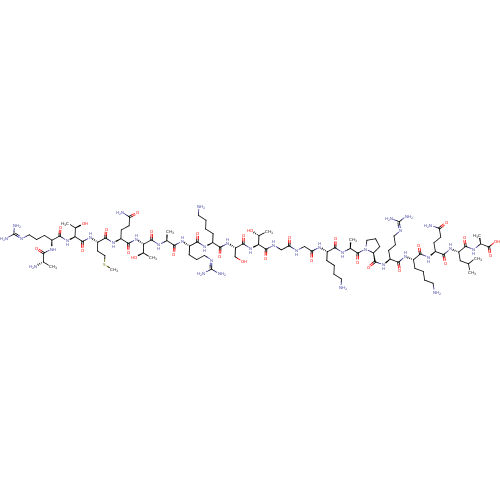
(CHEMBL4210908)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C94H173N35O27S/c1-47(2)43-64(84(148)114-51(6)91(155)156)124-80(144)62(31-32-67(100)134)121-76(140)56(24-12-16-35-96)118-78(142)60(28-20-39-108-93(103)104)122-86(150)66-30-22-41-129(66)90(154)50(5)113-75(139)55(23-11-15-34-95)115-69(136)45-110-68(135)44-111-87(151)70(52(7)131)126-85(149)65(46-130)125-79(143)57(25-13-17-36-97)119-77(141)59(27-19-38-107-92(101)102)117-74(138)49(4)112-88(152)71(53(8)132)127-82(146)58(26-14-18-37-98)120-81(145)63(33-42-157-10)123-89(153)72(54(9)133)128-83(147)61(116-73(137)48(3)99)29-21-40-109-94(105)106/h47-66,70-72,130-133H,11-46,95-99H2,1-10H3,(H2,100,134)(H,110,135)(H,111,151)(H,112,152)(H,113,139)(H,114,148)(H,115,136)(H,116,137)(H,117,138)(H,118,142)(H,119,141)(H,120,145)(H,121,140)(H,122,150)(H,123,153)(H,124,144)(H,125,143)(H,126,149)(H,127,146)(H,128,147)(H,155,156)(H4,101,102,107)(H4,103,104,108)(H4,105,106,109)/t48-,49-,50-,51-,52+,53+,54+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,70-,71-,72-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Inhibition of LSD1/CoREST (unknown origin) |
Eur J Med Chem 148: 210-220 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.098
BindingDB Entry DOI: 10.7270/Q2N0195V |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346870
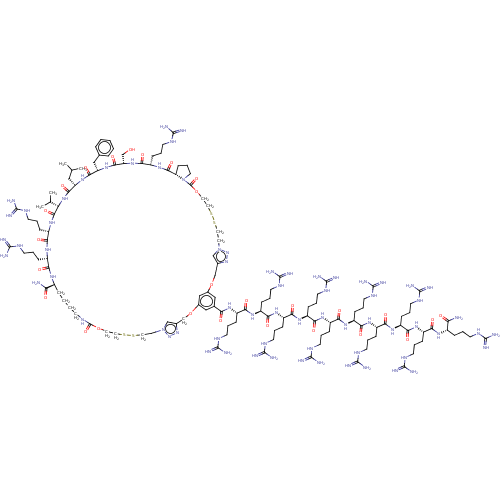
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586370
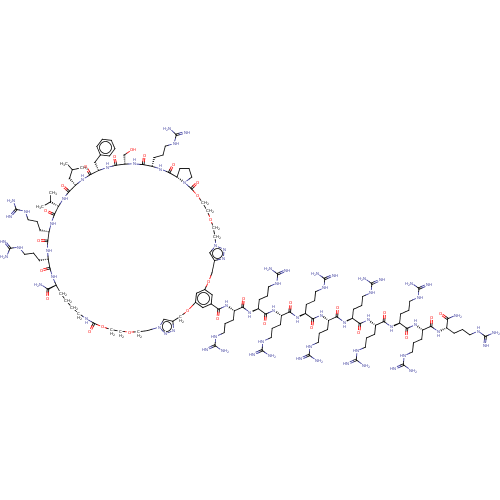
(CHEMBL5084197)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586371
(CHEMBL5089876)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCOCCn2cc(COc3cc(OCc4cn(CCOCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)cc(c3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594106
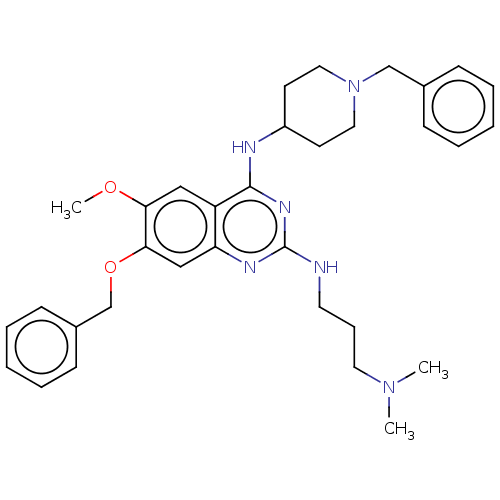
(CHEMBL5202637)Show SMILES COc1cc2c(NC3CCN(Cc4ccccc4)CC3)nc(NCCCN(C)C)nc2cc1OCc1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586363
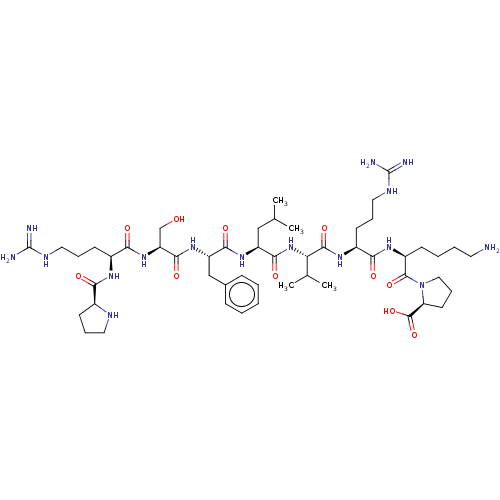
(CHEMBL5079374)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-noradrenaline induced contraction of rat prostatic vas deferens (alpha1A adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50158884

(CHEMBL3785550)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C51H87N17O10/c1-29(2)26-36(46(75)67-40(30(3)4)48(77)62-34(19-12-24-60-51(56)57)43(72)63-35(16-8-9-21-52)49(78)68-25-13-20-39(68)41(53)70)64-45(74)37(27-31-14-6-5-7-15-31)65-47(76)38(28-69)66-44(73)33(18-11-23-59-50(54)55)61-42(71)32-17-10-22-58-32/h5-7,14-15,29-30,32-40,58,69H,8-13,16-28,52H2,1-4H3,(H2,53,70)(H,61,71)(H,62,77)(H,63,72)(H,64,74)(H,65,76)(H,66,73)(H,67,75)(H4,54,55,59)(H4,56,57,60)/t32-,33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya City University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged LSD1 (171 to 836 residues)/GST-tagged CoREST (308 to 440 residues) complex using H3K4 peptide substrate by... |
Bioorg Med Chem 25: 1227-1234 (2017)
Article DOI: 10.1016/j.bmc.2016.12.033
BindingDB Entry DOI: 10.7270/Q2X92D9V |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594086

(CHEMBL5170197)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594083
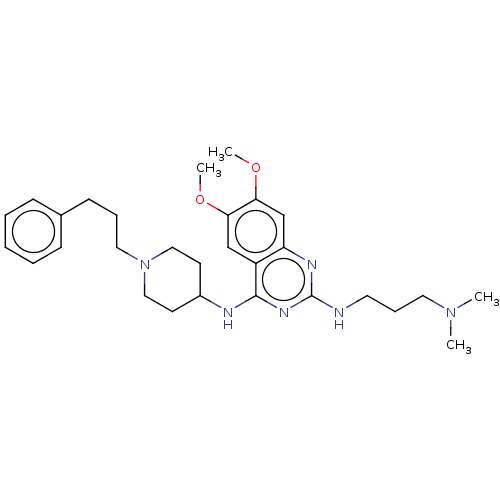
(CHEMBL5179091)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CCCc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594098
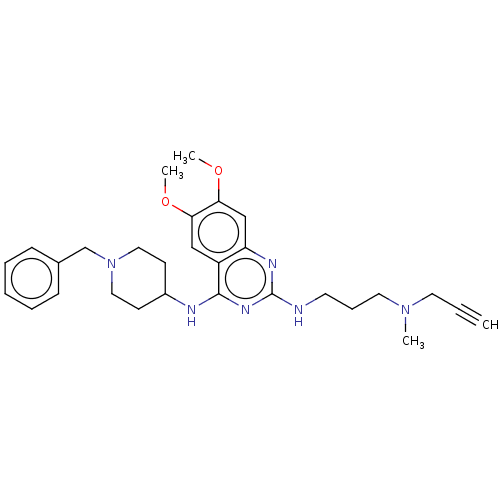
(CHEMBL5198334)Show SMILES COc1cc2nc(NCCCN(C)CC#C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594085
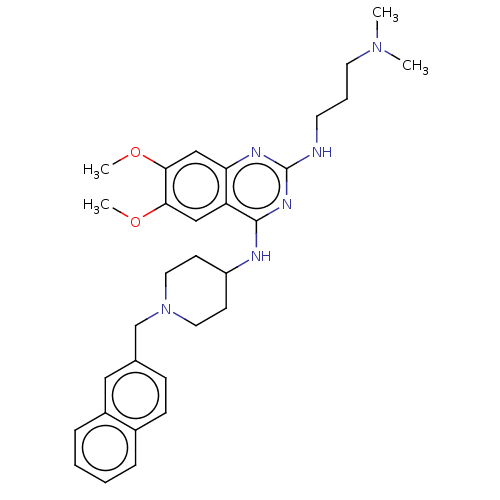
(CHEMBL5178765)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccc5ccccc5c4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5281080

Show SMILES C[C@@H]1NC(=O)[C@H](Cc2cccs2)NC(=O)CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CC3CCCCC3N2C(=O)[C@H]2Cc3ccccc3CN2C1=O |r| Show InChI InChI=1S/C39H53N9O6S/c1-23-37(53)47-22-26-11-3-2-9-24(26)19-32(47)38(54)48-30-14-5-4-10-25(30)20-31(48)36(52)46-28(13-6-17-43-39(40)41)34(50)42-16-7-15-33(49)45-29(35(51)44-23)21-27-12-8-18-55-27/h2-3,8-9,11-12,18,23,25,28-32H,4-7,10,13-17,19-22H2,1H3,(H,42,50)(H,44,51)(H,45,49)(H,46,52)(H4,40,41,43)/t23-,25?,28-,29-,30?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594101
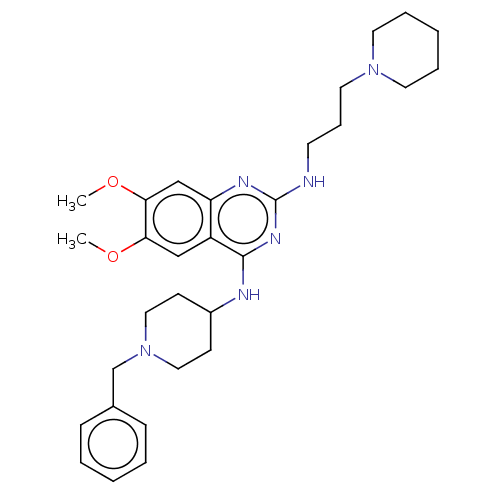
(CHEMBL5175171)Show SMILES COc1cc2nc(NCCCN3CCCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586373

(CHEMBL5075544)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)c1cc(OCc2cn(CCO)nn2)cc(OCc2cn(CCO)nn2)c1)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594099
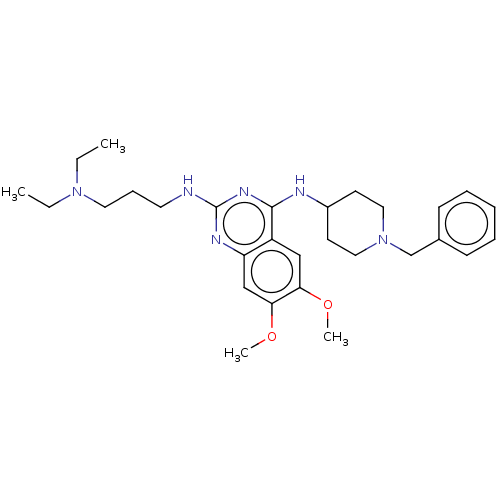
(CHEMBL5186174)Show SMILES CCN(CC)CCCNc1nc(NC2CCN(Cc3ccccc3)CC2)c2cc(OC)c(OC)cc2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594100

(CHEMBL5181017)Show SMILES COc1cc2nc(NCCCN3CCCC3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL1255711
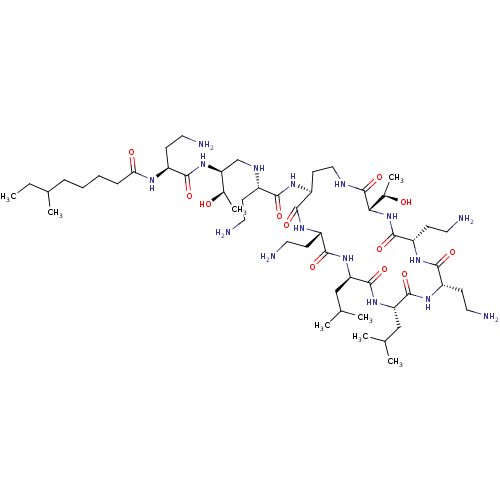
Show SMILES [#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C36H48N8O6/c37-20-31(45)41-27(17-22-9-2-1-3-10-22)33(47)43-21-25-13-5-4-11-23(25)18-30(43)34(48)44-28-15-7-6-12-24(28)19-29(44)32(46)42-26(35(49)50)14-8-16-40-36(38)39/h1-5,9-11,13,24,26-30H,6-8,12,14-21,37H2,(H,41,45)(H,42,46)(H,49,50)(H4,38,39,40)/t24?,26-,27-,28?,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mean functional activity against human H3 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594090
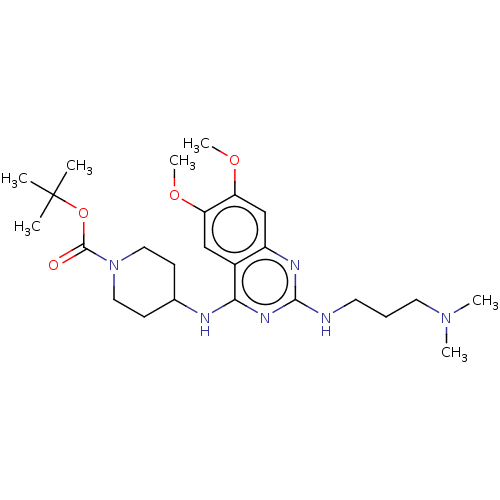
(CHEMBL5205888)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(CC3)C(=O)OC(C)(C)C)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5287743

Show InChI InChI=1S/C13H11N3OS2/c1-8-6-12(19-16-8)15-13(17)14-10-2-3-11-9(7-10)4-5-18-11/h2-7H,1H3,(H2,14,15,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594084
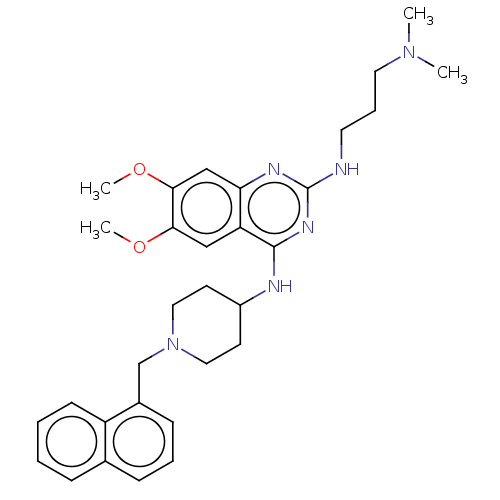
(CHEMBL5204694)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4cccc5ccccc45)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50507295
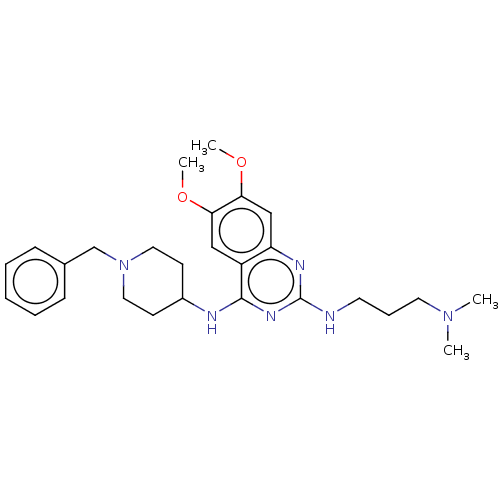
(CHEMBL1232432)Show SMILES COc1cc2nc(NCCCN(C)C)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C27H38N6O2/c1-32(2)14-8-13-28-27-30-23-18-25(35-4)24(34-3)17-22(23)26(31-27)29-21-11-15-33(16-12-21)19-20-9-6-5-7-10-20/h5-7,9-10,17-18,21H,8,11-16,19H2,1-4H3,(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50594087
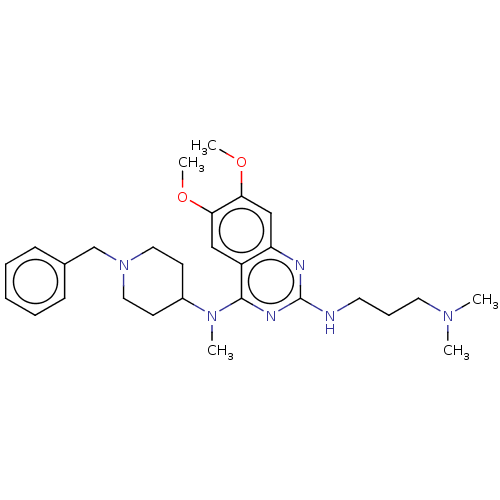
(CHEMBL5189687)Show SMILES COc1cc2nc(NCCCN(C)C)nc(N(C)C3CCN(Cc4ccccc4)CC3)c2cc1OC | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50586372
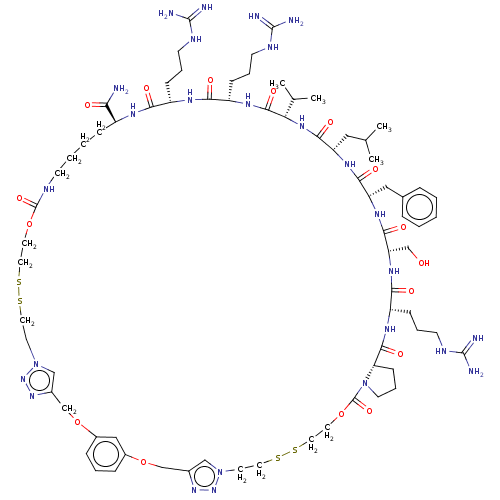
(CHEMBL5085737)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)OCCSSCCn2cc(COc3cccc(OCc4cn(CCSSCCOC(=O)NCCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC1=O)C(C)C)C(N)=O)nn4)c3)nn2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01371
BindingDB Entry DOI: 10.7270/Q2KS6WFP |
More data for this
Ligand-Target Pair | |
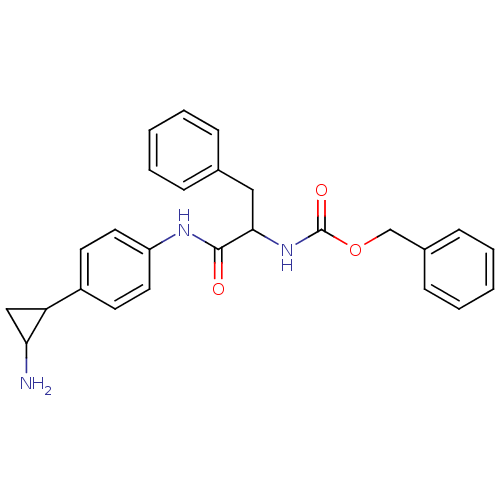
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346863
(CHEMBL1797640 | US8765820, 5b)Show InChI InChI=1S/C16H16N2O/c17-15-10-14(15)11-6-8-13(9-7-11)18-16(19)12-4-2-1-3-5-12/h1-9,14-15H,10,17H2,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
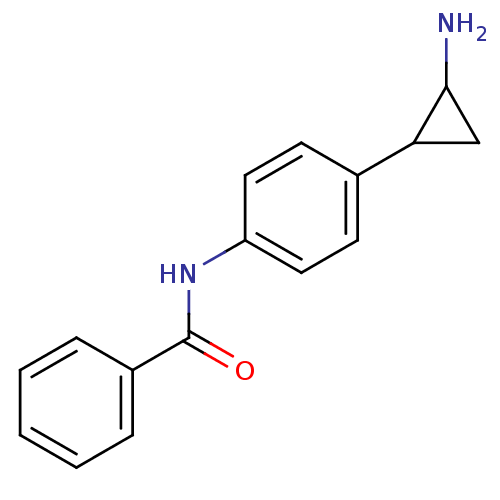
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346864
(CHEMBL1797641 | CHEMBL3104337 | US8765820, 8)Show SMILES NC1CC1c1ccc(NC(=O)C(Cc2ccccc2)NC(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O3/c27-23-16-22(23)20-11-13-21(14-12-20)28-25(30)24(15-18-7-3-1-4-8-18)29-26(31)32-17-19-9-5-2-6-10-19/h1-14,22-24H,15-17,27H2,(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50346586
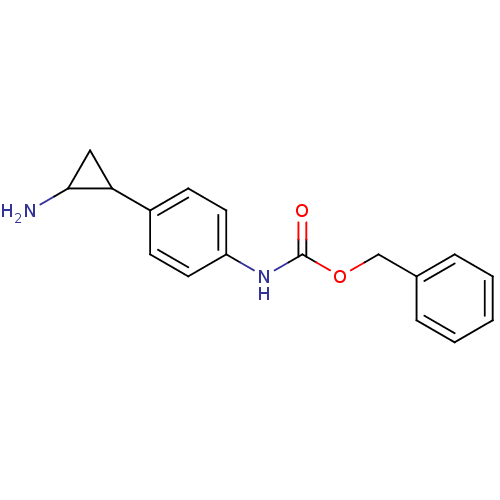
(CHEMBL1795981 | US8765820, 5a)Show InChI InChI=1S/C17H18N2O2/c18-16-10-15(16)13-6-8-14(9-7-13)19-17(20)21-11-12-4-2-1-3-5-12/h1-9,15-16H,10-11,18H2,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LSD1/CoREST expressed in Escherichia coli using histone H3 peptide monomethylated at Lys4 as substrate by peroxidase-coupled assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5266466
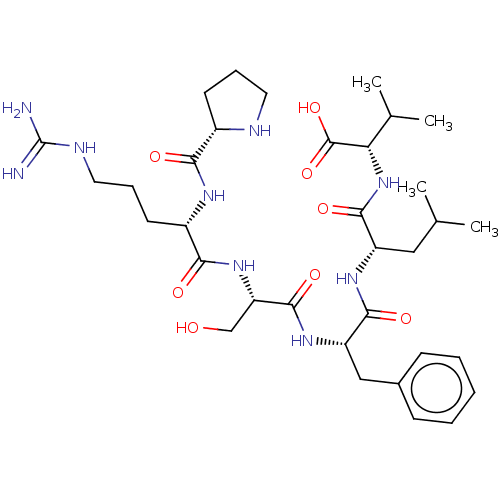
Show InChI InChI=1S/C14H14N4O2/c1-9-7-13(20-17-9)16-14(19)15-11-3-4-12-10(8-11)5-6-18(12)2/h3-8H,1-2H3,(H2,15,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Blocking activity was assessed by antagonism of (-)-phenylephrine induced contraction of rat spleen (alpha1B adrenoceptor) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50568523
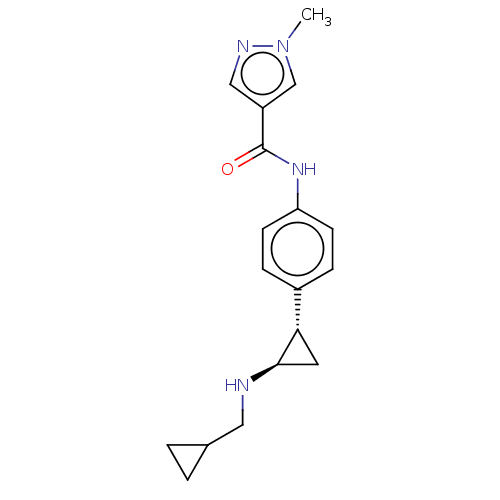
(CHEMBL4875015)Show SMILES Cl.Cn1cc(cn1)C(=O)Nc1ccc(cc1)[C@@H]1C[C@H]1NCC1CC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His-SUMO tagged human LSD1 (172 to 852 residues)/His-tagged CoREST (286 to 482 residues) expressed in Escherichia coli BL21 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00919
BindingDB Entry DOI: 10.7270/Q28K7DTB |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5291155

Show SMILES C\C(CCCN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)=N\OCCN1CCOCC1 Show InChI InChI=1S/C33H39F6N5O3/c1-23(41-47-16-13-42-11-14-46-15-12-42)5-4-8-43-9-10-44(28(22-43)19-25-21-40-30-7-3-2-6-29(25)30)31(45)24-17-26(32(34,35)36)20-27(18-24)33(37,38)39/h2-3,6-7,17-18,20-21,28,40H,4-5,8-16,19,22H2,1H3/b41-23-/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5280566
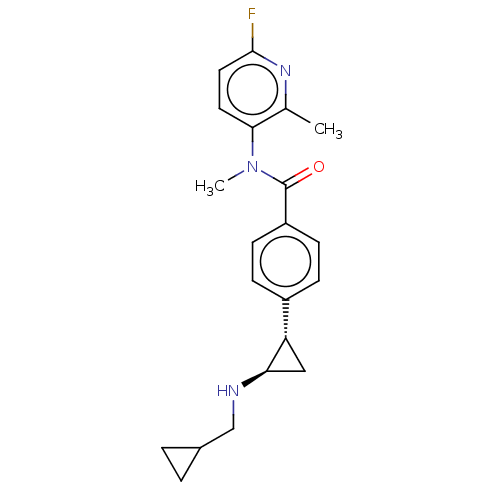
Show SMILES CN(C)CCO\N=C(\C)CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C29H33F6N5O2/c1-19(37-42-11-10-38(2)3)17-39-8-9-40(24(18-39)14-21-16-36-26-7-5-4-6-25(21)26)27(41)20-12-22(28(30,31)32)15-23(13-20)29(33,34)35/h4-7,12-13,15-16,24,36H,8-11,14,17-18H2,1-3H3/b37-19-/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155773
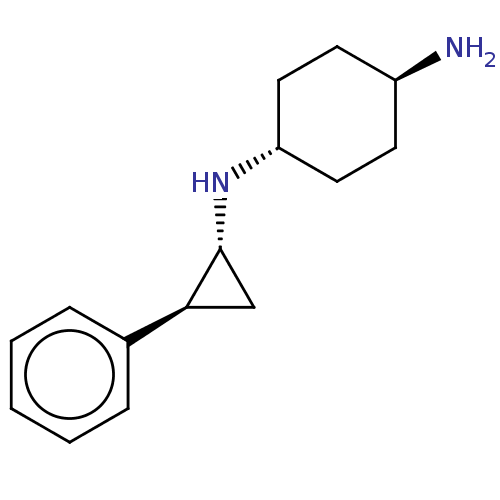
(CHEMBL3781751 | US9469597, 5)Show SMILES [H][C@@]1(CC[C@H](N)CC1)N[C@@H]1C[C@H]1c1ccccc1 |r,wU:9.9,wD:11.13,4.4,1.0,(-4.65,6.17,;-4.67,5.14,;-4.65,3.62,;-5.99,2.87,;-7.32,3.66,;-8.39,3.05,;-7.3,5.2,;-5.95,5.95,;-3.34,4.36,;-3.36,2.83,;-4.13,1.49,;-2.67,1.54,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,)| Show InChI InChI=1S/C15H22N2/c16-12-6-8-13(9-7-12)17-15-10-14(15)11-4-2-1-3-5-11/h1-5,12-15,17H,6-10,16H2/t12-,13-,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5277056
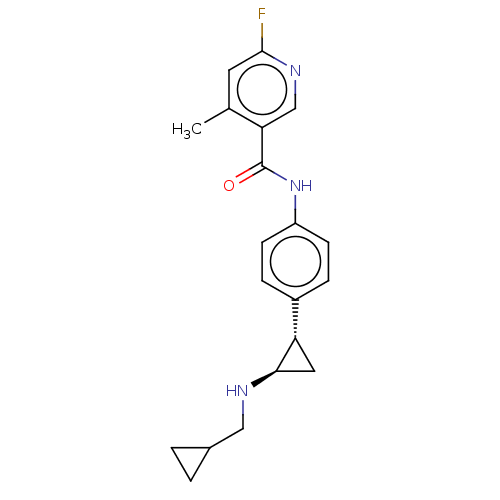
Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(CC2=NOC(CN3CCOCC3)C2)C[C@H]1Cc1c[nH]c2ccccc12 |t:22| Show InChI InChI=1S/C31H33F6N5O3/c32-30(33,34)22-11-20(12-23(14-22)31(35,36)37)29(43)42-6-5-41(17-24-15-26(45-39-24)19-40-7-9-44-10-8-40)18-25(42)13-21-16-38-28-4-2-1-3-27(21)28/h1-4,11-12,14,16,25-26,38H,5-10,13,15,17-19H2/t25-,26?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5281376

Show SMILES C\C(CCN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)=N\OCCN1CCOCC1 Show InChI InChI=1S/C32H37F6N5O3/c1-22(40-46-15-12-41-10-13-45-14-11-41)6-7-42-8-9-43(27(21-42)18-24-20-39-29-5-3-2-4-28(24)29)30(44)23-16-25(31(33,34)35)19-26(17-23)32(36,37)38/h2-5,16-17,19-20,27,39H,6-15,18,21H2,1H3/b40-22-/t27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155579
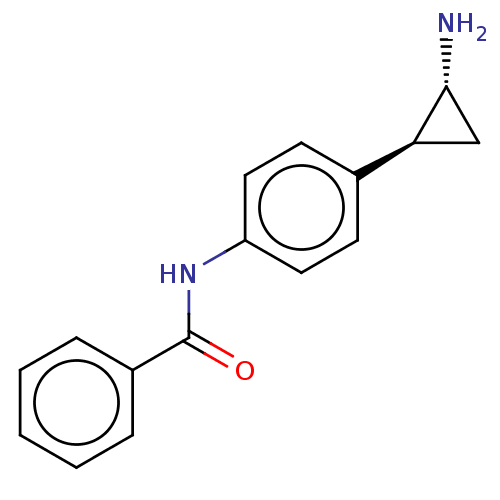
(CHEMBL3546846)Show SMILES N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccccc2)cc1 |r| Show InChI InChI=1S/C16H16N2O/c17-15-10-14(15)11-6-8-13(9-7-11)18-16(19)12-4-2-1-3-5-12/h1-9,14-15H,10,17H2,(H,18,19)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
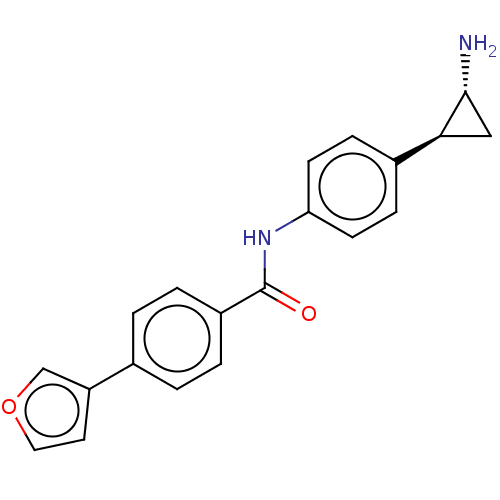
(Homo sapiens (Human)) | BDBM50155763
(CHEMBL3780391)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccc(cc2)-c2ccoc2)cc1 |r| Show InChI InChI=1S/C20H18N2O2.ClH/c21-19-11-18(19)14-5-7-17(8-6-14)22-20(23)15-3-1-13(2-4-15)16-9-10-24-12-16;/h1-10,12,18-19H,11,21H2,(H,22,23);1H/t18-,19+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5270114

Show SMILES C\C(CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)=N\OCCN1CCOCC1 Show InChI InChI=1S/C31H35F6N5O3/c1-21(39-45-13-10-40-8-11-44-12-9-40)19-41-6-7-42(26(20-41)16-23-18-38-28-5-3-2-4-27(23)28)29(43)22-14-24(30(32,33)34)17-25(15-22)31(35,36)37/h2-5,14-15,17-18,26,38H,6-13,16,19-20H2,1H3/b39-21-/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155766
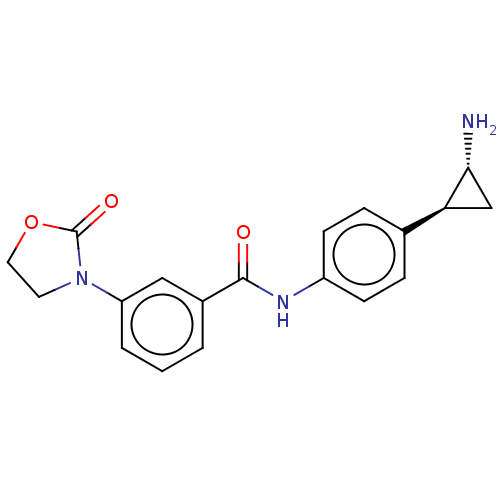
(CHEMBL3780374)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2cccc(c2)N2CCOC2=O)cc1 |r| Show InChI InChI=1S/C19H19N3O3.ClH/c20-17-11-16(17)12-4-6-14(7-5-12)21-18(23)13-2-1-3-15(10-13)22-8-9-25-19(22)24;/h1-7,10,16-17H,8-9,11,20H2,(H,21,23);1H/t16-,17+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50498143

(CHEMBL3407602)Show InChI InChI=1S/C11H14BrN.ClH/c1-2-11(13)7-10(11)8-4-3-5-9(12)6-8;/h3-6,10H,2,7,13H2,1H3;1H/t10-,11+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as s... |
Eur J Med Chem 92: 377-86 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.032
BindingDB Entry DOI: 10.7270/Q2X351F5 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155767
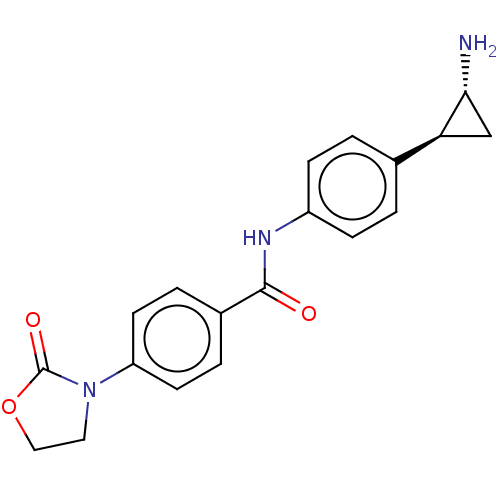
(CHEMBL3780524)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccc(cc2)N2CCOC2=O)cc1 |r| Show InChI InChI=1S/C19H19N3O3.ClH/c20-17-11-16(17)12-1-5-14(6-2-12)21-18(23)13-3-7-15(8-4-13)22-9-10-25-19(22)24;/h1-8,16-17H,9-11,20H2,(H,21,23);1H/t16-,17+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50498128

(CHEMBL3410944)Show InChI InChI=1S/C11H13BrFN.ClH/c1-2-11(14)6-8(11)7-3-4-9(12)10(13)5-7;/h3-5,8H,2,6,14H2,1H3;1H/t8-,11+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as s... |
Eur J Med Chem 92: 377-86 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.032
BindingDB Entry DOI: 10.7270/Q2X351F5 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50498142

(CHEMBL3407601)Show InChI InChI=1S/C11H14ClN.ClH/c1-2-11(13)7-10(11)8-4-3-5-9(12)6-8;/h3-6,10H,2,7,13H2,1H3;1H/t10-,11+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as s... |
Eur J Med Chem 92: 377-86 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.032
BindingDB Entry DOI: 10.7270/Q2X351F5 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155764
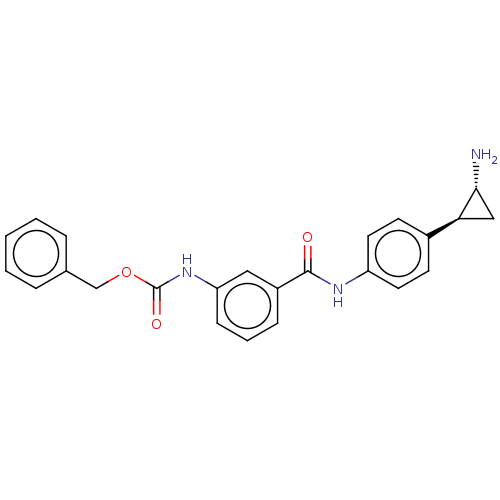
(CHEMBL3780674)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2cccc(NC(=O)OCc3ccccc3)c2)cc1 |r| Show InChI InChI=1S/C24H23N3O3.ClH/c25-22-14-21(22)17-9-11-19(12-10-17)26-23(28)18-7-4-8-20(13-18)27-24(29)30-15-16-5-2-1-3-6-16;/h1-13,21-22H,14-15,25H2,(H,26,28)(H,27,29);1H/t21-,22+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50498137
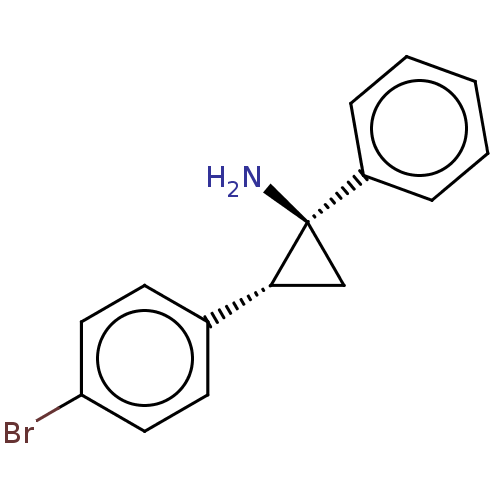
(CHEMBL3410941)Show SMILES Cl.N[C@]1(C[C@@H]1c1ccc(Br)cc1)c1ccccc1 |r| Show InChI InChI=1S/C15H14BrN.ClH/c16-13-8-6-11(7-9-13)14-10-15(14,17)12-4-2-1-3-5-12;/h1-9,14H,10,17H2;1H/t14-,15-;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as s... |
Eur J Med Chem 92: 377-86 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.032
BindingDB Entry DOI: 10.7270/Q2X351F5 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155768
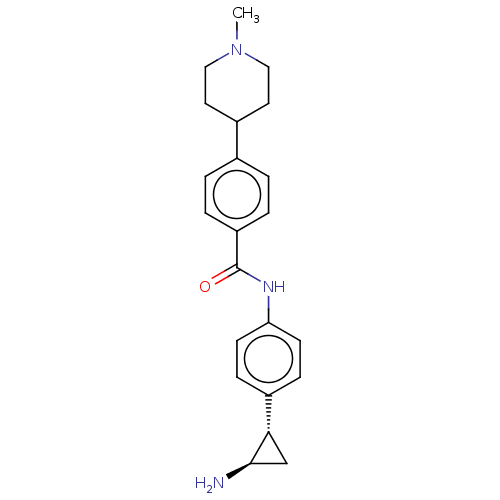
(CHEMBL3781494)Show SMILES Cl.CN1CCC(CC1)c1ccc(cc1)C(=O)Nc1ccc(cc1)[C@@H]1C[C@H]1N |r| Show InChI InChI=1S/C22H27N3O.ClH/c1-25-12-10-16(11-13-25)15-2-4-18(5-3-15)22(26)24-19-8-6-17(7-9-19)20-14-21(20)23;/h2-9,16,20-21H,10-14,23H2,1H3,(H,24,26);1H/t20-,21+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155769
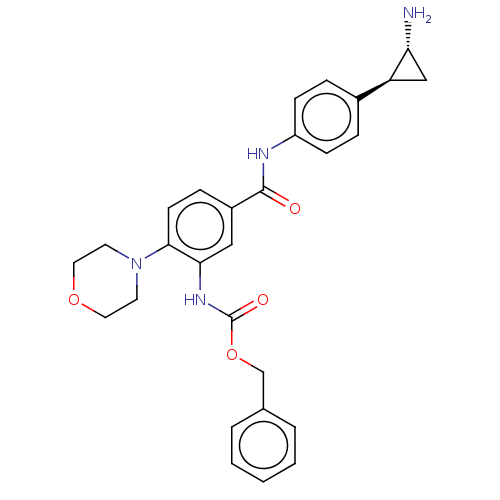
(CHEMBL3780542)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccc(N3CCOCC3)c(NC(=O)OCc3ccccc3)c2)cc1 |r| Show InChI InChI=1S/C28H30N4O4.ClH/c29-24-17-23(24)20-6-9-22(10-7-20)30-27(33)21-8-11-26(32-12-14-35-15-13-32)25(16-21)31-28(34)36-18-19-4-2-1-3-5-19;/h1-11,16,23-24H,12-15,17-18,29H2,(H,30,33)(H,31,34);1H/t23-,24+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155765
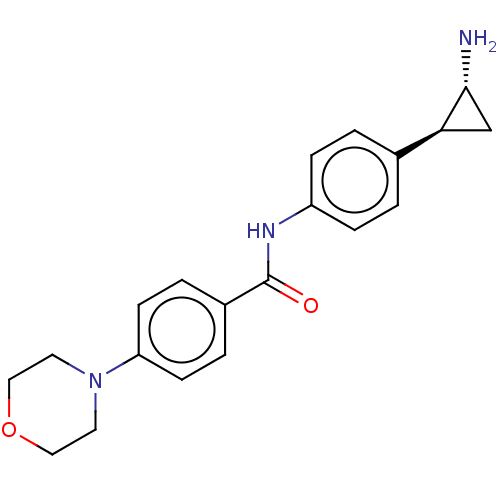
(CHEMBL3781329)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccc(cc2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C20H23N3O2.ClH/c21-19-13-18(19)14-1-5-16(6-2-14)22-20(24)15-3-7-17(8-4-15)23-9-11-25-12-10-23;/h1-8,18-19H,9-13,21H2,(H,22,24);1H/t18-,19+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | CHEMBL5270703
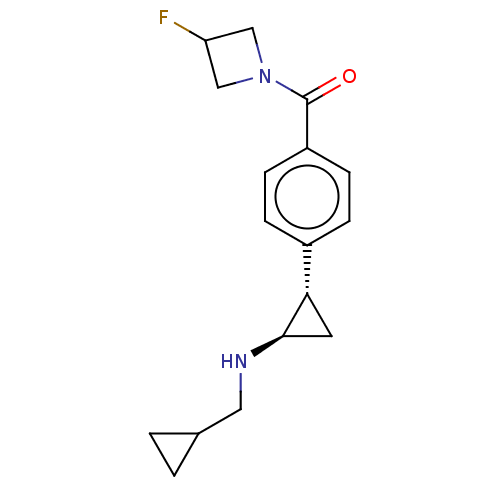
Show SMILES CO\N=C(\C)CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H26F6N4O2/c1-16(34-38-2)14-35-7-8-36(21(15-35)11-18-13-33-23-6-4-3-5-22(18)23)24(37)17-9-19(25(27,28)29)12-20(10-17)26(30,31)32/h3-6,9-10,12-13,21,33H,7-8,11,14-15H2,1-2H3/b34-16-/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
REST corepressor 1 [4-485]
(Homo sapiens (Human)) | BDBM50155770
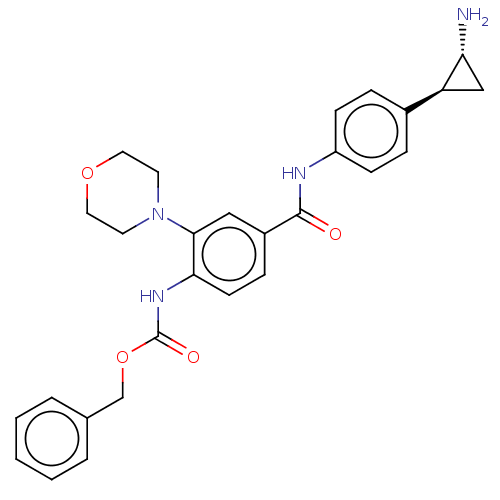
(CHEMBL3781885)Show SMILES Cl.N[C@@H]1C[C@H]1c1ccc(NC(=O)c2ccc(NC(=O)OCc3ccccc3)c(c2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C28H30N4O4.ClH/c29-24-17-23(24)20-6-9-22(10-7-20)30-27(33)21-8-11-25(26(16-21)32-12-14-35-15-13-32)31-28(34)36-18-19-4-2-1-3-5-19;/h1-11,16,23-24H,12-15,17-18,29H2,(H,30,33)(H,31,34);1H/t23-,24+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST expressed in Escherichia coli using mono-methylated H3-K4 peptide as substrate assessed as H2O2 release ... |
J Med Chem 59: 1501-17 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01209
BindingDB Entry DOI: 10.7270/Q2086767 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50498150

(CHEMBL3407599)Show InChI InChI=1S/C11H14FN.ClH/c1-2-11(13)7-10(11)8-4-3-5-9(12)6-8;/h3-6,10H,2,7,13H2,1H3;1H/t10-,11+;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A/CoREST complex expressed in Escherichia coli using mono-methylated H3-K4 peptide containing 21 amino acids as s... |
Eur J Med Chem 92: 377-86 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.032
BindingDB Entry DOI: 10.7270/Q2X351F5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data