 Found 4548 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein phosphatase non-receptor type 11'
Found 4548 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein phosphatase non-receptor type 11' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50553786

(CHEMBL4789106)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(Cl)c2Cl)c(N)n1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112356
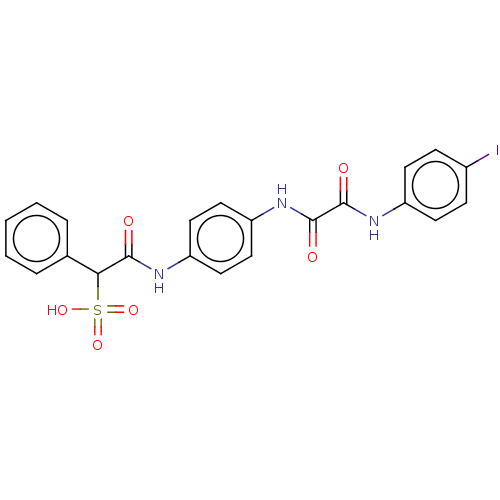
(CHEMBL3609373)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(I)cc2)cc1)c1ccccc1 Show InChI InChI=1S/C22H18IN3O6S/c23-15-6-8-16(9-7-15)25-21(28)22(29)26-18-12-10-17(11-13-18)24-20(27)19(33(30,31)32)14-4-2-1-3-5-14/h1-13,19H,(H,24,27)(H,25,28)(H,26,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | CHEMBL4063482
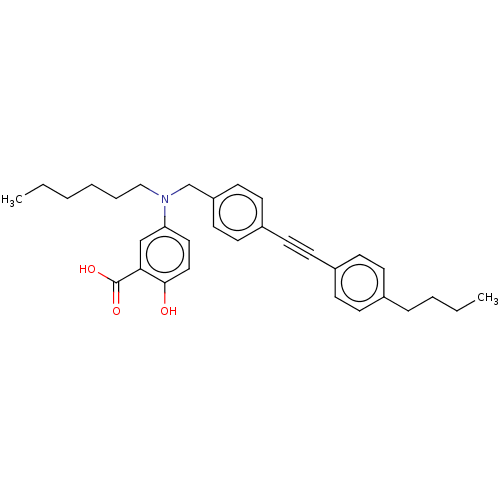
| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | 652 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342004

(4-(2-(3-(4-nitrophenyl)-5-oxo-1-phenyl-1H-pyrazol-...)Show SMILES OS(=O)(=O)c1ccc(cc1)N=Nc1c([nH]n(-c2ccccc2)c1=O)-c1ccc(cc1)[N+]([O-])=O |w:10.10| Show InChI InChI=1S/C21H15N5O6S/c27-21-20(23-22-15-8-12-18(13-9-15)33(30,31)32)19(14-6-10-17(11-7-14)26(28)29)24-25(21)16-4-2-1-3-5-16/h1-13,24H,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of SHP-2 |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | CHEMBL5288934

Show SMILES CC(=O)c1ccc2-c3[nH]c4ccccc4c3C[C@@H](COC(=O)CCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)n2c1=O Show InChI InChI=1S/C37H42N4O6/c1-25(42)29-13-14-33-36-31(30-11-3-4-12-32(30)39-36)22-27(41(33)37(29)45)24-47-35(44)16-15-34(43)38-17-8-20-46-28-10-7-9-26(21-28)23-40-18-5-2-6-19-40/h3-4,7,9-14,21,27,39H,2,5-6,8,15-20,22-24H2,1H3,(H,38,43)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Ras Farnesyltransferase enzyme from pig brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM199180
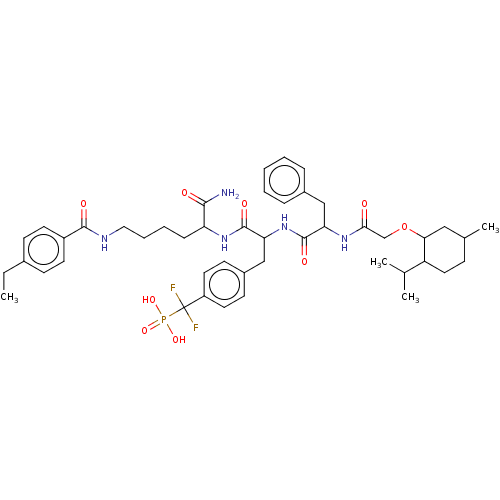
(US9217012, 10)Show SMILES CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)COC1CC(C)CCC1C(C)C)C(N)=O Show InChI InChI=1S/C46H62F2N5O9P/c1-5-31-15-19-34(20-16-31)43(56)50-24-10-9-13-37(42(49)55)52-45(58)39(27-33-17-21-35(22-18-33)46(47,48)63(59,60)61)53-44(57)38(26-32-11-7-6-8-12-32)51-41(54)28-62-40-25-30(4)14-23-36(40)29(2)3/h6-8,11-12,15-22,29-30,36-40H,5,9-10,13-14,23-28H2,1-4H3,(H2,49,55)(H,50,56)(H,51,54)(H,52,58)(H,53,57)(H2,59,60,61) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation
US Patent
| Assay Description
PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... |
US Patent US9217012 (2015)
BindingDB Entry DOI: 10.7270/Q2FX788H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50348092
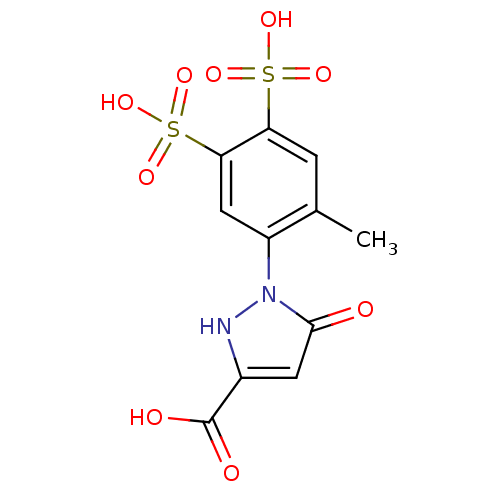
(CHEMBL1800273)Show SMILES Cc1cc(c(cc1-n1[nH]c(cc1=O)C(O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C11H10N2O9S2/c1-5-2-8(23(17,18)19)9(24(20,21)22)4-7(5)13-10(14)3-6(12-13)11(15)16/h2-4,12H,1H3,(H,15,16)(H,17,18,19)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Competitive inhibition at SHP2 catalytic domain assessed as inhibition of pNPP to p-nitrophenol conversion by spectrophotometry |
Bioorg Med Chem Lett 21: 4238-42 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.078
BindingDB Entry DOI: 10.7270/Q2JQ11CJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50348092

(CHEMBL1800273)Show SMILES Cc1cc(c(cc1-n1[nH]c(cc1=O)C(O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C11H10N2O9S2/c1-5-2-8(23(17,18)19)9(24(20,21)22)4-7(5)13-10(14)3-6(12-13)11(15)16/h2-4,12H,1H3,(H,15,16)(H,17,18,19)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Reversible inhibition at SHP2 catalytic domain assessed as inhibition of pNPP to p-nitrophenol conversion by spectrophotometry |
Bioorg Med Chem Lett 21: 4238-42 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.078
BindingDB Entry DOI: 10.7270/Q2JQ11CJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50308158

(3-(1-(3-(Biphenyl-4-ylamino)-3-oxopropyl)-1H-1,2,3...)Show SMILES Cn1c(c(-c2cn(CCC(=O)Nc3ccc(cc3)-c3ccccc3)nn2)c2cc(C(O)=O)c(O)cc12)-c1ccccc1 Show InChI InChI=1S/C33H27N5O4/c1-37-28-19-29(39)26(33(41)42)18-25(28)31(32(37)23-10-6-3-7-11-23)27-20-38(36-35-27)17-16-30(40)34-24-14-12-22(13-15-24)21-8-4-2-5-9-21/h2-15,18-20,39H,16-17H2,1H3,(H,34,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 Src homology-2 domain expressed in Escherichia coli BL21 (DE3) assessed as inhibition of p-nitrophenyl phosphate hydrolysis by Lin... |
J Med Chem 53: 2482-93 (2010)
Article DOI: 10.1021/jm901645u
BindingDB Entry DOI: 10.7270/Q2639PVD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50420258

(CEFSULODIN)Show SMILES NC(=O)c1cc[n+](CC2=C(N3[C@H](SC2)[C@H](NC(=O)C(c2ccccc2)S(O)(=O)=O)C3=O)C(O)=O)cc1 |t:8| Show InChI InChI=1S/C22H20N4O8S2/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34)/p+1/t15-,17?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24516

((2E,5E)-5-[(4-methoxyphenyl)methylidene]-2-(1,3-th...)Show InChI InChI=1S/C14H11N3O2S2/c1-19-10-4-2-9(3-5-10)8-11-12(18)16-14(21-11)17-13-15-6-7-20-13/h2-8H,1H3,(H,15,16,17,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24524

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-metho...)Show SMILES COc1ccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc1 Show InChI InChI=1S/C18H13N3O2S2/c1-23-12-8-6-11(7-9-12)10-15-16(22)20-18(25-15)21-17-19-13-4-2-3-5-14(13)24-17/h2-10H,1H3,(H,19,20,21,22)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | -28.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24523

((2E,5E)-2-{[4-(adamantan-1-yl)-1,3-thiazol-2-yl]im...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc(cs2)C23CC4CC(CC(C4)C2)C3)ccc1O |TLB:21:22:26:20.25.19,THB:21:20:26:22.27.23,23:24:22.21.27:19,23:22:24.25.26:19| Show InChI InChI=1S/C24H25N3O3S2/c1-30-18-7-13(2-3-17(18)28)8-19-21(29)26-23(32-19)27-22-25-20(12-31-22)24-9-14-4-15(10-24)6-16(5-14)11-24/h2-3,7-8,12,14-16,28H,4-6,9-11H2,1H3,(H,25,26,27,29)/b19-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24526
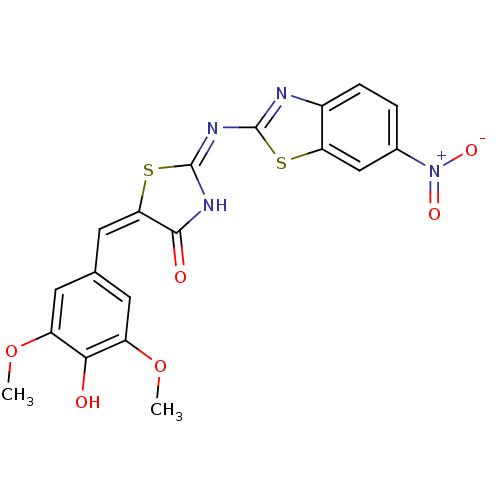
((2E,5E)-5-[(4-hydroxy-3,5-dimethoxyphenyl)methylid...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc3ccc(cc3s2)[N+]([O-])=O)cc(OC)c1O Show InChI InChI=1S/C19H14N4O6S2/c1-28-12-5-9(6-13(29-2)16(12)24)7-15-17(25)21-19(31-15)22-18-20-11-4-3-10(23(26)27)8-14(11)30-18/h3-8,24H,1-2H3,(H,20,21,22,25)/b15-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24527

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-nitro...)Show SMILES [O-][N+](=O)c1ccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc1 Show InChI InChI=1S/C17H10N4O3S2/c22-15-14(9-10-5-7-11(8-6-10)21(23)24)26-17(19-15)20-16-18-12-3-1-2-4-13(12)25-16/h1-9H,(H,18,19,20,22)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+4 | -27.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24525

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(4-hydro...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)cc(OC)c1O Show InChI InChI=1S/C19H15N3O4S2/c1-25-12-7-10(8-13(26-2)16(12)23)9-15-17(24)21-19(28-15)22-18-20-11-5-3-4-6-14(11)27-18/h3-9,23H,1-2H3,(H,20,21,22,24)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.81E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24529
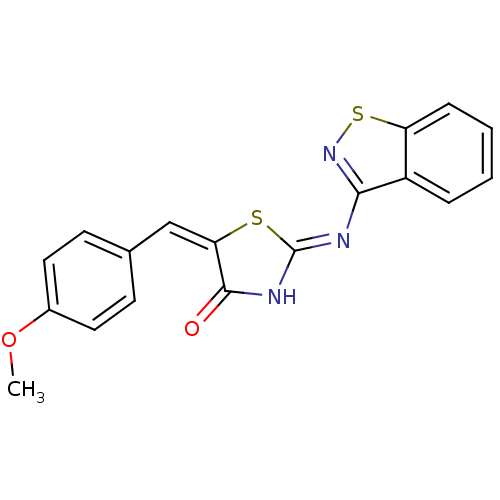
((2Z,5E)-2-(1,2-benzothiazol-3-ylimino)-5-[(4-metho...)Show SMILES COc1ccc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)cc1 Show InChI InChI=1S/C18H13N3O2S2/c1-23-12-8-6-11(7-9-12)10-15-17(22)20-18(24-15)19-16-13-4-2-3-5-14(13)25-21-16/h2-10H,1H3,(H,19,20,21,22)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.28E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24515
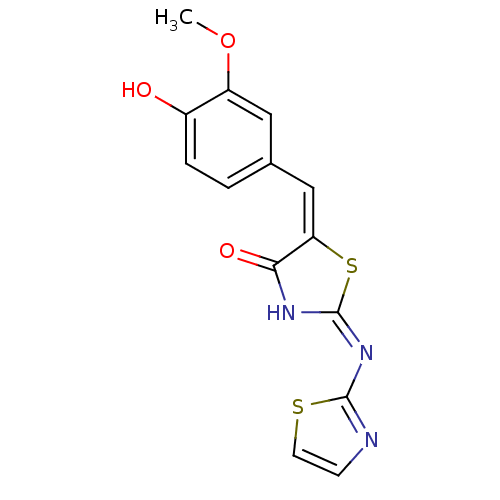
((2E,5E)-5-[(4-hydroxy-3-methoxyphenyl)methylidene]...)Show InChI InChI=1S/C14H11N3O3S2/c1-20-10-6-8(2-3-9(10)18)7-11-12(19)16-14(22-11)17-13-15-4-5-21-13/h2-7,18H,1H3,(H,15,16,17,19)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.56E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24528

((2E,5E)-2-(1,3-benzothiazol-2-ylimino)-5-[(3-nitro...)Show SMILES [O-][N+](=O)c1cccc(\C=C2\S\C(NC2=O)=N\c2nc3ccccc3s2)c1 Show InChI InChI=1S/C17H10N4O3S2/c22-15-14(9-10-4-3-5-11(8-10)21(23)24)26-17(19-15)20-16-18-12-6-1-2-7-13(12)25-16/h1-9H,(H,18,19,20,22)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50132460
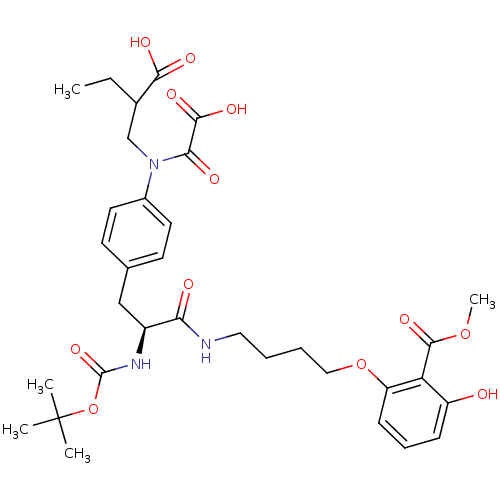
(2-[4-((S)-2-tert-Butoxycarbonylamino-3-{4-[(2-carb...)Show SMILES CCC(CN(C(=O)C(O)=O)c1ccc(C[C@H](NC(=O)OC(C)(C)C)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)cc1)C(O)=O Show InChI InChI=1S/C33H43N3O12/c1-6-21(29(40)41)19-36(28(39)30(42)43)22-14-12-20(13-15-22)18-23(35-32(45)48-33(2,3)4)27(38)34-16-7-8-17-47-25-11-9-10-24(37)26(25)31(44)46-5/h9-15,21,23,37H,6-8,16-19H2,1-5H3,(H,34,38)(H,35,45)(H,40,41)(H,42,43)/t21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Tyrosine phosphatase SHP2 |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50132461
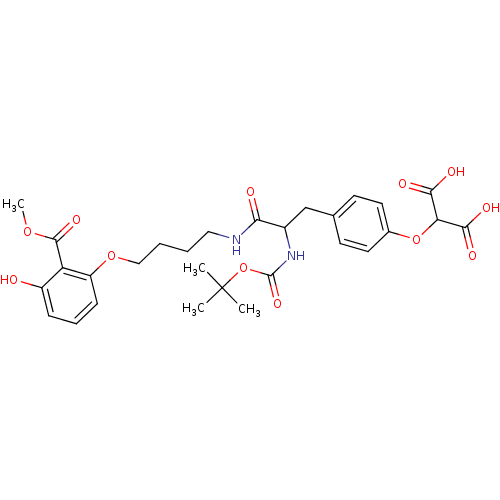
(2-(4-{2-tert-Butoxycarbonylamino-2-[4-(3-hydroxy-2...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)C(Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C29H36N2O12/c1-29(2,3)43-28(39)31-19(16-17-10-12-18(13-11-17)42-23(25(34)35)26(36)37)24(33)30-14-5-6-15-41-21-9-7-8-20(32)22(21)27(38)40-4/h7-13,19,23,32H,5-6,14-16H2,1-4H3,(H,30,33)(H,31,39)(H,34,35)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Tyrosine phosphatase SHP2 |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50132465
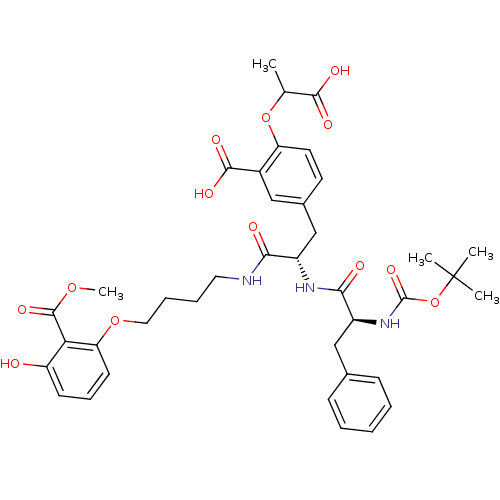
(5-{(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phenyl-...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OC(C)C(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C39H47N3O13/c1-23(35(46)47)54-30-17-16-25(20-26(30)36(48)49)22-27(33(44)40-18-9-10-19-53-31-15-11-14-29(43)32(31)37(50)52-5)41-34(45)28(21-24-12-7-6-8-13-24)42-38(51)55-39(2,3)4/h6-8,11-17,20,23,27-28,43H,9-10,18-19,21-22H2,1-5H3,(H,40,44)(H,41,45)(H,42,51)(H,46,47)(H,48,49)/t23?,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Tyrosine phosphatase SHP2 |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24521
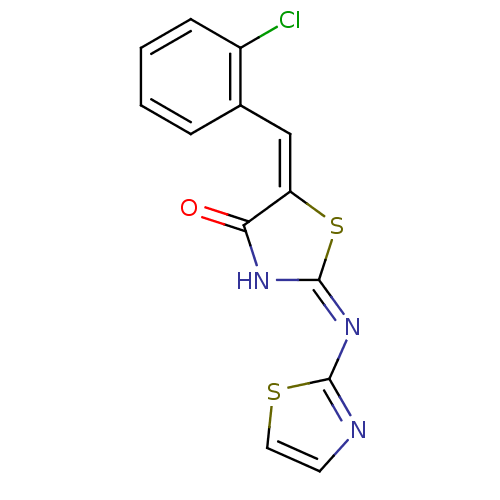
((2E,5E)-5-[(2-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-4-2-1-3-8(9)7-10-11(18)16-13(20-10)17-12-15-5-6-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24519
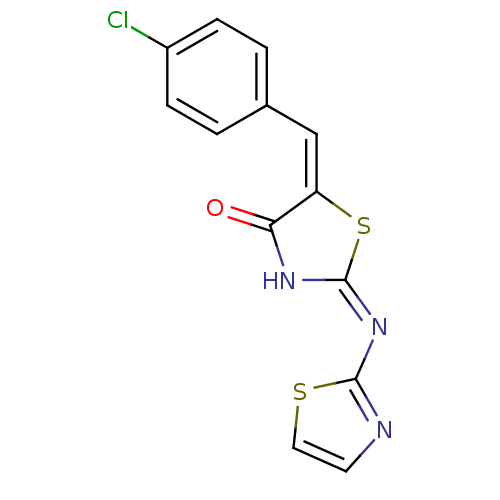
((2E,5E)-5-[(4-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-3-1-8(2-4-9)7-10-11(18)16-13(20-10)17-12-15-5-6-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.45E+4 | -24.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24530

((2Z,5E)-2-(1,2-benzothiazol-3-ylimino)-5-[(4-hydro...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N/c2nsc3ccccc23)ccc1O Show InChI InChI=1S/C18H13N3O3S2/c1-24-13-8-10(6-7-12(13)22)9-15-17(23)20-18(25-15)19-16-11-4-2-3-5-14(11)26-21-16/h2-9,22H,1H3,(H,19,20,21,23)/b15-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.08E+4 | -23.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24522

((2E,5E)-5-[(4-hydroxy-3-methoxyphenyl)methylidene]...)Show SMILES COc1cc(\C=C2\S\C(NC2=O)=N\c2nc(cs2)-c2ccccc2)ccc1O Show InChI InChI=1S/C20H15N3O3S2/c1-26-16-9-12(7-8-15(16)24)10-17-18(25)22-20(28-17)23-19-21-14(11-27-19)13-5-3-2-4-6-13/h2-11,24H,1H3,(H,21,22,23,25)/b17-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+5 | -22.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24513
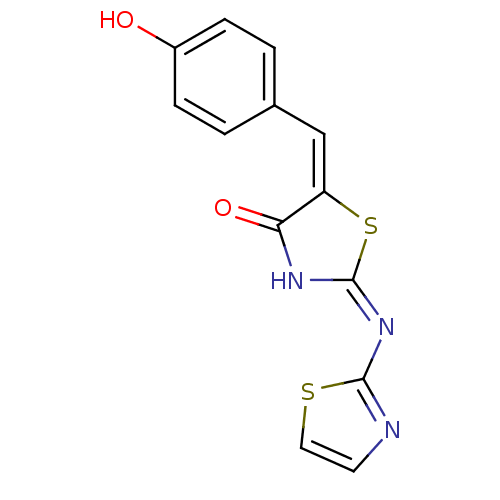
((2E,5E)-5-[(4-hydroxyphenyl)methylidene]-2-(1,3-th...)Show InChI InChI=1S/C13H9N3O2S2/c17-9-3-1-8(2-4-9)7-10-11(18)15-13(20-10)16-12-14-5-6-19-12/h1-7,17H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.88E+5 | -19.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24517

((2E,5E)-5-[(4-nitrophenyl)methylidene]-2-(1,3-thia...)Show SMILES [O-][N+](=O)c1ccc(\C=C2\S\C(NC2=O)=N\c2nccs2)cc1 Show InChI InChI=1S/C13H8N4O3S2/c18-11-10(7-8-1-3-9(4-2-8)17(19)20)22-13(15-11)16-12-14-5-6-21-12/h1-7H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.74E+5 | -19.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24518
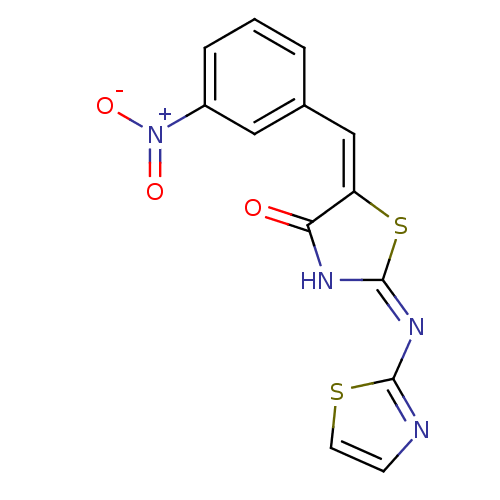
((2E,5E)-5-[(3-nitrophenyl)methylidene]-2-(1,3-thia...)Show SMILES [O-][N+](=O)c1cccc(\C=C2\S\C(NC2=O)=N\c2nccs2)c1 Show InChI InChI=1S/C13H8N4O3S2/c18-11-10(7-8-2-1-3-9(6-8)17(19)20)22-13(15-11)16-12-14-4-5-21-12/h1-7H,(H,14,15,16,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.48E+5 | -18.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM24520
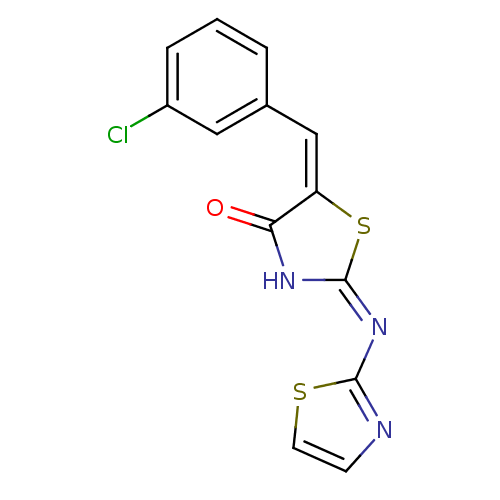
((2E,5E)-5-[(3-chlorophenyl)methylidene]-2-(1,3-thi...)Show InChI InChI=1S/C13H8ClN3OS2/c14-9-3-1-2-8(6-9)7-10-11(18)16-13(20-10)17-12-15-4-5-19-12/h1-7H,(H,15,16,17,18)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+6 | -16.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Aristotle University
| Assay Description
SHP-2 inhibitory activity was tested using human recombinant GST-fusion SHP-2 (Calbiochem). Test compound was preincubated with enzyme mixture before... |
J Med Chem 51: 5221-8 (2008)
Article DOI: 10.1021/jm8004306
BindingDB Entry DOI: 10.7270/Q2251GGD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50571444

(CHEMBL4858291)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cccc(N)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50546219
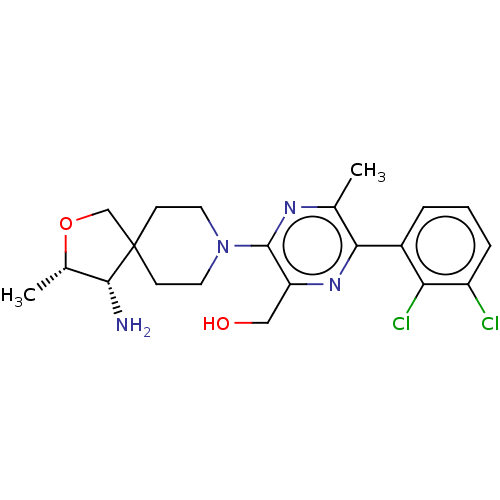
(CHEMBL4752026 | US11596633, Compound B | US1170239...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2nc(C)c(nc2CO)-c2cccc(Cl)c2Cl)[C@@H]1N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.0583 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50571445
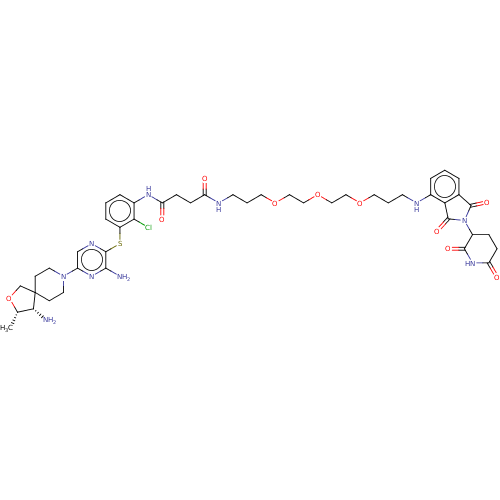
(CHEMBL4871539)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cccc(NC(=O)CCC(=O)NCCCOCCOCCOCCCNc4cccc5C(=O)N(C6CCC(=O)NC6=O)C(=O)c45)c3Cl)c(N)n2)[C@@H]1N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.202 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human SHP2 catalytic activity in human MV4-11 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113341
BindingDB Entry DOI: 10.7270/Q2K0782R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM497105
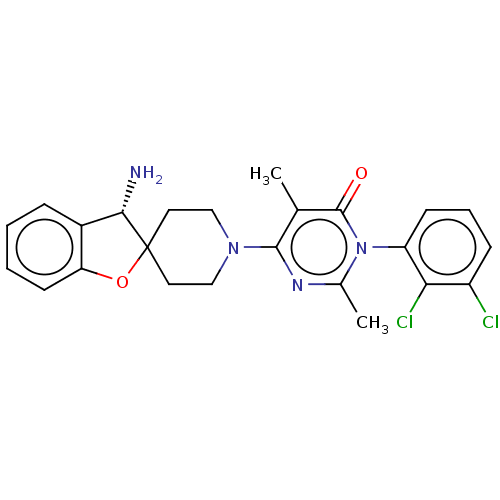
(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM636747
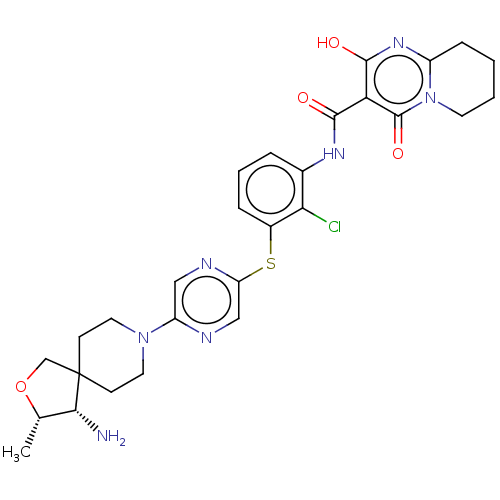
(US11827644, Example 11)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2cnc(Sc3cccc(NC(=O)c4c(O)nc5CCCCn5c4=O)c3Cl)cn2)[C@@H]1N |r,$;;;;;;;;;;;;;;;;;;;;HN;;;;;;;;;;;;;;;;;;;;$| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50087879

(CHEMBL472004 | NSC-87877)Show SMILES Oc1c(cc(c2cccnc12)S(O)(=O)=O)\N=N\c1ccc2cc(ccc2c1)S(O)(=O)=O Show InChI InChI=1S/C19H13N3O7S2/c23-19-16(10-17(31(27,28)29)15-2-1-7-20-18(15)19)22-21-13-5-3-12-9-14(30(24,25)26)6-4-11(12)8-13/h1-10,23H,(H,24,25,26)(H,27,28,29)/b22-21+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.318 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM488485
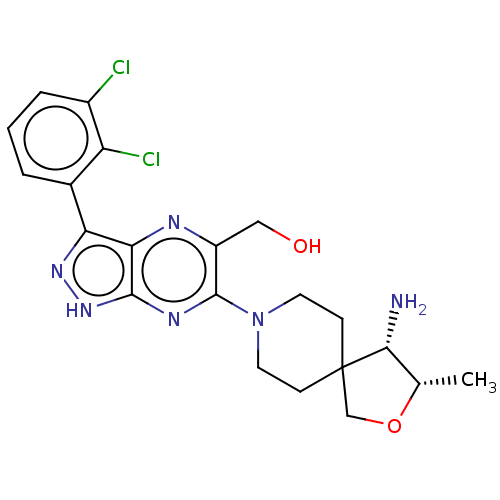
(US10954243, Example 16)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2nc3[nH]nc(-c4cccc(Cl)c4Cl)c3nc2CO)[C@@H]1N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01132
BindingDB Entry DOI: 10.7270/Q2JS9VC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497105

(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM50546219

(CHEMBL4752026 | US11596633, Compound B | US1170239...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2nc(C)c(nc2CO)-c2cccc(Cl)c2Cl)[C@@H]1N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.583 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | CHEMBL5287751
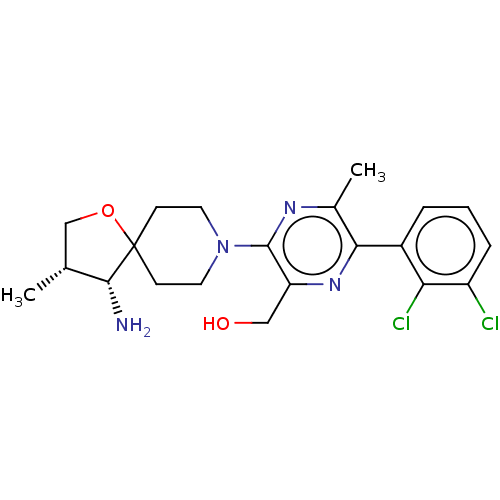
Show SMILES [O-][N+](=O)c1ccc(NCCCCCC(=O)NCC[N-]C(NCCCOc2cccc(CN3CCCCC3)c2)=[NH+]C#N)c2[n-][o+]nc12 |w:38.40| Show InChI InChI=1S/C31H42N10O5/c32-23-37-31(35-15-8-20-45-25-10-7-9-24(21-25)22-40-18-5-2-6-19-40)36-17-16-34-28(42)11-3-1-4-14-33-26-12-13-27(41(43)44)30-29(26)38-46-39-30/h7,9-10,12-13,21,33H,1-6,8,11,14-20,22H2,(H3,34,35,36,37,42) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.583 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM575655
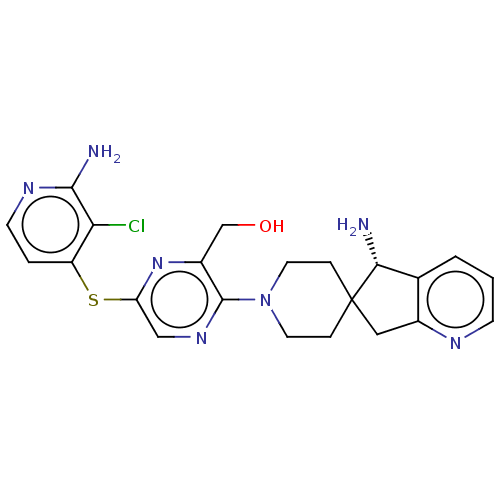
((S)-(6-((2-amino-3-chloropyridin-4-yl)thio)- 3-(5-...)Show SMILES N[C@@H]1c2cccnc2CC11CCN(CC1)c1ncc(Sc2ccnc(N)c2Cl)nc1CO |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
SHP2 possesses two N-terminal Src homology 2 (SH2) domains, a central protein-tyrosine phosphatase (PTP) domain, and C-terminal tail. At the basal st... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2028VS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | CHEMBL5270661

| PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM497078
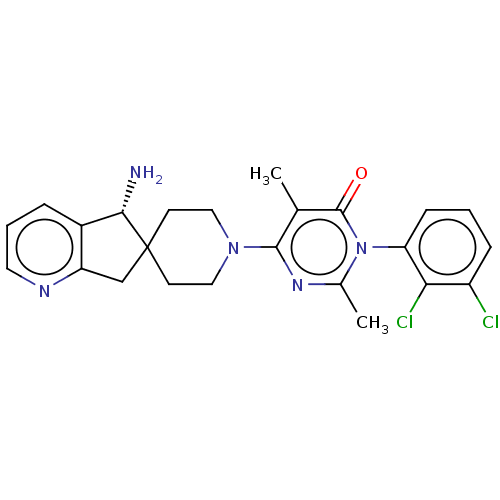
(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM628564
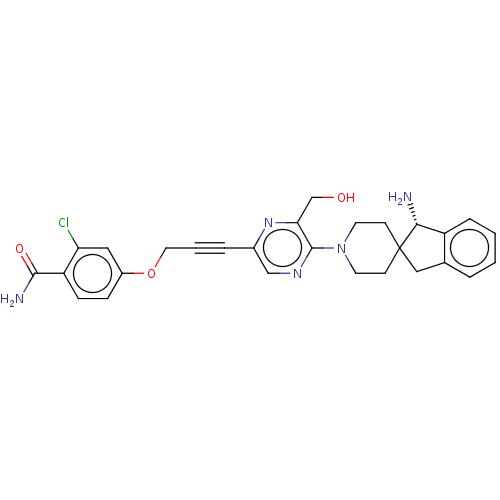
(US20230339882, Example A54 | US20230339882, Exampl...)Show SMILES N[C@@H]1c2ccccc2CC11CCN(CC1)c1ncc(nc1CO)C#CCOc1ccc(C(N)=O)c(Cl)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM628494

(US20230339882, Example A02)Show SMILES COc1cccc(OCC#Cc2cnc(N3CCC4(Cc5ccccc5[C@H]4N)CC3)c(CO)n2)c1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM497145

(US11001561, Compound 119b | US11702392, Compound 1...)Show SMILES Cc1nc(cc(=O)n1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@@H]2N)CC1 |wU:27.31,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;.11,-5.1,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,)| Show InChI InChI=1S/C24H24Cl2N4O/c1-15-28-20(13-21(31)30(15)19-8-4-7-18(25)22(19)26)29-11-9-24(10-12-29)14-16-5-2-3-6-17(16)23(24)27/h2-8,13,23H,9-12,14,27H2,1H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens) | BDBM497144
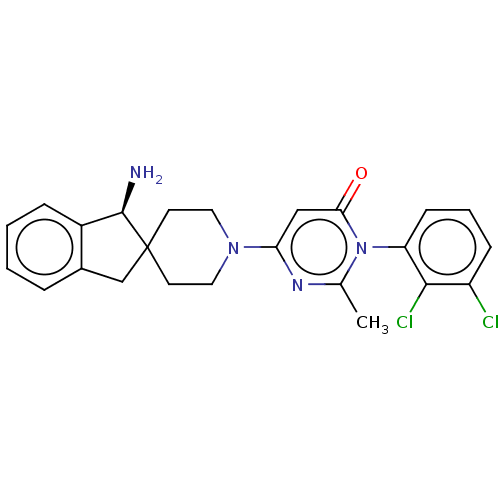
(US11001561, Compound 119a | US11702392, Compound 1...)Show SMILES Cc1nc(cc(=O)n1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@H]2N)CC1 |wD:27.31,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;.11,-5.1,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,)| Show InChI InChI=1S/C24H24Cl2N4O/c1-15-28-20(13-21(31)30(15)19-8-4-7-18(25)22(19)26)29-11-9-24(10-12-29)14-16-5-2-3-6-17(16)23(24)27/h2-8,13,23H,9-12,14,27H2,1H3/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human N-terminal His6-tagged full length SHP2 expressed in Escherichia coli using DiFMUP as substrate measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00621
BindingDB Entry DOI: 10.7270/Q2ST7TFF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497145

(US11001561, Compound 119b | US11702392, Compound 1...)Show SMILES Cc1nc(cc(=O)n1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@@H]2N)CC1 |wU:27.31,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;.11,-5.1,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,)| Show InChI InChI=1S/C24H24Cl2N4O/c1-15-28-20(13-21(31)30(15)19-8-4-7-18(25)22(19)26)29-11-9-24(10-12-29)14-16-5-2-3-6-17(16)23(24)27/h2-8,13,23H,9-12,14,27H2,1H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data