

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50469035 (CHEMBL4282693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50430584 (CHEMBL2337806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (RAT) | BDBM50022784 ((R)-N-methyl-3-phenyl-3-(o-tolyloxy)propan-1-amine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropsychopharmacology 27: 699-711 (2002) Article DOI: 10.1016/S0893-133X(02)00346-9 BindingDB Entry DOI: 10.7270/Q2GQ6W98 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (RAT) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropsychopharmacology 25: 871-80 (2001) Article DOI: 10.1016/S0893-133X(01)00298-6 BindingDB Entry DOI: 10.7270/Q25M648W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (RAT) | BDBM82071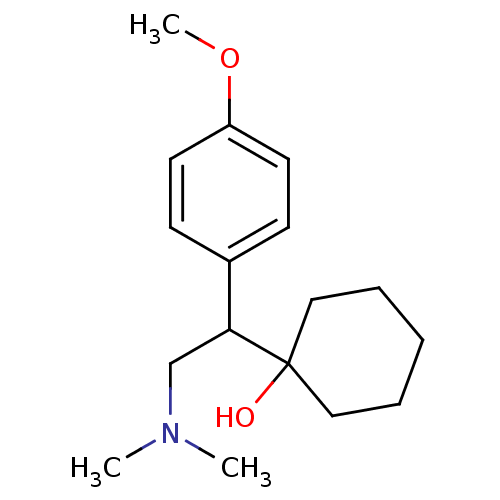 (CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by PDSP Ki Database | Neuropsychopharmacology 25: 871-80 (2001) Article DOI: 10.1016/S0893-133X(01)00298-6 BindingDB Entry DOI: 10.7270/Q25M648W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586357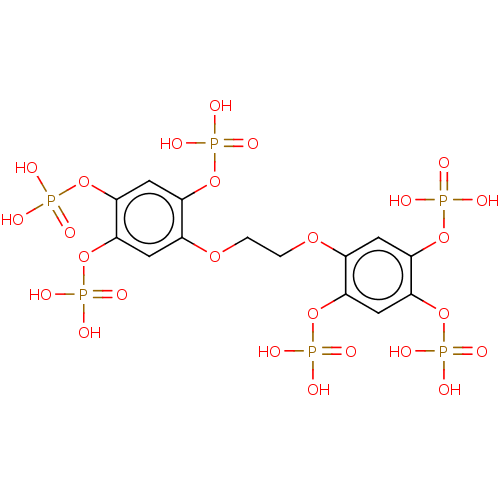 (CHEMBL5080660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (RAT) | BDBM50010685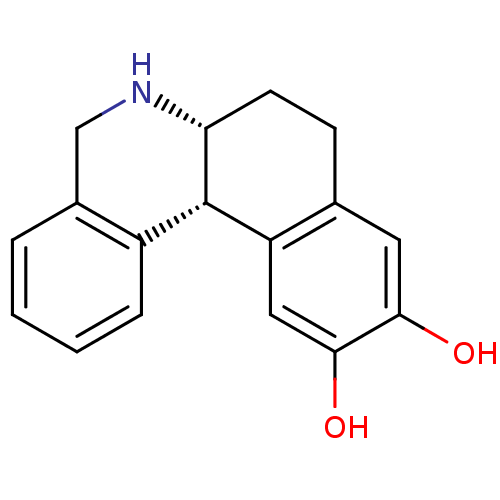 ((+/-)-trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by PDSP Ki Database | J Pharmacol Exp Ther 262: 383-93 (1992) BindingDB Entry DOI: 10.7270/Q218350W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586355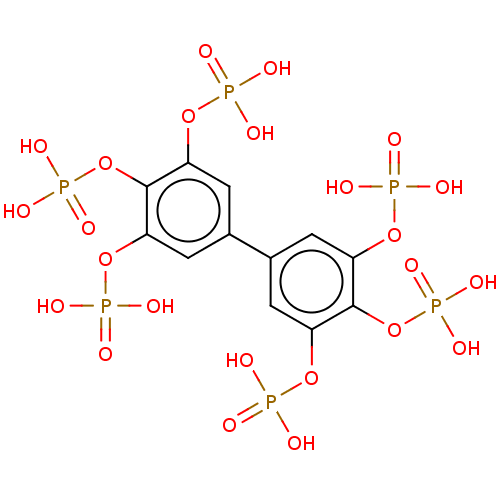 (CHEMBL5087243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586354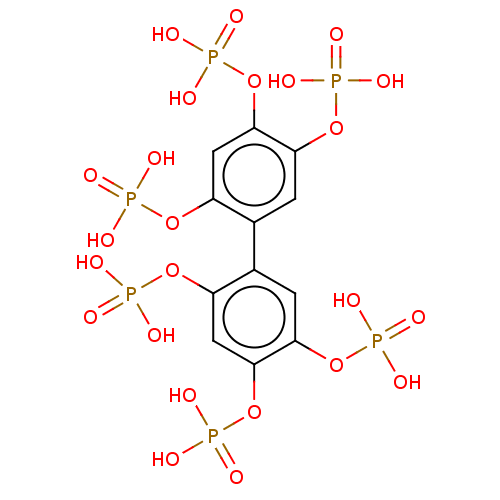 (CHEMBL5078323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586353 (CHEMBL5092991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586357 (CHEMBL5080660) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586356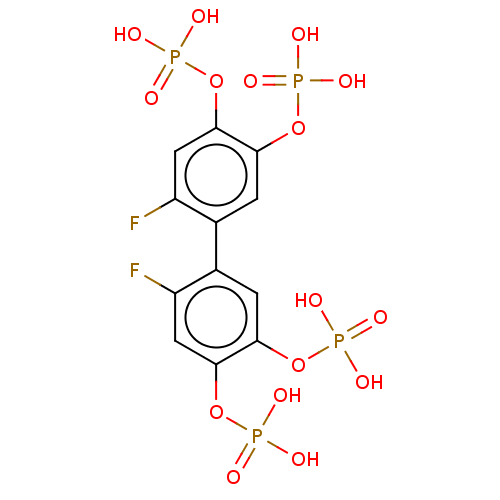 (CHEMBL5081948) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054344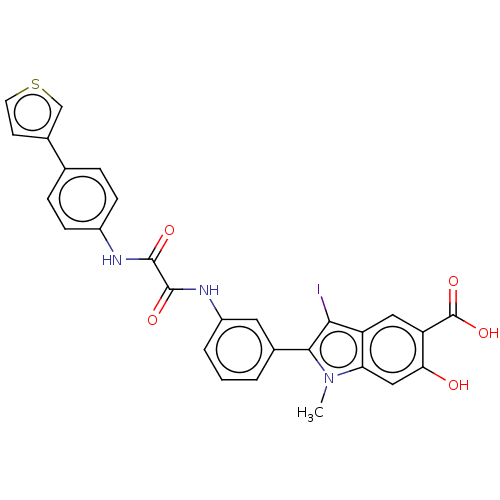 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description For selectivity studies, the PTPs, including LYP, mPTPA, SHP1-D1C, PTP1B, LMPTP, VHR, Laforin and PTPα-D1D2 were expressed and purified from E. ... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054257 (CHEMBL3319376 | US9522881, 11a-21 L97L08 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054257 (CHEMBL3319376 | US9522881, 11a-21 L97L08 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054258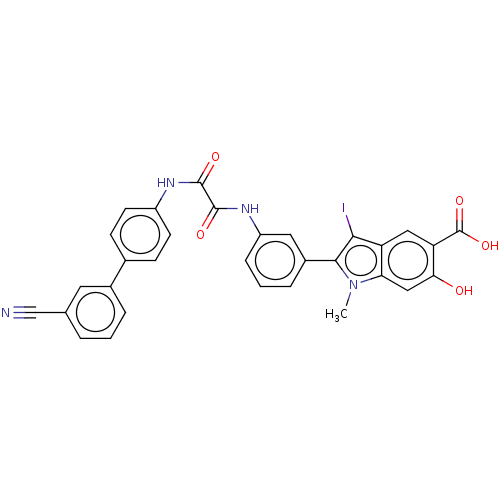 (CHEMBL3319377 | US9522881, 11a-22 L97L07 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 310 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054258 (CHEMBL3319377 | US9522881, 11a-22 L97L07 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054259 (CHEMBL3319378 | US9522881, 11a-23 L97L03) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM362880 (US9844535, ID 11a-23 L97L03) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054260 (CHEMBL3319379 | US9522881, 11a-24 L97L05 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054260 (CHEMBL3319379 | US9522881, 11a-24 L97L05 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Mus musculus) | BDBM50511342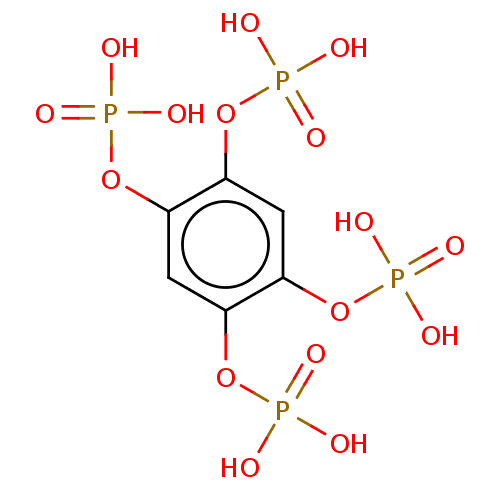 (CHEMBL4538474) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SHIP2 in serum-starved mouse Mm1 cells assessed as reduction in Akt phosphorylation incubated for 30 mins by Western blot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054261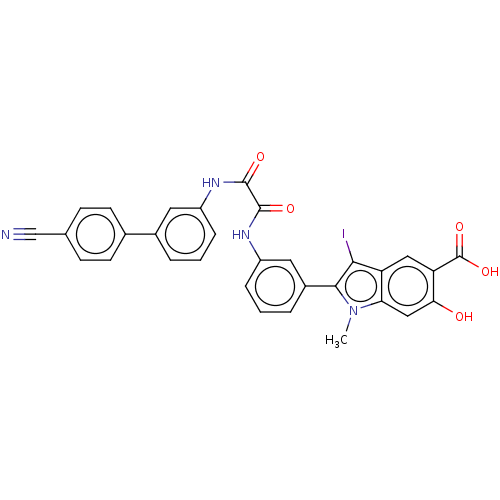 (CHEMBL3319380 | US9522881, 11a-25 L97L06 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054261 (CHEMBL3319380 | US9522881, 11a-25 L97L06 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054406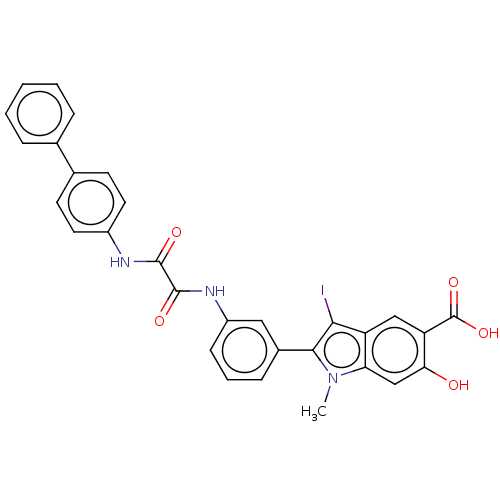 (CHEMBL3319357 | US9522881, 11a-2 (L97N08) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50430584 (CHEMBL2337806) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of SHIP2 (unknown origin) | Eur J Med Chem 62: 649-60 (2013) Article DOI: 10.1016/j.ejmech.2013.01.014 BindingDB Entry DOI: 10.7270/Q2D79CS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054406 (CHEMBL3319357 | US9522881, 11a-2 (L97N08) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054262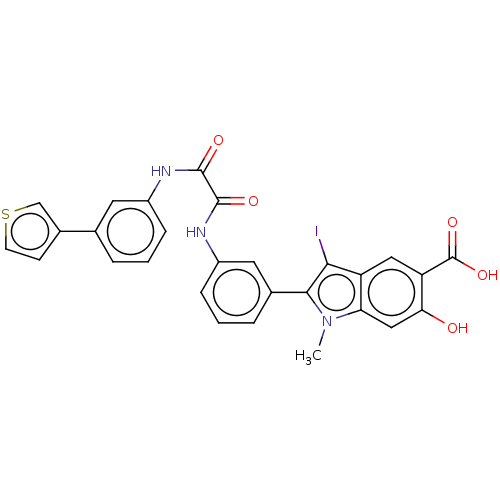 (CHEMBL3319381 | US9522881, 11a-26 L97L02 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054262 (CHEMBL3319381 | US9522881, 11a-26 L97L02 | US98445...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054405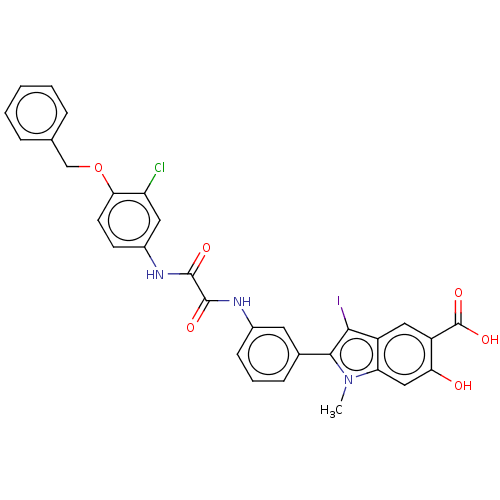 (CHEMBL3319358 | US9522881, 11a-3 (L97M50) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054405 (CHEMBL3319358 | US9522881, 11a-3 (L97M50) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586355 (CHEMBL5087243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as inhibition of Ins(1,3,4,5)P4 production using Ins(1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054404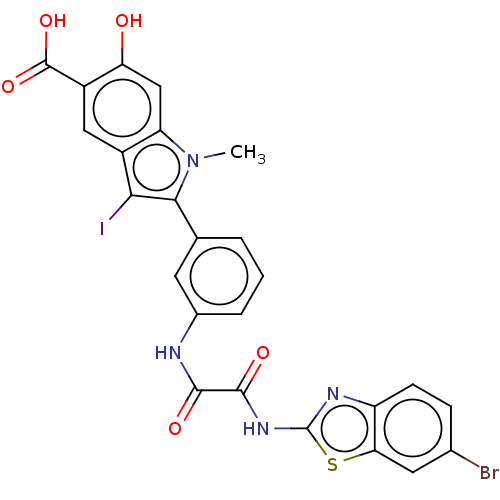 (CHEMBL3319359 | US9522881, 11a-4 (L97M61) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054404 (CHEMBL3319359 | US9522881, 11a-4 (L97M61) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054403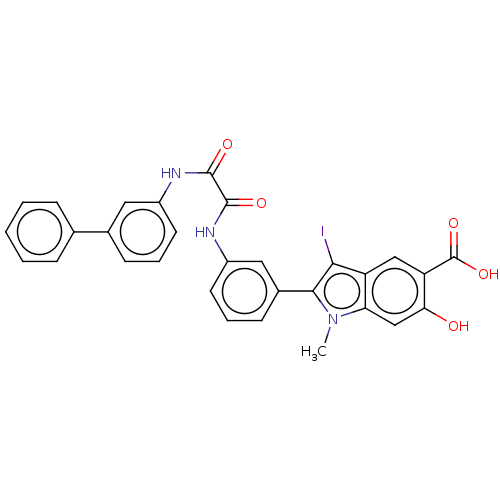 (CHEMBL3319360 | US9522881, 11a-5 (L97M48) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054403 (CHEMBL3319360 | US9522881, 11a-5 (L97M48) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054402 (CHEMBL3319361 | US9522881, 11a-6 (L97M52) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054402 (CHEMBL3319361 | US9522881, 11a-6 (L97M52) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586354 (CHEMBL5078323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as inhibition of Ins(1,3,4,5)P4 production using Ins(1... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50586355 (CHEMBL5087243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as phosphate release using Ins(1,3,4,5)P4 as substrate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01944 BindingDB Entry DOI: 10.7270/Q2V98CZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50511346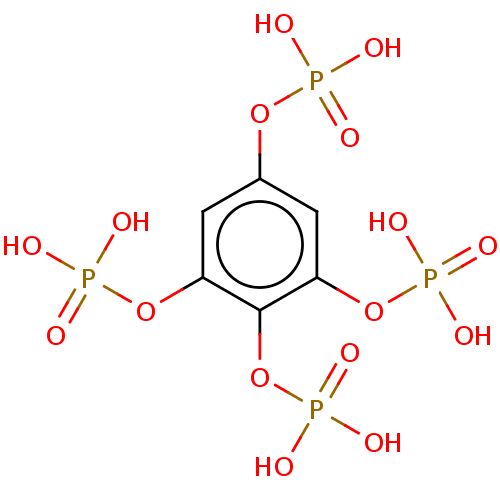 (CHEMBL4454154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Displacement of 2FAMInsP5 from recombinant human N-terminal His-tagged SHIP2 (419 to 832 residues) expressed in Escherichia coli Rosetta2 (DE3) cells... | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50511342 (CHEMBL4538474) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Displacement of 2FAMInsP5 from recombinant human N-terminal His-tagged SHIP2 (419 to 832 residues) expressed in Escherichia coli Rosetta2 (DE3) cells... | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054401 (CHEMBL3319362 | US9522881, 11a-7 (L97M93) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054401 (CHEMBL3319362 | US9522881, 11a-7 (L97M93) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description The inhibition assays were performed at 25° C. in 50 mM 3,3-dimethylglutarate buffer, pH 7.0, containing 1 mM EDTA with an ionic strength of 0.15M ad... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50430583 (CHEMBL2337807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of SHIP2 (unknown origin) | Eur J Med Chem 62: 649-60 (2013) Article DOI: 10.1016/j.ejmech.2013.01.014 BindingDB Entry DOI: 10.7270/Q2D79CS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2 (Homo sapiens (Human)) | BDBM50054400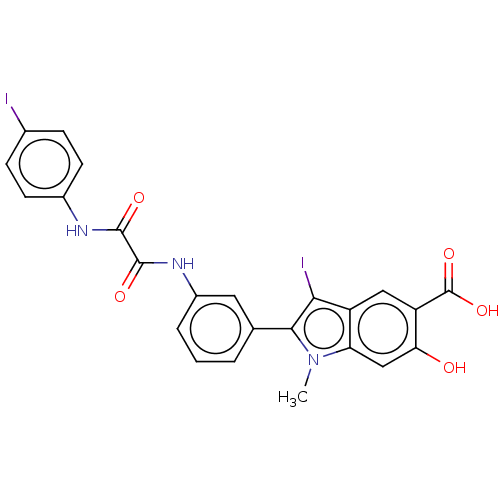 (CHEMBL3319363 | US9522881, 11a-8 (L97M24) | US9844...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP assays were conducted as previously described in Kontaridis et al., J Biol Chem. 2006; 281:6785-6792, using para-nitrophenyl phosphate (pNPP, obt... | US Patent US9844535 (2017) BindingDB Entry DOI: 10.7270/Q2NZ89X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 154 total ) | Next | Last >> |