

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM18796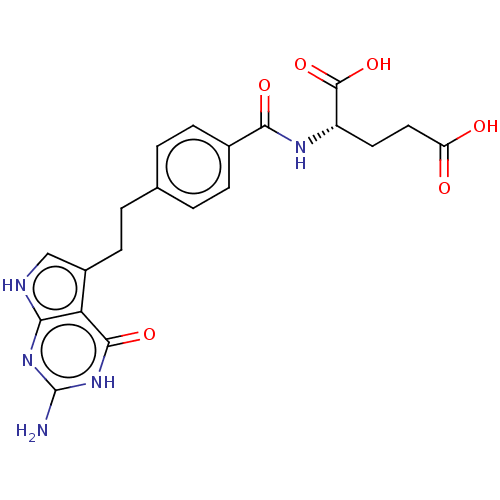 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic by spectrophotometry | Eur J Med Chem 46: 1616-21 (2011) Article DOI: 10.1016/j.ejmech.2011.02.009 BindingDB Entry DOI: 10.7270/Q22V2H35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM18796 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic measured by slope intercepts of mixed-type inhibition against compound con... | Eur J Med Chem 46: 1616-21 (2011) Article DOI: 10.1016/j.ejmech.2011.02.009 BindingDB Entry DOI: 10.7270/Q22V2H35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM18796 ((2S)-2-{[4-(2-{2-amino-4-oxo-1H,4H,7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Mixed-type inhibition of human recombinant Serine hydroxymethyltransferase, cytosolic measured by Y-axes intercepts of mixed-type inhibition against ... | Eur J Med Chem 46: 1616-21 (2011) Article DOI: 10.1016/j.ejmech.2011.02.009 BindingDB Entry DOI: 10.7270/Q22V2H35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285658 (US10077273, Compound 4 | US10584132, Compound 4 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285663 (US10077273, Compound 11 | US10584132, Compound 11 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285659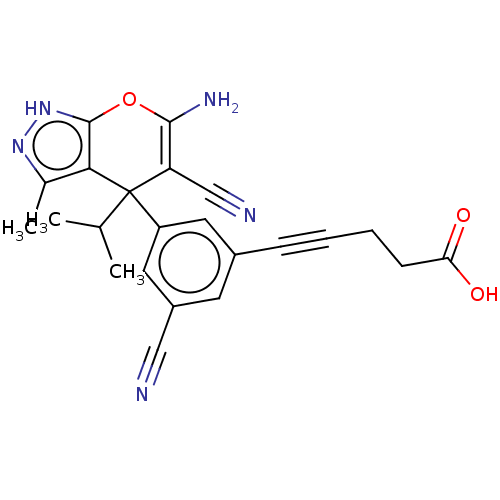 (US10077273, Compound 68 | US10584132, Compound 68 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285663 (US10077273, Compound 11 | US10584132, Compound 11 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285660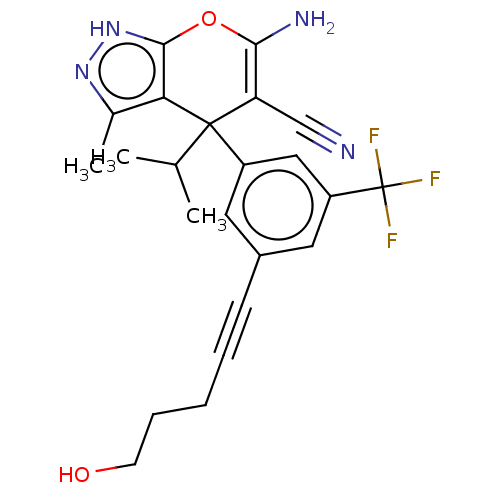 (US10077273, Compound 1 | US10584132, Compound 1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285660 (US10077273, Compound 1 | US10584132, Compound 1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285661 (US10077273, Compound 69 | US10584132, Compound 69 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285662 (US10077273, Compound 70 | US10584132, Compound 70 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285663 (US10077273, Compound 11 | US10584132, Compound 11 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285664 (US10077273, Compound 71 | US10584132, Compound 71 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285659 (US10077273, Compound 68 | US10584132, Compound 68 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285659 (US10077273, Compound 68 | US10584132, Compound 68 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285666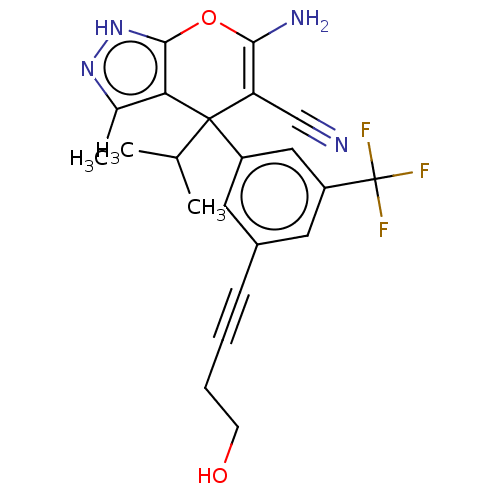 (US10077273, Compound 2 | US10584132, Compound 2 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285666 (US10077273, Compound 2 | US10584132, Compound 2 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285665 (US10077273, Compound 72 | US10584132, Compound 72 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285664 (US10077273, Compound 71 | US10584132, Compound 71 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285664 (US10077273, Compound 71 | US10584132, Compound 71 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285665 (US10077273, Compound 72 | US10584132, Compound 72 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285665 (US10077273, Compound 72 | US10584132, Compound 72 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285666 (US10077273, Compound 2 | US10584132, Compound 2 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285688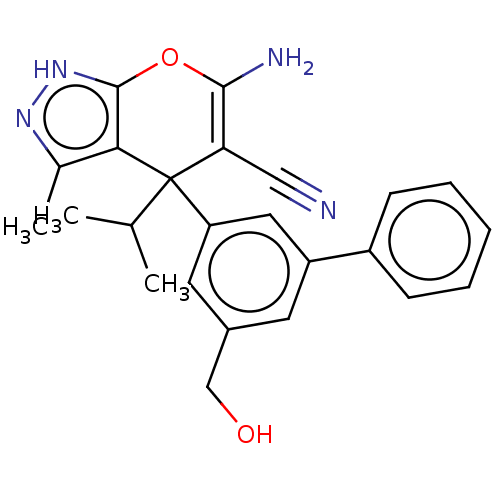 (US10077273, Compound HK-X2 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285686 (US10077273, Compound HK-16 | US10077273, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285686 (US10077273, Compound HK-16 | US10077273, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285667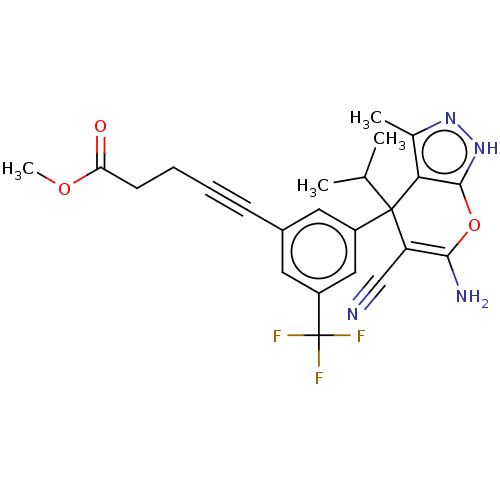 (US10077273, Compound 73 | US10584132, Compound 73 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285689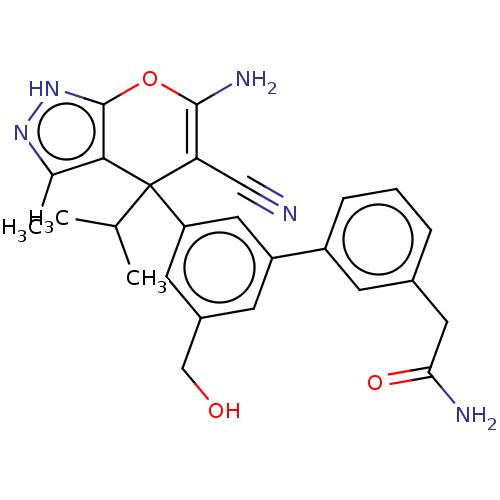 (US10077273, Compound HK-X3 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285668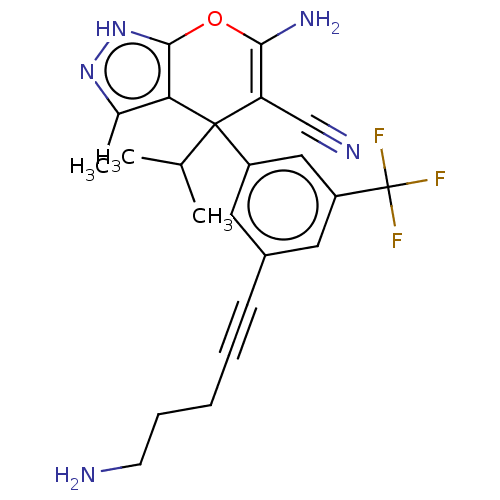 (US10077273, Compound 8 | US10584132, Compound 8 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285668 (US10077273, Compound 8 | US10584132, Compound 8 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285687 (US10077273, Compound HK-X1 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285688 (US10077273, Compound HK-X2 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM485192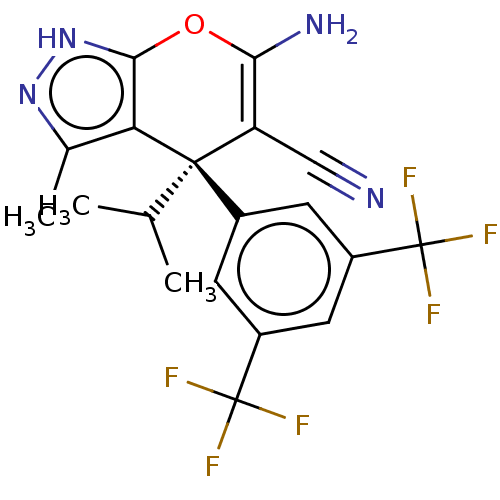 (US10934305, Compound HK-16 (PK-2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285689 (US10077273, Compound HK-X3 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285669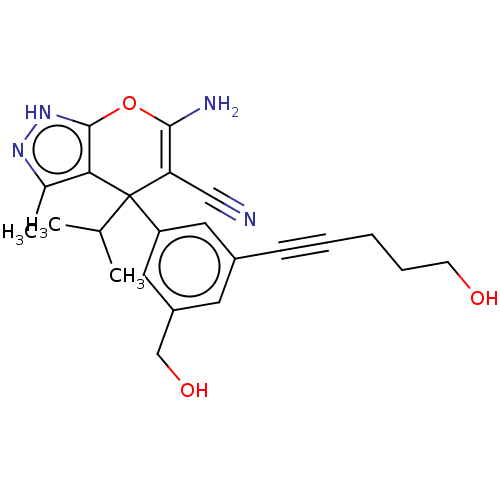 (US10077273, Compound 74 | US10584132, Compound 74 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | US Patent US10584132 (2020) BindingDB Entry DOI: 10.7270/Q2K939XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285669 (US10077273, Compound 74 | US10584132, Compound 74 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285669 (US10077273, Compound 74 | US10584132, Compound 74 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285685 (US10077273, Compound HK-15 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285686 (US10077273, Compound HK-16 | US10077273, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285686 (US10077273, Compound HK-16 | US10077273, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285685 (US10077273, Compound HK-15 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285690 (6-amino-4-(3-bromo-5-(trifluoromethyl)phenyl)-4-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285668 (US10077273, Compound 8 | US10584132, Compound 8 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285687 (US10077273, Compound HK-X1 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285684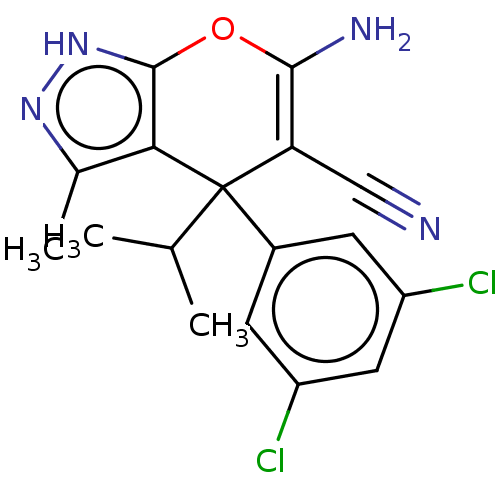 (US10077273, Compound HK-14 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285685 (US10077273, Compound HK-15 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285684 (US10077273, Compound HK-14 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285682 (US10077273, Compound HK-12 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM285682 (US10077273, Compound HK-12 | US10934305, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 206 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10077273 (2018) BindingDB Entry DOI: 10.7270/Q2DR2XHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine hydroxymethyltransferase, cytosolic (Homo sapiens (Human)) | BDBM485179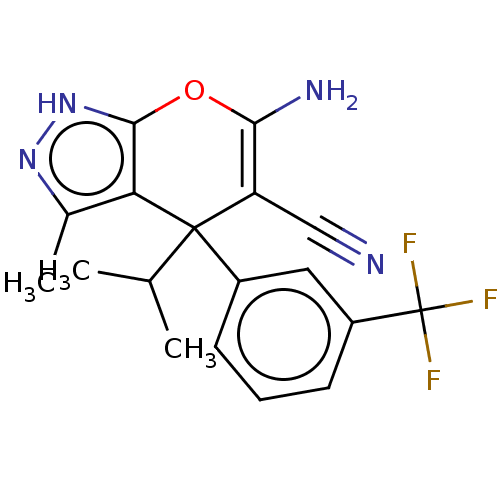 (US10934305, Compound HK-5 | US10934305, Compound H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Princeton University US Patent | Assay Description For the SHMT1 and SHMT2 in vitro enzymatic assays, the rate of 5,10-methylene tetrahydrofolate formation catalyzed by SHMT1/2 was indirectly evaluate... | US Patent US10934305 (2021) BindingDB Entry DOI: 10.7270/Q2KK9FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |