

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM21079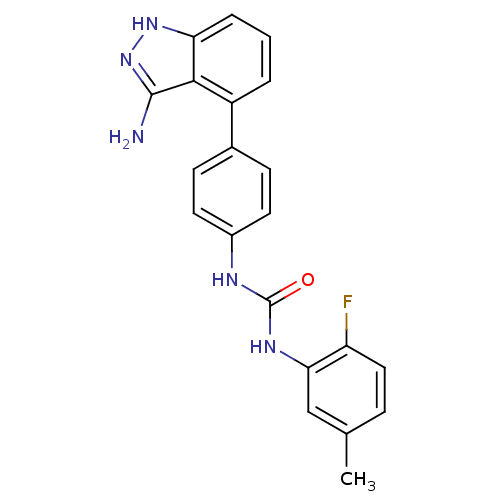 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM81374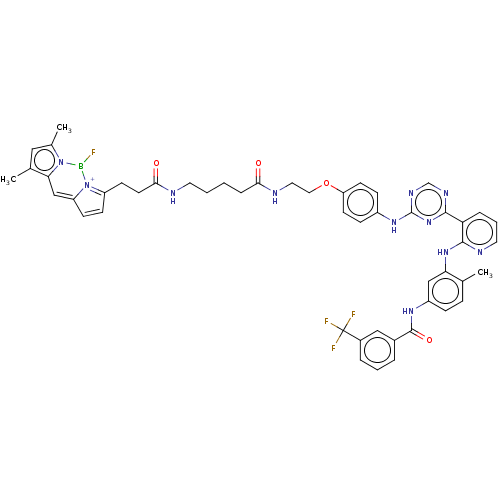 (BODIPY-labeled probe, 4a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 27 | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description In vitro activity assay using various kinases. | Chem Biol 17: 195-206 (2010) Article DOI: 10.1016/j.chembiol.2010.01.008 BindingDB Entry DOI: 10.7270/Q2833QG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PCBioAssay | n/a | n/a | n/a | 880 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PCBioAssay | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM31090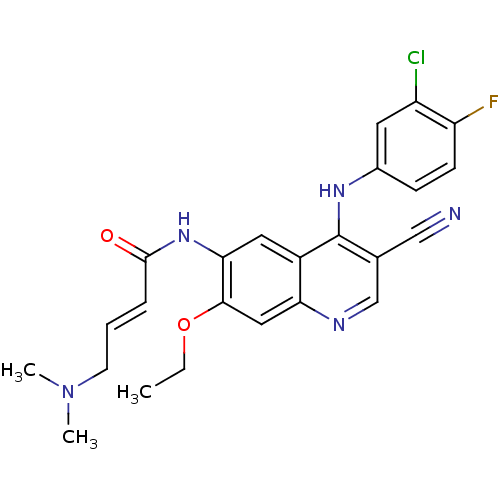 ((E)-N-[4-(3-chloro-4-fluoro-anilino)-3-cyano-7-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM31099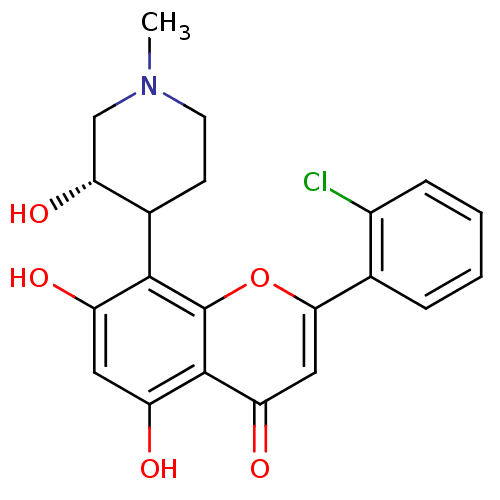 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S)-3-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 27 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM4814 (CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM21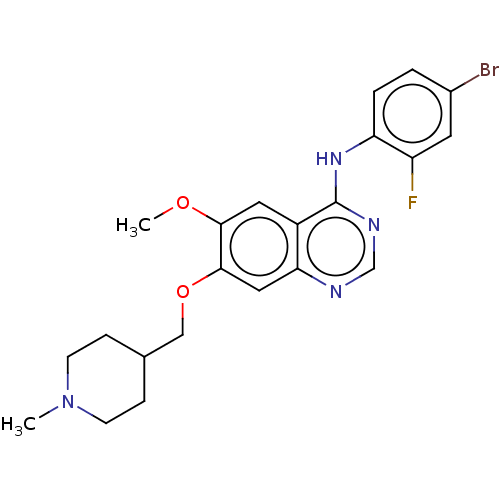 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 3 [1-975] (Homo sapiens (Human)) | BDBM31085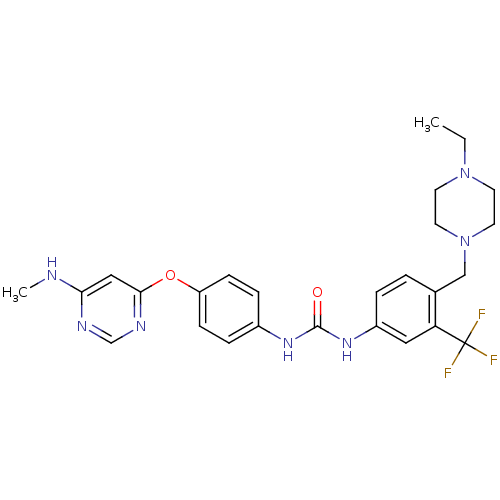 (1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | 80 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2NC5ZHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||