

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM13066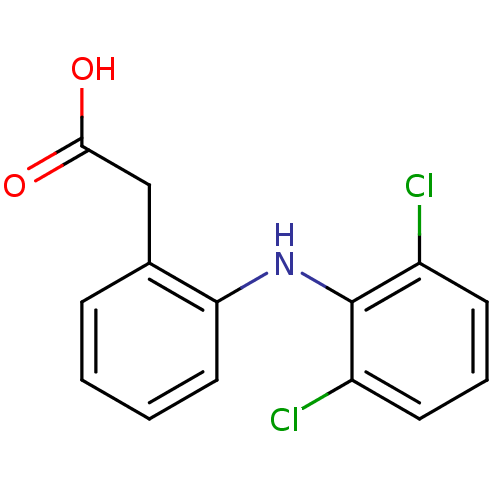 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia Curated by ChEMBL | Assay Description Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A9 | Pharmacol Ther 106: 97-132 (2005) Article DOI: 10.1016/j.pharmthera.2004.10.013 BindingDB Entry DOI: 10.7270/Q2959HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Drug metabolism assessed as recombinant human UGT1A9 assessed as O-glucuronidation measured as inhibition constant at 10 to 400 uM incubated for 60 m... | Drug Metab Dispos 40: 240-8 (2012) Article DOI: 10.1124/dmd.111.042150 BindingDB Entry DOI: 10.7270/Q2KS6T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50088502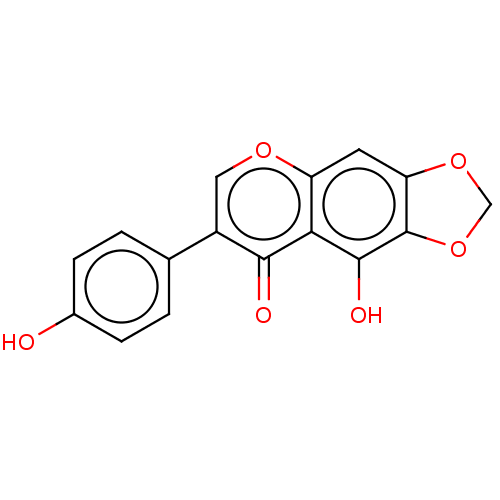 (CHEBI:5970 | CHEMBL3527329) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Rubner-Institute Curated by ChEMBL | Assay Description Substrate inhibition of human recombinant UGT1A9 assessed as IRI-O-5-monoglucuronide formation incubated for 5 mins prior to UDPGA addition measured ... | Drug Metab Dispos 39: 610-6 (2011) Article DOI: 10.1124/dmd.110.033076 BindingDB Entry DOI: 10.7270/Q20V8FHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50008347 (5-(2-(diethylamino)ethylamino)-8-hydroxy-6H-imidaz...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gdansk University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A9 activity expressed in baculovirus-infected Sf9 cells | Drug Metab Dispos 40: 1736-43 (2012) Article DOI: 10.1124/dmd.112.045401 BindingDB Entry DOI: 10.7270/Q25H7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50088499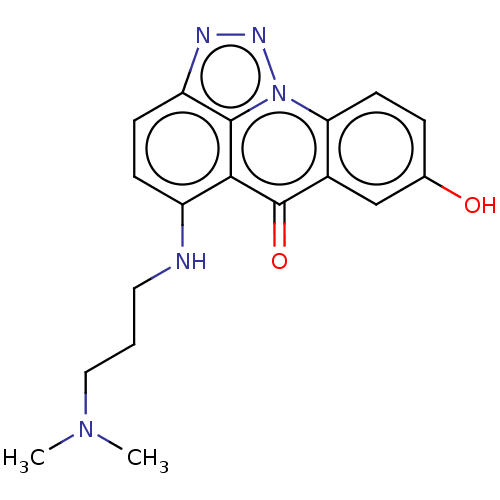 (CHEMBL329092) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gdansk University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant UGT1A9 activity expressed in baculovirus-infected Sf9 cells | Drug Metab Dispos 40: 1736-43 (2012) Article DOI: 10.1124/dmd.112.045401 BindingDB Entry DOI: 10.7270/Q25H7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50206509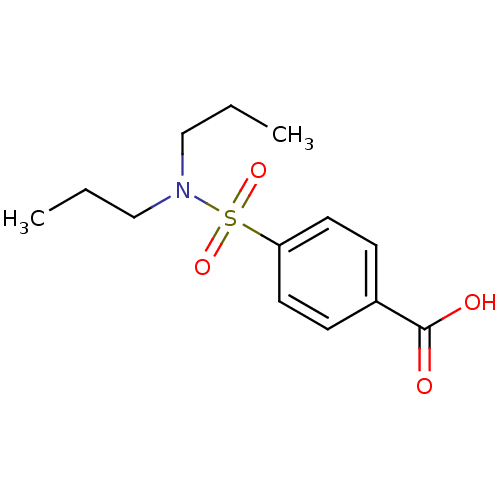 (4-Dipropylsulfamoyl-benzoic acid | 4-Dipropylsulfa...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia Curated by ChEMBL | Assay Description Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A9 | Pharmacol Ther 106: 97-132 (2005) Article DOI: 10.1016/j.pharmthera.2004.10.013 BindingDB Entry DOI: 10.7270/Q2959HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50511112 (CHEMBL4567446) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of UGT1A9 in human liver microsomes incubated for 30 mins in presence of UDPGA by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00640 BindingDB Entry DOI: 10.7270/Q2RR22VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50508419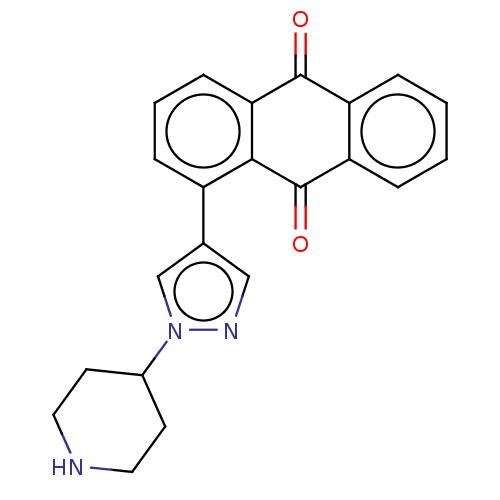 (CHEMBL4471264) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of UGT1A9 in human liver microsomes assessed as reduction in mycophenolic acid glucuronidation by tandem mass spectrometry analysis | J Med Chem 62: 575-588 (2019) Article DOI: 10.1021/acs.jmedchem.8b01168 BindingDB Entry DOI: 10.7270/Q2QZ2F8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A9 (Homo sapiens (Human)) | BDBM50103451 (CHEMBL3398252) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibition of of UGT1A9 in pooled mixed-gender human liver microsomes using 4-methylumbelliferone substrate by HPLC method | Bioorg Med Chem Lett 25: 1269-73 (2015) Article DOI: 10.1016/j.bmcl.2015.01.050 BindingDB Entry DOI: 10.7270/Q2KW5HTQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||