

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM85095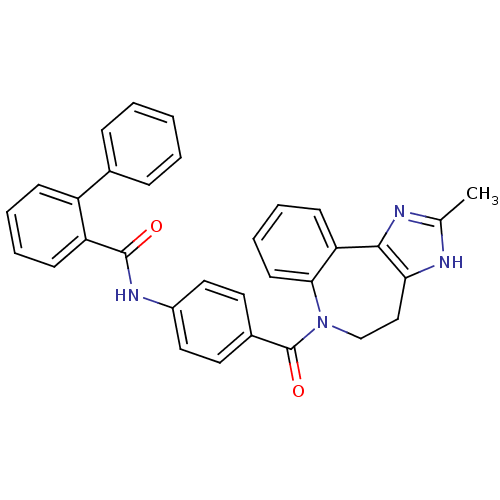 (CAS_151171 | CONIVAPTAN | NSC_151171 | YM087) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement [3H]Arg human recombinant Vasopressin V2 receptor | Bioorg Med Chem Lett 20: 3742-5 (2010) Article DOI: 10.1016/j.bmcl.2010.04.068 BindingDB Entry DOI: 10.7270/Q24F1QW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50291823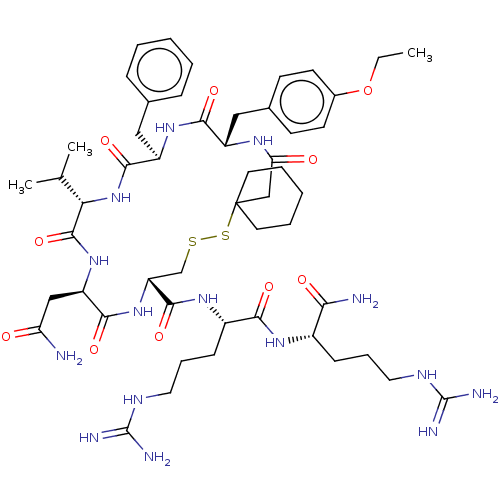 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50307117 (CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in CHO cell membranes by radioligand binding assay | J Med Chem 61: 8670-8692 (2018) Article DOI: 10.1021/acs.jmedchem.8b00697 BindingDB Entry DOI: 10.7270/Q24J0HRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50307117 (CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50307117 (CHEMBL603708 | N-(4-(2,3,4,5-tetrahydro-1H-benzo[b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50326722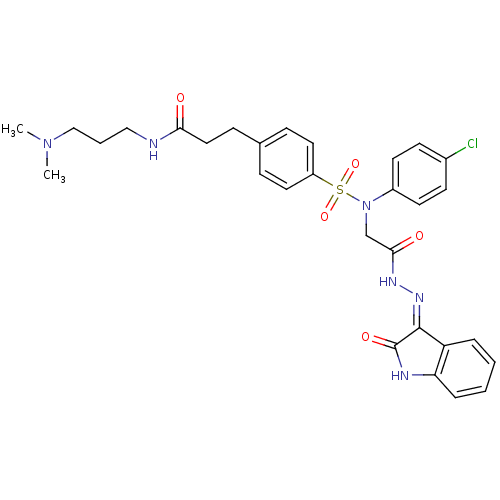 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V2 receptor in human kidney tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50291822 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50397209 (CHEMBL2172291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50291821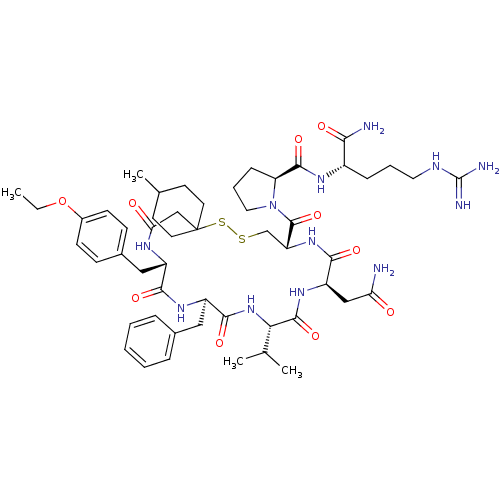 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50291821 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50020673 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V2 receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50291824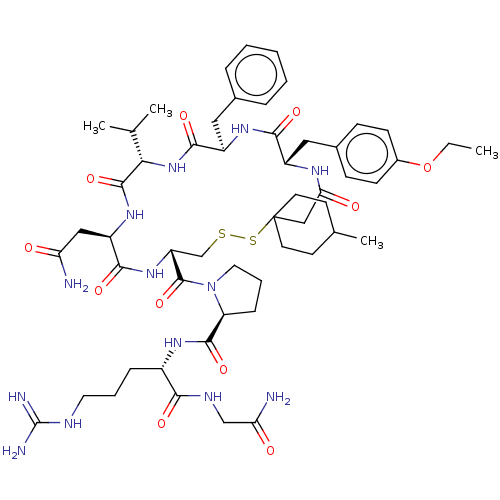 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50020667 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound towards Vasopressin receptor by binding [3H]-LVP to dog renal medullary preparation. | J Med Chem 32: 880-4 (1989) BindingDB Entry DOI: 10.7270/Q2ZK5FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50108498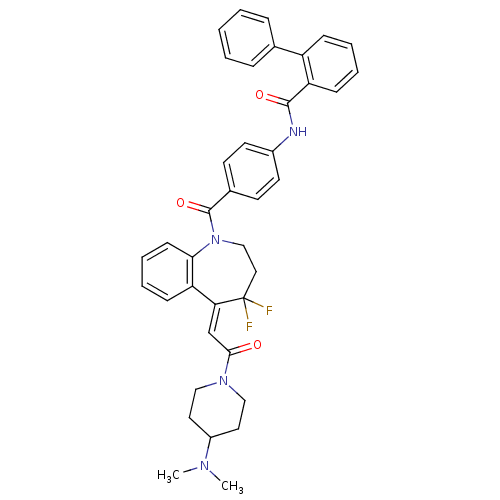 (Biphenyl-2-carboxylic acid (4-{5-[2-(4-dimethylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Binding affinity at cloned human Vasopressin V2 receptor stably expressed in CHO cells, using [3H]-AVP as radioligand | J Med Chem 45: 2589-98 (2002) BindingDB Entry DOI: 10.7270/Q2NC60H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50145118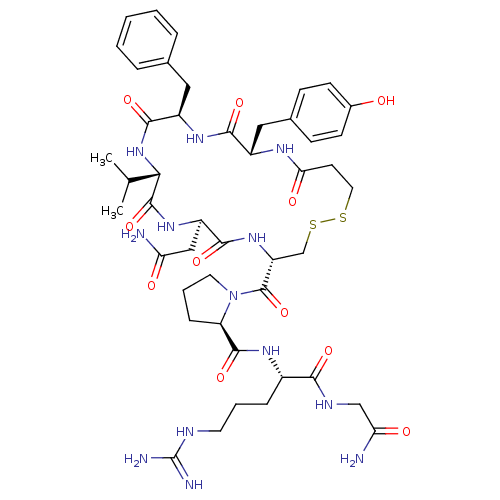 (CHEMBL412353 | d[Val4]AVP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio Curated by ChEMBL | Assay Description Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor... | J Med Chem 47: 2375-88 (2004) Article DOI: 10.1021/jm030611c BindingDB Entry DOI: 10.7270/Q2GF0V8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins by saturation binding assay | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146293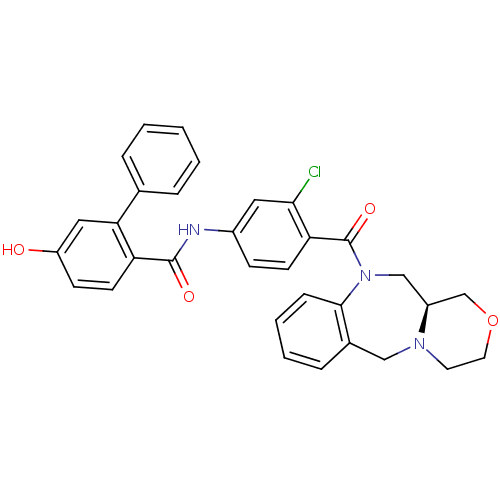 (5-Hydroxy-biphenyl-2-carboxylic acid [3-chloro-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50145111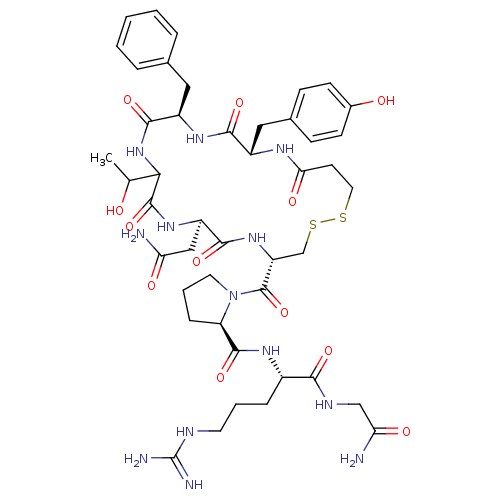 ((2S)-2-{[(2R)-1-{[(4S,7R,13R,16S)-13-benzyl-7-(car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio Curated by ChEMBL | Assay Description Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor... | J Med Chem 47: 2375-88 (2004) Article DOI: 10.1021/jm030611c BindingDB Entry DOI: 10.7270/Q2GF0V8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016752 (CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00011 BindingDB Entry DOI: 10.7270/Q29C72HD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146309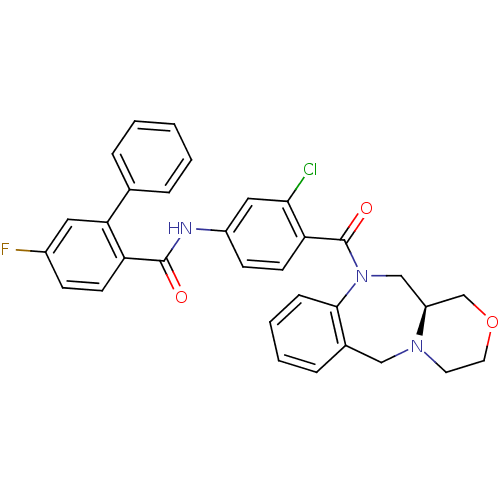 (5-Fluoro-biphenyl-2-carboxylic acid [3-chloro-4-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50397210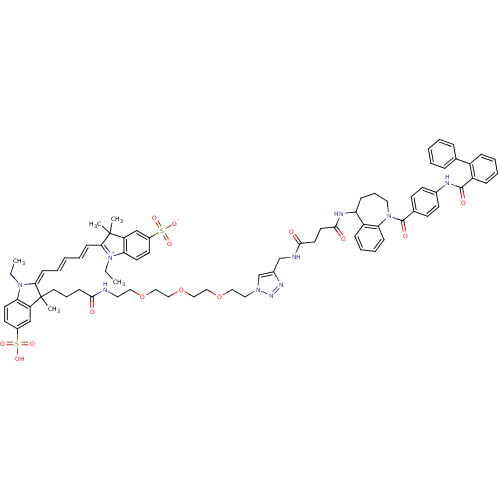 (CHEMBL2172290) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from SNAP-tagged vasopressin V2 receptor expressed in HEK293 cells by FRET assay | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146304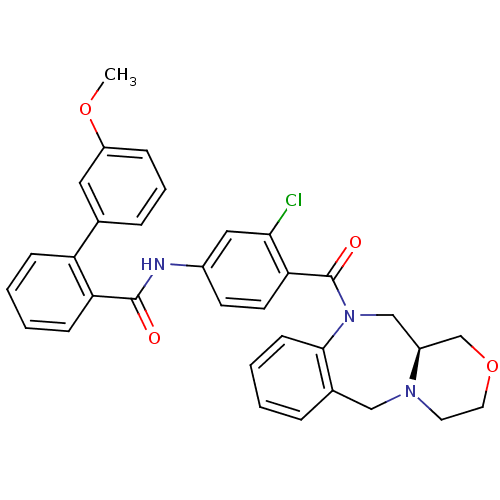 (3'-Methoxy-biphenyl-2-carboxylic acid [3-chloro-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146309 (5-Fluoro-biphenyl-2-carboxylic acid [3-chloro-4-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50145109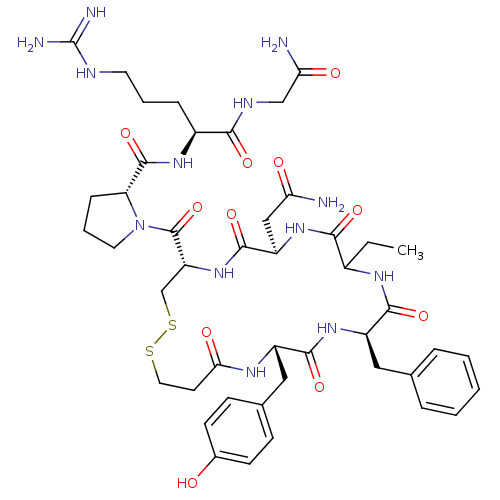 ((2S)-2-{[(2R)-1-{[(4S,7R,13R,16S)-13-benzyl-7-(car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio Curated by ChEMBL | Assay Description Binding affinity against human vasopressin V2 receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT receptor... | J Med Chem 47: 2375-88 (2004) Article DOI: 10.1021/jm030611c BindingDB Entry DOI: 10.7270/Q2GF0V8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00567 BindingDB Entry DOI: 10.7270/Q2RR2392 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50052954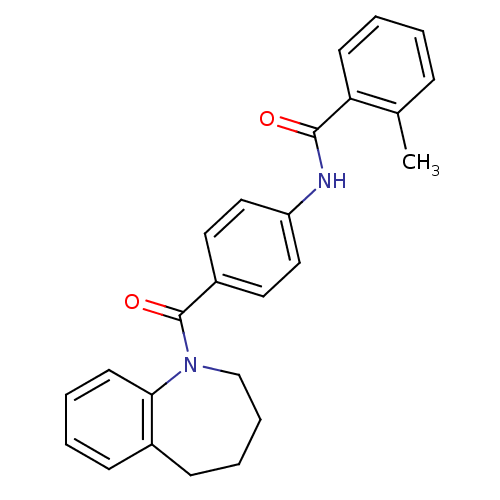 (2-Methyl-N-[4-(2,3,4,5-tetrahydro-benzo[b]azepine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146283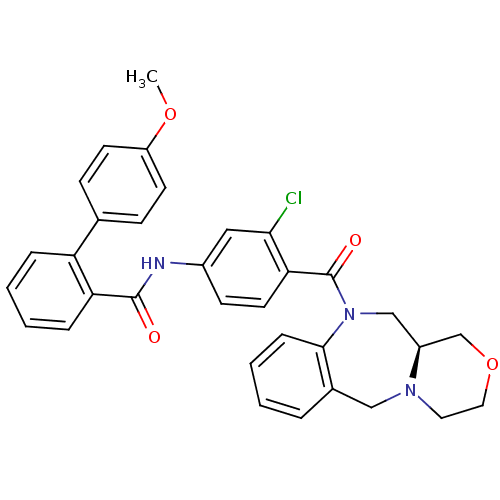 (4'-Methoxy-biphenyl-2-carboxylic acid [3-chloro-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146283 (4'-Methoxy-biphenyl-2-carboxylic acid [3-chloro-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146291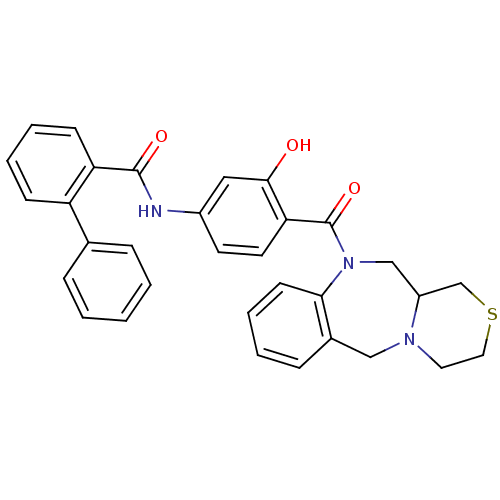 (Biphenyl-2-carboxylic acid [3-hydroxy-4-(3,4,11,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146285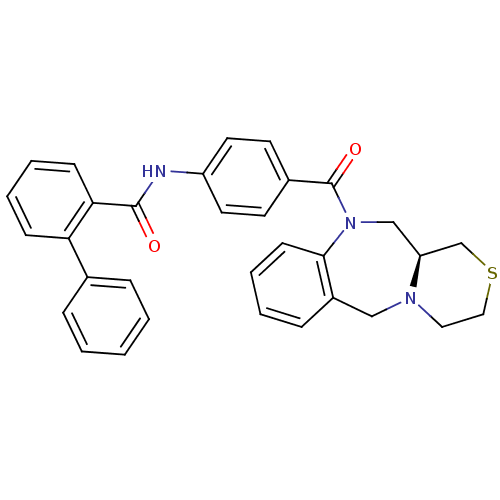 (Biphenyl-2-carboxylic acid [4-((S)-3,4,11,11a-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016760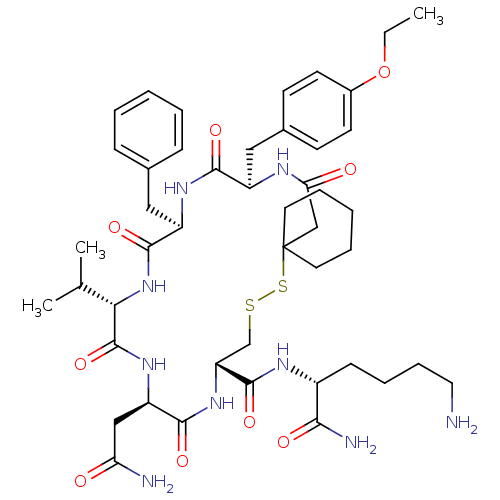 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35683 (5H-1-benzazepin-5-ylidene acetamide, 1k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 23 |
Astellas Pharma Inc. | Assay Description The affinities of test compounds for human V2 receptor were evaluated by the radioligand binding study using membrane fractions isolated from CHO cel... | Bioorg Med Chem 17: 3130-41 (2009) Article DOI: 10.1016/j.bmc.2009.03.001 BindingDB Entry DOI: 10.7270/Q24T6GQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146310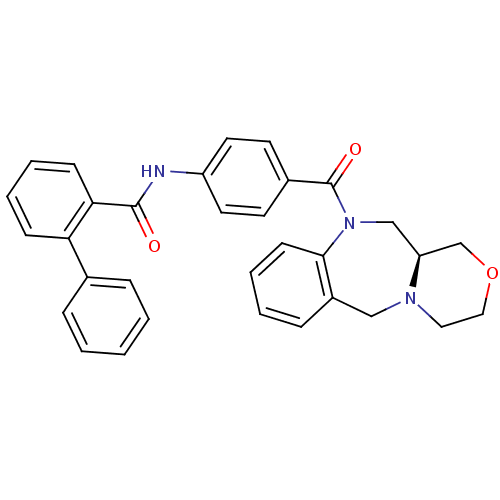 (Biphenyl-2-carboxylic acid [4-((S)-3,4,11,11a-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016756 (CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146297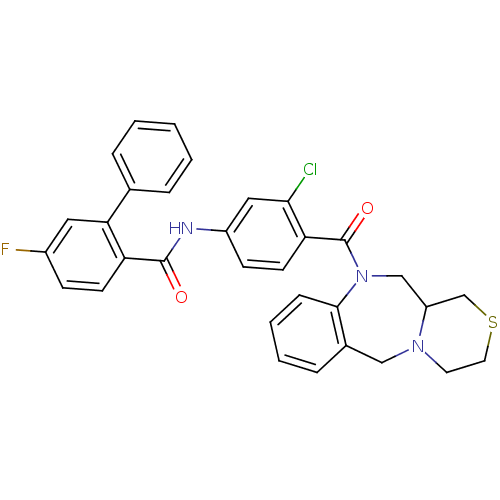 (5-Fluoro-biphenyl-2-carboxylic acid [3-chloro-4-(3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146303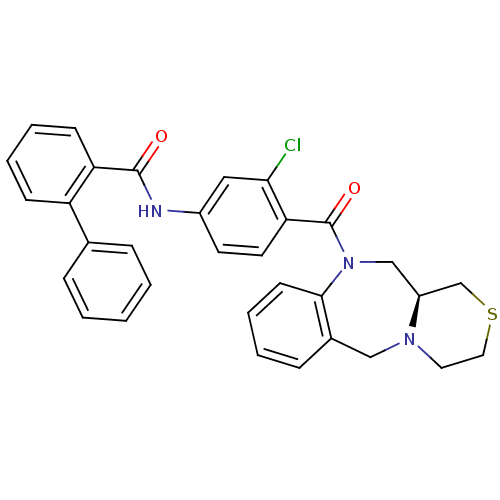 (Biphenyl-2-carboxylic acid [3-chloro-4-((S)-3,4,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146304 (3'-Methoxy-biphenyl-2-carboxylic acid [3-chloro-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50604776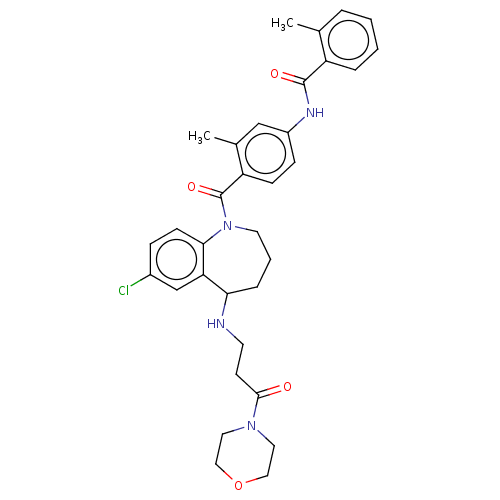 (CHEMBL5207224) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00011 BindingDB Entry DOI: 10.7270/Q29C72HD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146284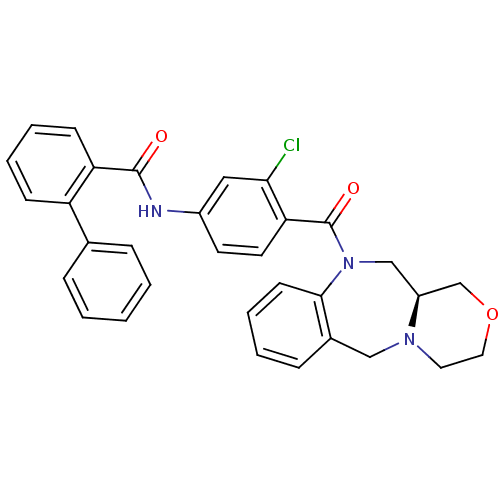 (Biphenyl-2-carboxylic acid [3-chloro-4-((S)-3,4,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50397215 (CHEMBL2172295) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2 receptor expressed in CHO cells after 30 mins | J Med Chem 55: 8588-602 (2012) Article DOI: 10.1021/jm3006146 BindingDB Entry DOI: 10.7270/Q28G8MT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146284 (Biphenyl-2-carboxylic acid [3-chloro-4-((S)-3,4,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146301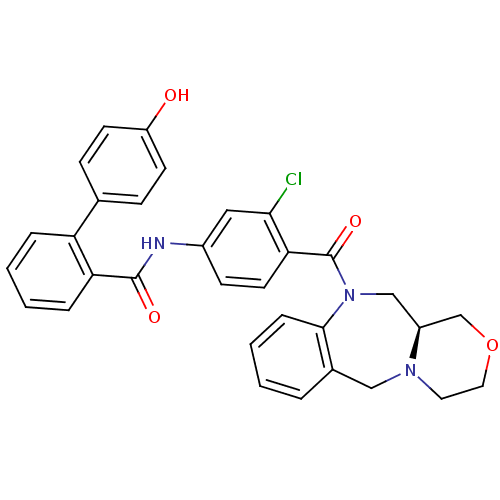 (4'-Hydroxy-biphenyl-2-carboxylic acid [3-chloro-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 2747-52 (2004) Article DOI: 10.1016/j.bmcl.2004.03.083 BindingDB Entry DOI: 10.7270/Q2QN679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016753 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1427 total ) | Next | Last >> |