

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50131270 (2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]prop...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of AADAC in human kidney microsome | Drug Metab Dispos 40: 671-9 (2012) Article DOI: 10.1124/dmd.111.043067 BindingDB Entry DOI: 10.7270/Q2H133RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50131270 (2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]prop...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of AADAC in human jejunum microsome | Drug Metab Dispos 40: 671-9 (2012) Article DOI: 10.1124/dmd.111.043067 BindingDB Entry DOI: 10.7270/Q2H133RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of AADAC in human liver microsome | Drug Metab Dispos 40: 671-9 (2012) Article DOI: 10.1124/dmd.111.043067 BindingDB Entry DOI: 10.7270/Q2H133RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of human recombinant AADAC | Drug Metab Dispos 40: 671-9 (2012) Article DOI: 10.1124/dmd.111.043067 BindingDB Entry DOI: 10.7270/Q2H133RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of AADAC in human jejunum microsome | Drug Metab Dispos 40: 671-9 (2012) Article DOI: 10.1124/dmd.111.043067 BindingDB Entry DOI: 10.7270/Q2H133RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50454599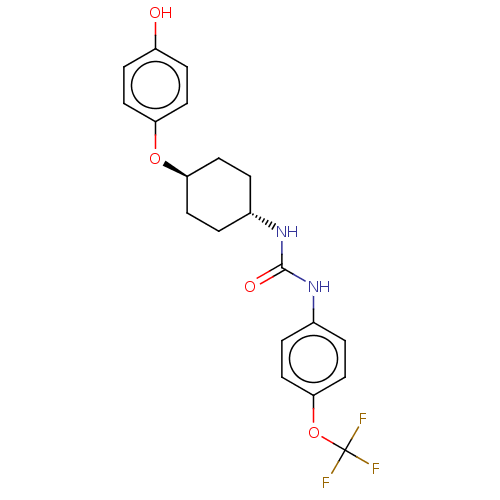 (CHEMBL4216368) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of AADAC (unknown origin) expressed in baculovirus system using CMNA substrate by fluorescence based assay | Bioorg Med Chem Lett 28: 762-768 (2018) Article DOI: 10.1016/j.bmcl.2018.01.003 BindingDB Entry DOI: 10.7270/Q2PV6NZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM50454598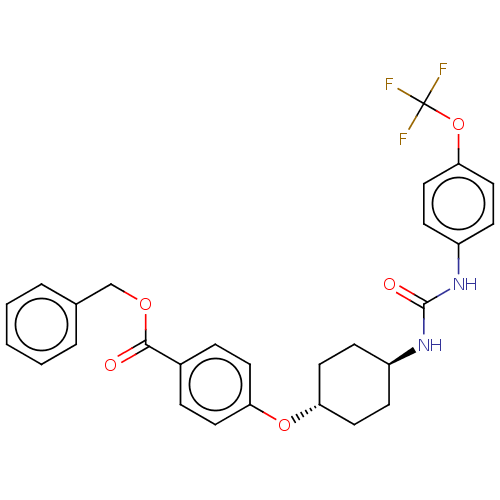 (CHEMBL4215208) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of AADAC (unknown origin) expressed in baculovirus system using CMNA substrate by fluorescence based assay | Bioorg Med Chem Lett 28: 762-768 (2018) Article DOI: 10.1016/j.bmcl.2018.01.003 BindingDB Entry DOI: 10.7270/Q2PV6NZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arylacetamide deacetylase (Homo sapiens (Human)) | BDBM158481 (US9029401, 1728 (t-TUCB)) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of AADAC (unknown origin) expressed in baculovirus system using CMNA substrate by fluorescence based assay | Bioorg Med Chem Lett 28: 762-768 (2018) Article DOI: 10.1016/j.bmcl.2018.01.003 BindingDB Entry DOI: 10.7270/Q2PV6NZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||