 Found 139 hits Enz. Inhib. hit(s) with Target = 'M-phase inducer phosphatase 3' AND taxid = 9606
Found 139 hits Enz. Inhib. hit(s) with Target = 'M-phase inducer phosphatase 3' AND taxid = 9606 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504063

(CHEMBL4570006)Show InChI InChI=1S/C19H17NO2/c1-2-22-19(21)16-11-5-6-12-18(16)20-17-13-7-9-14-8-3-4-10-15(14)17/h3-13,20H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... |
J Med Chem 62: 7089-7110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00632
BindingDB Entry DOI: 10.7270/Q20005B5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50129576
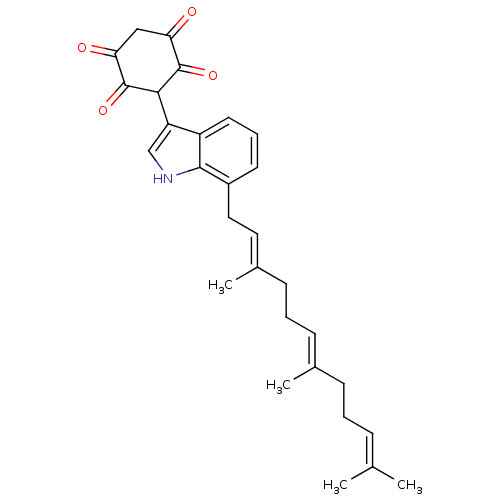
(2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...)Show SMILES CC(C)=CCC\C(C)=C\CC\C(C)=C\Cc1cccc2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O |(-9.11,4.99,;-7.6,4.77,;-6.64,5.99,;-7.04,3.33,;-5.51,3.1,;-4.95,1.67,;-3.42,1.44,;-2.47,2.65,;-2.86,.02,;-1.35,-.22,;-.77,-1.66,;.75,-1.87,;1.31,-3.32,;1.71,-.68,;3.23,-.91,;4.18,.3,;3.62,1.73,;4.58,2.93,;6.11,2.7,;6.67,1.26,;8.1,.72,;8.03,-.82,;6.56,-1.21,;5.72,.06,;9.43,1.51,;9.4,3.06,;8.03,3.83,;10.72,3.84,;10.69,5.38,;12.07,3.1,;12.09,1.54,;13.46,.77,;10.76,.76,;10.79,-.78,)| Show InChI InChI=1S/C29H33NO4/c1-18(2)8-5-9-19(3)10-6-11-20(4)14-15-21-12-7-13-22-23(17-30-27(21)22)26-28(33)24(31)16-25(32)29(26)34/h7-8,10,12-14,17,26,30H,5-6,9,11,15-16H2,1-4H3/b19-10+,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against Cell division cycle 25 was determined |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504074
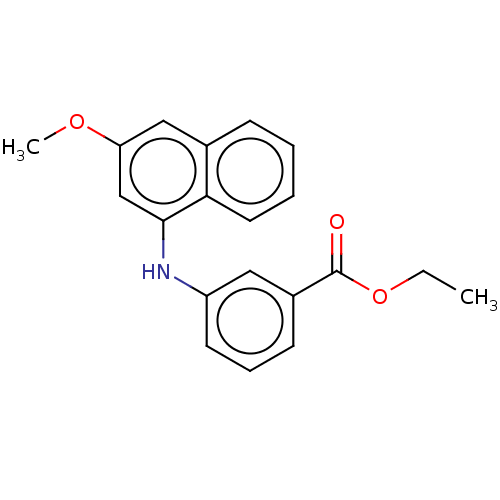
(CHEMBL4447602)Show InChI InChI=1S/C20H19NO3/c1-3-24-20(22)15-8-6-9-16(11-15)21-19-13-17(23-2)12-14-7-4-5-10-18(14)19/h4-13,21H,3H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... |
J Med Chem 62: 7089-7110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00632
BindingDB Entry DOI: 10.7270/Q20005B5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25 degree C (Cdc25 C) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504077
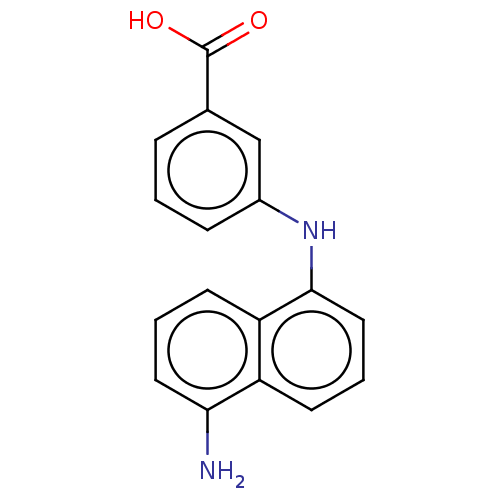
(CHEMBL4442406)Show InChI InChI=1S/C17H14N2O2/c18-15-8-2-7-14-13(15)6-3-9-16(14)19-12-5-1-4-11(10-12)17(20)21/h1-10,19H,18H2,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... |
J Med Chem 62: 7089-7110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00632
BindingDB Entry DOI: 10.7270/Q20005B5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50132461
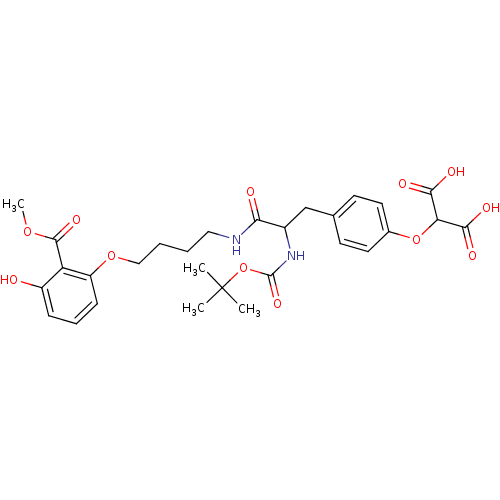
(2-(4-{2-tert-Butoxycarbonylamino-2-[4-(3-hydroxy-2...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)C(Cc1ccc(OC(C(O)=O)C(O)=O)cc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C29H36N2O12/c1-29(2,3)43-28(39)31-19(16-17-10-12-18(13-11-17)42-23(25(34)35)26(36)37)24(33)30-14-5-6-15-41-21-9-7-8-20(32)22(21)27(38)40-4/h7-13,19,23,32H,5-6,14-16H2,1-4H3,(H,30,33)(H,31,39)(H,34,35)(H,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Cell division cycle 25 degree C |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50132460
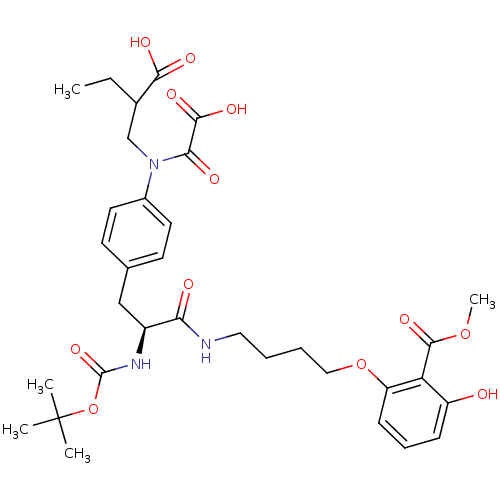
(2-[4-((S)-2-tert-Butoxycarbonylamino-3-{4-[(2-carb...)Show SMILES CCC(CN(C(=O)C(O)=O)c1ccc(C[C@H](NC(=O)OC(C)(C)C)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)cc1)C(O)=O Show InChI InChI=1S/C33H43N3O12/c1-6-21(29(40)41)19-36(28(39)30(42)43)22-14-12-20(13-15-22)18-23(35-32(45)48-33(2,3)4)27(38)34-16-7-8-17-47-25-11-9-10-24(37)26(25)31(44)46-5/h9-15,21,23,37H,6-8,16-19H2,1-5H3,(H,34,38)(H,35,45)(H,40,41)(H,42,43)/t21?,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Cell division cycle 25 degree C |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50132465
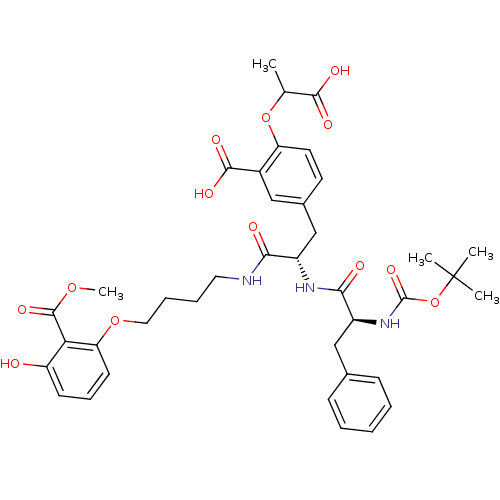
(5-{(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phenyl-...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(OC(C)C(O)=O)c(c1)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C39H47N3O13/c1-23(35(46)47)54-30-17-16-25(20-26(30)36(48)49)22-27(33(44)40-18-9-10-19-53-31-15-11-14-29(43)32(31)37(50)52-5)41-34(45)28(21-24-12-7-6-8-13-24)42-38(51)55-39(2,3)4/h6-8,11-17,20,23,27-28,43H,9-10,18-19,21-22H2,1-5H3,(H,40,44)(H,41,45)(H,42,51)(H,46,47)(H,48,49)/t23?,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency of the compound against Cell division cycle 25 degree C |
Bioorg Med Chem Lett 13: 3129-32 (2003)
BindingDB Entry DOI: 10.7270/Q24B30QV |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50131545
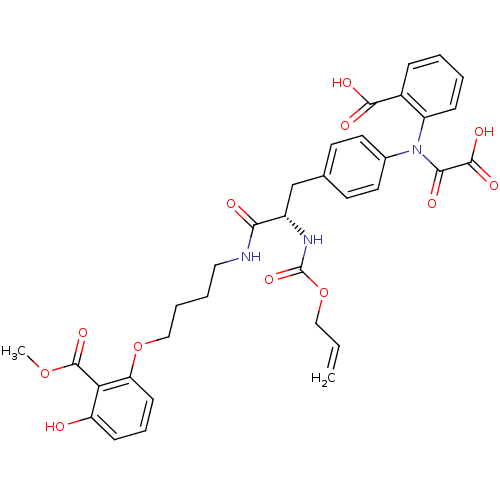
((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cell division cycle 25 degree C was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612011
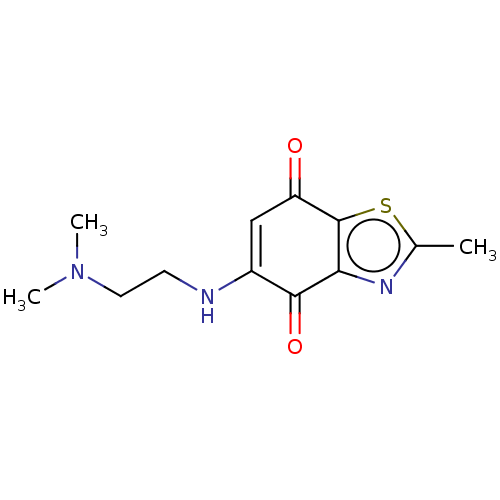
(CHEMBL5288122) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612010

(CHEMBL5271462) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50208827

(2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...)Show InChI InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50208827

(2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...)Show InChI InChI=1S/C14H14O4S2/c15-5-7-19-13-11(17)9-3-1-2-4-10(9)12(18)14(13)20-8-6-16/h1-4,15-16H,5-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504275
(CHEMBL4521092)Show SMILES Cc1ccc(Cn2cc(CNC3=C(Cl)C(=O)c4cccnc4C3=O)nn2)cc1 |c:11| Show InChI InChI=1S/C20H16ClN5O2/c1-12-4-6-13(7-5-12)10-26-11-14(24-25-26)9-23-18-16(21)19(27)15-3-2-8-22-17(15)20(18)28/h2-8,11,23H,9-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111696
BindingDB Entry DOI: 10.7270/Q21R6TS1 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251697

(CHEMBL4093222)Show SMILES O=C1C=CC(=O)c2c1ccc1c3ccc4ccccc4c3oc(=O)c21 |c:2| Show InChI InChI=1S/C21H10O4/c22-16-9-10-17(23)18-15(16)8-7-13-14-6-5-11-3-1-2-4-12(11)20(14)25-21(24)19(13)18/h1-10H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]-cytisine binding from high-affinity Nicotinic acetylcholine receptor in rat brain (principall... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50175216

(5-(2-(dimethylamino)ethylamino)-2-ethylbenzo[d]oxa...)Show InChI InChI=1S/C13H17N3O3/c1-4-10-15-11-12(18)8(14-5-6-16(2)3)7-9(17)13(11)19-10/h5,7,17-18H,4,6H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50175215

(6-(2-(dimethylamino)ethylamino)-2-ethylbenzo[d]oxa...)Show InChI InChI=1S/C13H17N3O3/c1-4-10-15-11-9(17)7-8(12(18)13(11)19-10)14-5-6-16(2)3/h5,7,17-18H,4,6H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50175218
(5-(2-(dimethylamino)ethylamino)-2-methylbenzo[d]th...)Show InChI InChI=1S/C12H15N3O2S/c1-7-14-10-11(17)8(13-4-5-15(2)3)6-9(16)12(10)18-7/h4,6,16-17H,5H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50341995

(5-(2-(dimethylamino)ethylamino)-2,6-dimethylbenzo[...)Show InChI InChI=1S/C13H17N3O2S/c1-7-9(14-5-6-16(3)4)12(18)10-13(11(7)17)19-8(2)15-10/h5,17-18H,6H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504277
(CHEMBL4566962)Show SMILES ClC1=C(NCc2cn(Cc3cccc(c3)C#N)nn2)C(=O)c2ncccc2C1=O |c:1| Show InChI InChI=1S/C20H13ClN6O2/c21-16-18(20(29)17-15(19(16)28)5-2-6-23-17)24-9-14-11-27(26-25-14)10-13-4-1-3-12(7-13)8-22/h1-7,11,24H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111696
BindingDB Entry DOI: 10.7270/Q21R6TS1 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50175217

(5-(2-(dimethylamino)ethylamino)-2-phenylbenzo[d]ox...)Show SMILES CN(C)CC=Nc1cc(O)c2oc(nc2c1O)-c1ccccc1 |w:5.5| Show InChI InChI=1S/C17H17N3O3/c1-20(2)9-8-18-12-10-13(21)16-14(15(12)22)19-17(23-16)11-6-4-3-5-7-11/h3-8,10,21-22H,9H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50175219

(6-(2-(dimethylamino)ethylamino)-2-phenylbenzo[d]ox...)Show SMILES CN(C)CC=Nc1cc(O)c2nc(oc2c1O)-c1ccccc1 |w:5.5| Show InChI InChI=1S/C17H17N3O3/c1-20(2)9-8-18-12-10-13(21)14-16(15(12)22)23-17(19-14)11-6-4-3-5-7-11/h3-8,10,21-22H,9H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant CDC25C |
Bioorg Med Chem Lett 16: 171-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.030
BindingDB Entry DOI: 10.7270/Q2T1535S |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251724
(CHEMBL4066424)Show SMILES CCN(CC)c1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:19| Show InChI InChI=1S/C21H17NO4/c1-3-22(4-2)12-5-6-13-14-7-8-15-16(23)9-10-17(24)19(15)20(14)21(25)26-18(13)11-12/h5-11H,3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251670
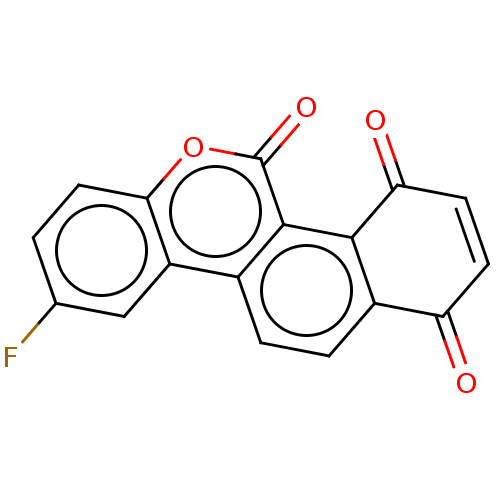
(CHEMBL4085485)Show SMILES Fc1ccc2oc(=O)c3c4C(=O)C=CC(=O)c4ccc3c2c1 |c:12| Show InChI InChI=1S/C17H7FO4/c18-8-1-6-14-11(7-8)9-2-3-10-12(19)4-5-13(20)15(10)16(9)17(21)22-14/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]-cytisine binding from high-affinity Nicotinic acetylcholine receptor in rat brain (principall... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50504276

(CHEMBL4466461)Show SMILES ClC1=C(NCc2cn(Cc3ccccc3C#N)nn2)C(=O)c2ncccc2C1=O |c:1| Show InChI InChI=1S/C20H13ClN6O2/c21-16-18(20(29)17-15(19(16)28)6-3-7-23-17)24-9-14-11-27(26-25-14)10-13-5-2-1-4-12(13)8-22/h1-7,11,24H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111696
BindingDB Entry DOI: 10.7270/Q21R6TS1 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251672

(CHEMBL4060132)Show SMILES Fc1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:15| Show InChI InChI=1S/C17H7FO4/c18-8-1-2-9-10-3-4-11-12(19)5-6-13(20)15(11)16(10)17(21)22-14(9)7-8/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111696
BindingDB Entry DOI: 10.7270/Q21R6TS1 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251671

(CHEMBL4097725)Show SMILES Clc1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:15| Show InChI InChI=1S/C17H7ClO4/c18-8-1-2-9-10-3-4-11-12(19)5-6-13(20)15(11)16(10)17(21)22-14(9)7-8/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251688
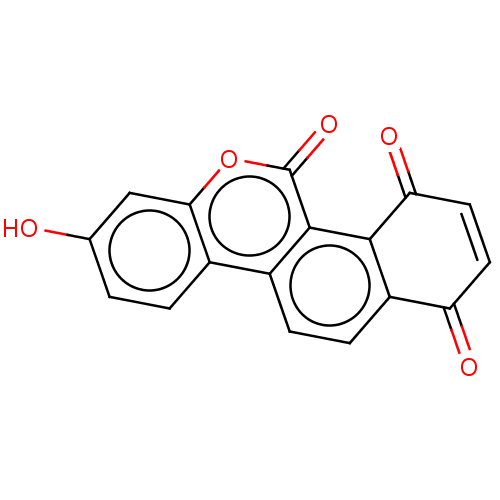
(CHEMBL4086414)Show SMILES Oc1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:15| Show InChI InChI=1S/C17H8O5/c18-8-1-2-9-10-3-4-11-12(19)5-6-13(20)15(11)16(10)17(21)22-14(9)7-8/h1-7,18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612014

(CHEMBL3623325) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251687
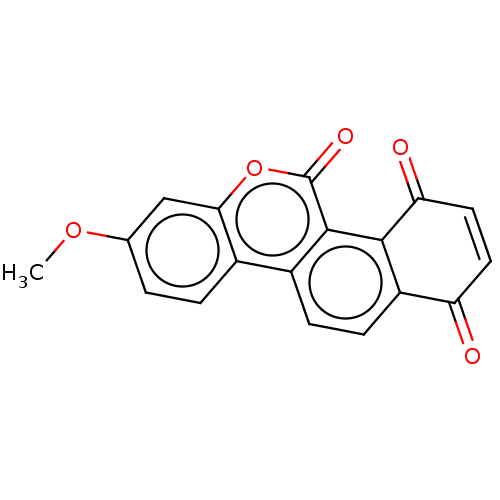
(CHEMBL4066643)Show SMILES COc1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:16| Show InChI InChI=1S/C18H10O5/c1-22-9-2-3-10-11-4-5-12-13(19)6-7-14(20)16(12)17(11)18(21)23-15(10)8-9/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]-cytisine binding from high-affinity Nicotinic acetylcholine receptor in rat brain (principall... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
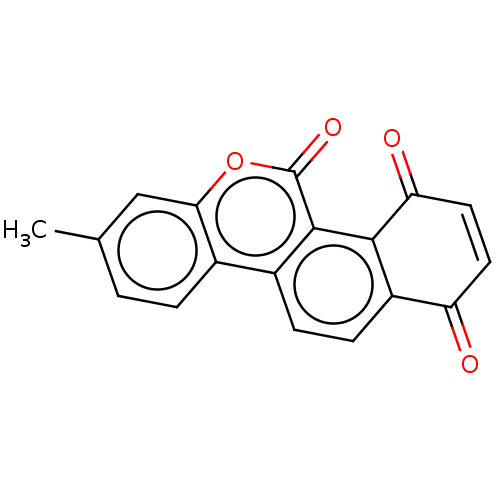
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251723
(CHEMBL4094178)Show SMILES Cc1ccc2c(c1)oc(=O)c1c3C(=O)C=CC(=O)c3ccc21 |c:15| Show InChI InChI=1S/C18H10O4/c1-9-2-3-10-11-4-5-12-13(19)6-7-14(20)16(12)17(11)18(21)22-15(10)8-9/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251732
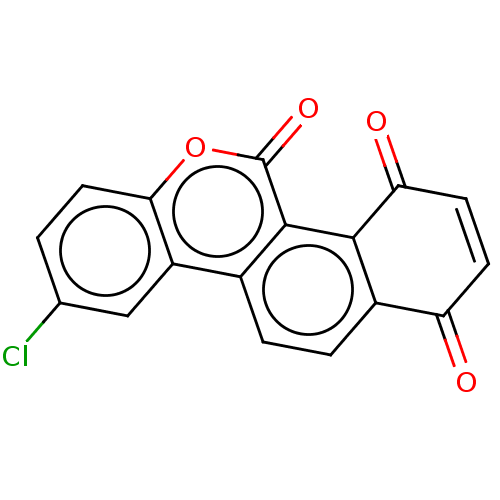
(CHEMBL4075821)Show SMILES Clc1ccc2oc(=O)c3c4C(=O)C=CC(=O)c4ccc3c2c1 |c:12| Show InChI InChI=1S/C17H7ClO4/c18-8-1-6-14-11(7-8)9-2-3-10-12(19)4-5-13(20)15(10)16(9)17(21)22-14/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612003

(CHEMBL5278031) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612004

(CHEMBL5284545) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50177318
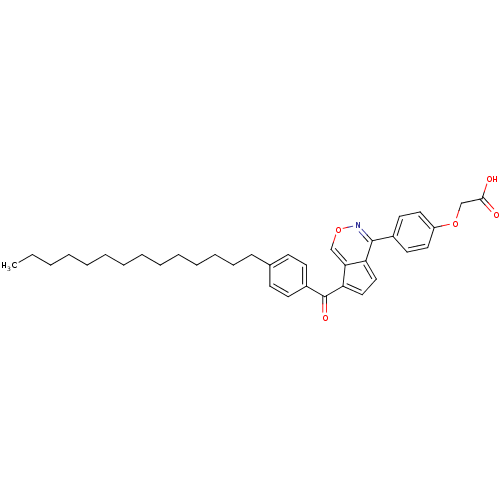
(2-(4-(7-(4-tetradecylbenzoyl)cyclopenta[d][1,2]oxa...)Show SMILES CCCCCCCCCCCCCCc1ccc(cc1)C(=O)c1ccc2c(nocc12)-c1ccc(OCC(O)=O)cc1 Show InChI InChI=1S/C36H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-27-15-17-29(18-16-27)36(40)32-24-23-31-33(32)25-42-37-35(31)28-19-21-30(22-20-28)41-26-34(38)39/h15-25H,2-14,26H2,1H3,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against cdc25C phosphatase |
Bioorg Med Chem Lett 16: 499-502 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.062
BindingDB Entry DOI: 10.7270/Q2319VFG |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251722

(CHEMBL4098262)Show SMILES O=C1C=CC(=O)c2c1ccc1c3ccccc3oc(=O)c21 |c:2| Show InChI InChI=1S/C17H8O4/c18-12-7-8-13(19)15-11(12)6-5-10-9-3-1-2-4-14(9)21-17(20)16(10)15/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CDC25C expressed in Escherichia coli BL21-DE3 pLysS using 3-O-methylfluorescein phosphate as s... |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251722

(CHEMBL4098262)Show SMILES O=C1C=CC(=O)c2c1ccc1c3ccccc3oc(=O)c21 |c:2| Show InChI InChI=1S/C17H8O4/c18-12-7-8-13(19)15-11(12)6-5-10-9-3-1-2-4-14(9)21-17(20)16(10)15/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612005

(CHEMBL5269565) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50612002
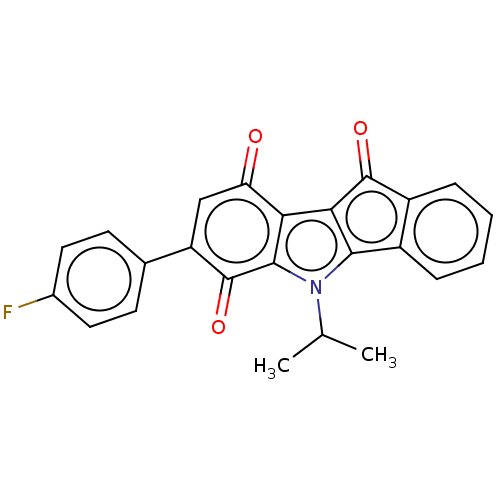
(CHEMBL5269423) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50129579

(2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...)Show SMILES Cc1ccccc1Cc1cccc2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O Show InChI InChI=1S/C22H17NO4/c1-12-5-2-3-6-13(12)9-14-7-4-8-15-16(11-23-20(14)15)19-21(26)17(24)10-18(25)22(19)27/h2-8,11,19,23H,9-10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Eight point inhibitory concentration against Cell division cycle 25 degree C was determined |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50514451

(CHEMBL1967787)Show InChI InChI=1S/C20H18O5/c21-11-5-7-15-17(9-11)25-18-10-12(22)6-8-16(18)19(15)13-3-1-2-4-14(13)20(23)24/h5-10,13-14,21H,1-4H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50129576

(2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...)Show SMILES CC(C)=CCC\C(C)=C\CC\C(C)=C\Cc1cccc2c(c[nH]c12)C1C(=O)C(=O)CC(=O)C1=O |(-9.11,4.99,;-7.6,4.77,;-6.64,5.99,;-7.04,3.33,;-5.51,3.1,;-4.95,1.67,;-3.42,1.44,;-2.47,2.65,;-2.86,.02,;-1.35,-.22,;-.77,-1.66,;.75,-1.87,;1.31,-3.32,;1.71,-.68,;3.23,-.91,;4.18,.3,;3.62,1.73,;4.58,2.93,;6.11,2.7,;6.67,1.26,;8.1,.72,;8.03,-.82,;6.56,-1.21,;5.72,.06,;9.43,1.51,;9.4,3.06,;8.03,3.83,;10.72,3.84,;10.69,5.38,;12.07,3.1,;12.09,1.54,;13.46,.77,;10.76,.76,;10.79,-.78,)| Show InChI InChI=1S/C29H33NO4/c1-18(2)8-5-9-19(3)10-6-11-20(4)14-15-21-12-7-13-22-23(17-30-27(21)22)26-28(33)24(31)16-25(32)29(26)34/h7-8,10,12-14,17,26,30H,5-6,9,11,15-16H2,1-4H3/b19-10+,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Eight point inhibitory concentration against Cell division cycle 25 degree C was determined |
J Med Chem 46: 2580-8 (2003)
Article DOI: 10.1021/jm0300835
BindingDB Entry DOI: 10.7270/Q2RJ4HVW |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50251698
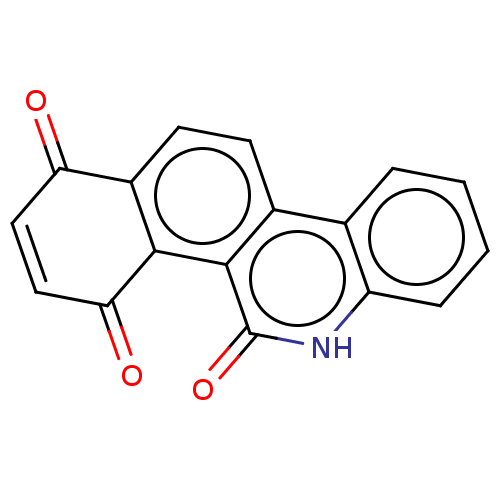
(CHEMBL4105005)Show SMILES O=C1C=CC(=O)c2c1ccc1c3ccccc3[nH]c(=O)c21 |c:2| Show InChI InChI=1S/C17H9NO3/c19-13-7-8-14(20)15-11(13)6-5-10-9-3-1-2-4-12(9)18-17(21)16(10)15/h1-8H,(H,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [125 I]alpha-bungarotoxin (alpha-BgT) from torpedo alpha1-beta1-gamma1 electroplax |
Eur J Med Chem 134: 316-333 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.012
BindingDB Entry DOI: 10.7270/Q2WD4311 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50155597
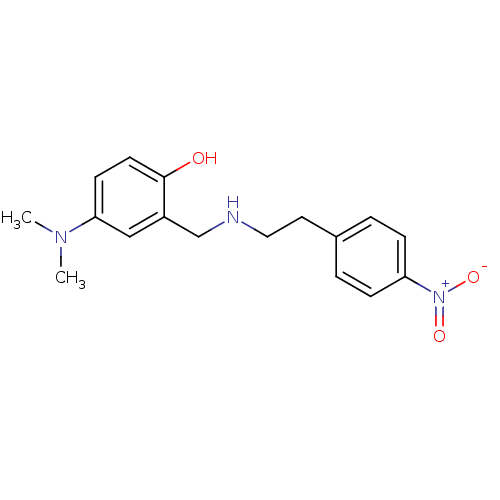
(4-Dimethylamino-2-{[2-(4-nitro-phenyl)-ethylamino]...)Show SMILES CN(C)c1ccc(O)c(CNCCc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C17H21N3O3/c1-19(2)16-7-8-17(21)14(11-16)12-18-10-9-13-3-5-15(6-4-13)20(22)23/h3-8,11,18,21H,9-10,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human cell division cycle 25 degree C phosphatase |
Bioorg Med Chem Lett 14: 5809-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.041
BindingDB Entry DOI: 10.7270/Q2H41S67 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50155595
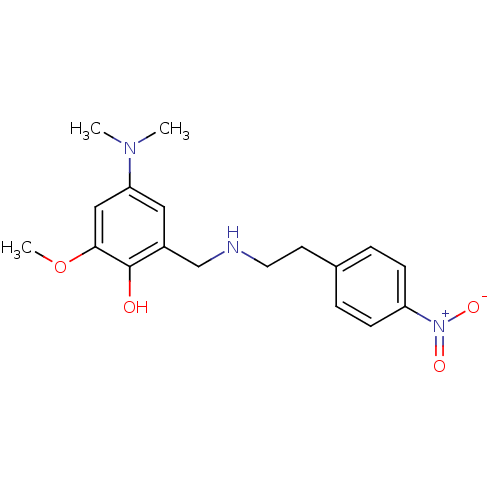
(4-Dimethylamino-2-methoxy-6-{[2-(4-nitro-phenyl)-e...)Show SMILES COc1cc(cc(CNCCc2ccc(cc2)[N+]([O-])=O)c1O)N(C)C Show InChI InChI=1S/C18H23N3O4/c1-20(2)16-10-14(18(22)17(11-16)25-3)12-19-9-8-13-4-6-15(7-5-13)21(23)24/h4-7,10-11,19,22H,8-9,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration for human cell division cycle 25 degree C phosphatase |
Bioorg Med Chem Lett 14: 5809-12 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.041
BindingDB Entry DOI: 10.7270/Q2H41S67 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25C expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25C |
Bioorg Med Chem 19: 2145-55 (2011)
Article DOI: 10.1016/j.bmc.2011.02.047
BindingDB Entry DOI: 10.7270/Q2BR8SHK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data