

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choline O-acetyltransferase (RAT) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition constant determined against Acetylcholinesterase (AChE) receptor. | J Med Chem 41: 4186-9 (1998) Checked by Author Article DOI: 10.1021/jm9810452 BindingDB Entry DOI: 10.7270/Q2HH6KRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50133817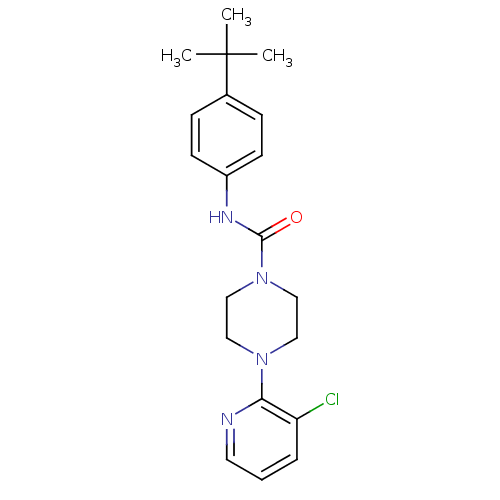 (4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pudue Pharma Discovery Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 377-86 (2003) Article DOI: 10.1124/jpet.102.045674 BindingDB Entry DOI: 10.7270/Q2TX3CX5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026220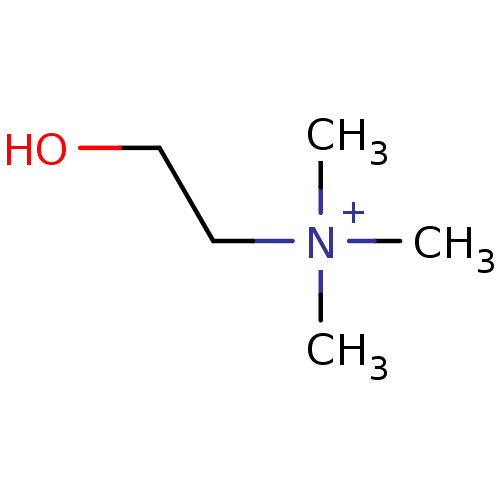 (2-hydroxy-N,N,N-trimethylethanaminium | CHEMBL2824...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026470 ((2-Hydroxy-ethyl)-dimethyl-sulfonium; iodide | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026480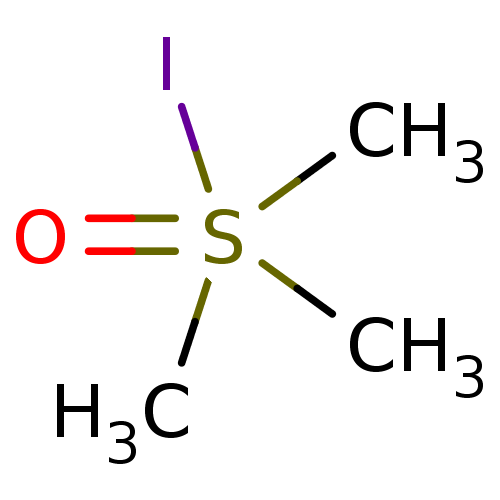 (CHEMBL283149 | Trimethyl-sulfoniumoxide; iodide) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50031187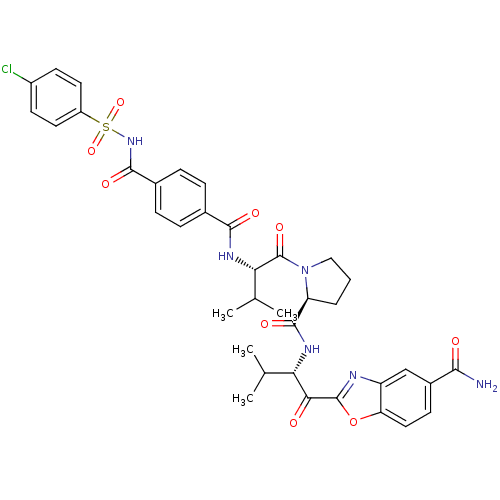 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of the compound towards Acetylcholinesterase | J Med Chem 38: 3972-82 (1995) BindingDB Entry DOI: 10.7270/Q2T152NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026468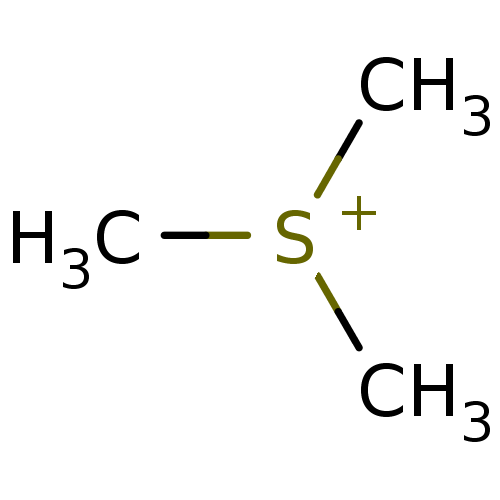 (CHEMBL25340 | Trimethyl-sulfonium; iodide) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026469 (2-Trimethylsilanyl-ethanol | CHEMBL25482) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents | PubMed | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026475 (Acetic acid 2-methanesulfonyl-ethyl ester | CHEMBL...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 6.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026475 (Acetic acid 2-methanesulfonyl-ethyl ester | CHEMBL...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 6.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026468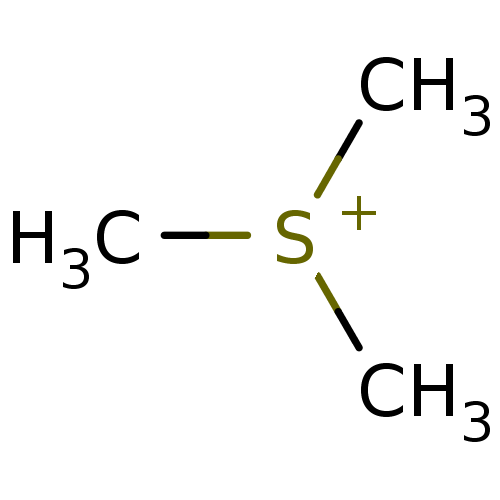 (CHEMBL25340 | Trimethyl-sulfonium; iodide) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | 7.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026220 (2-hydroxy-N,N,N-trimethylethanaminium | CHEMBL2824...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 7.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026477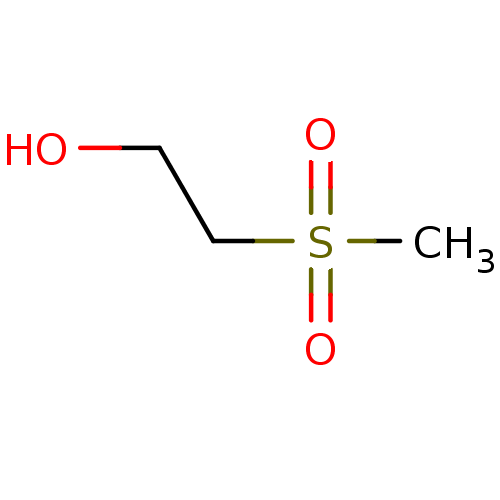 (2-Methanesulfonyl-ethanol | CHEMBL281616) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 8.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026470 ((2-Hydroxy-ethyl)-dimethyl-sulfonium; iodide | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026478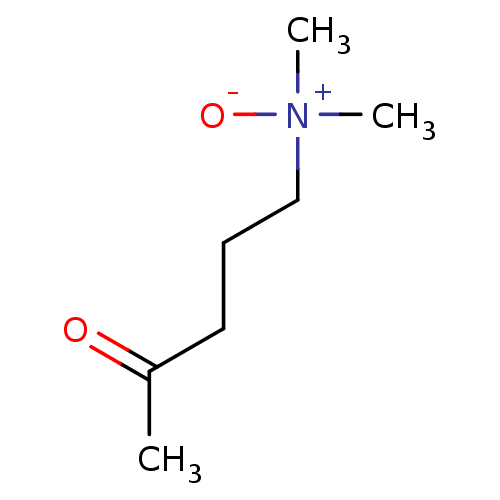 (5-dimethylamino-2-pentanone-N-oxide | CHEMBL25004) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.40E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis of acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50048111 ((2,3-Dihydroxy-phenyl)-trimethyl-ammonium; iodide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Enzyme inhibition dissociation constant for choline acetyl transferase (ChAcT) was determined | J Med Chem 36: 1893-901 (1993) BindingDB Entry DOI: 10.7270/Q2RX9B5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026474 (3,3-Dimethyl-butan-1-ol | CHEMBL25029) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(competitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026474 (3,3-Dimethyl-butan-1-ol | CHEMBL25029) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026472 ((CH3)2SO | (methanesulfinyl)methanedimethyl sulfox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(com) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50048110 (CHEMBL57055 | [2-(3,4-Dihydroxy-phenyl)-2-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Enzyme inhibition dissociation constant for choline acetyl transferase (ChAcT) was determined | J Med Chem 36: 1893-901 (1993) BindingDB Entry DOI: 10.7270/Q2RX9B5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026473 (CHEMBL25028 | Sulfonylbismethane | dimethyl sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026479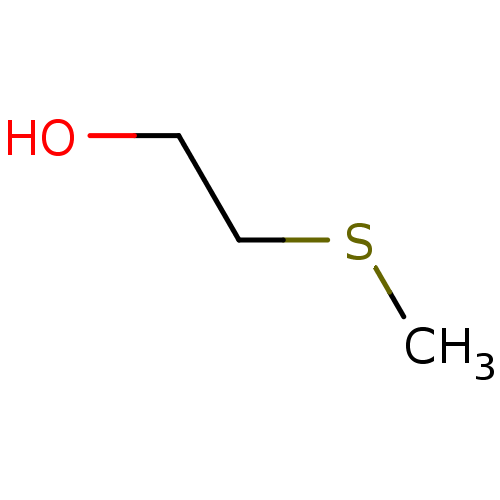 (2-Methylsulfanyl-ethanol | CHEMBL277871) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(com) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM36173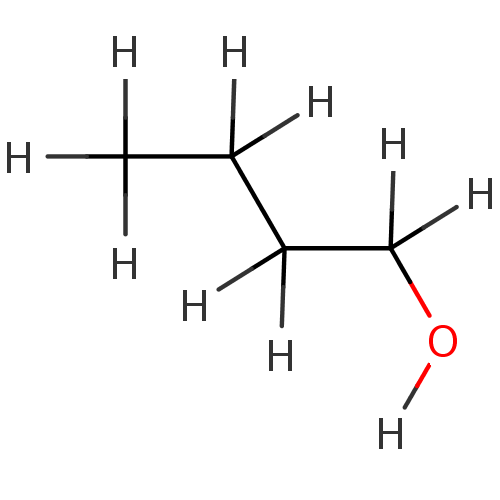 (1-butanol | 1-butanol-d10 | CHEMBL14245) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(com) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026477 (2-Methanesulfonyl-ethanol | CHEMBL281616) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(com) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50026473 (CHEMBL25028 | Sulfonylbismethane | dimethyl sulfon...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2.60E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for reversible inhibition of hydrolysis acetylcholine by acetylcholinesterase and represented as KI(noncompetitive) | J Med Chem 28: 1309-13 (1985) BindingDB Entry DOI: 10.7270/Q26W994C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against acetylcholinesterase | J Med Chem 35: 584-9 (1992) BindingDB Entry DOI: 10.7270/Q20G3J3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020521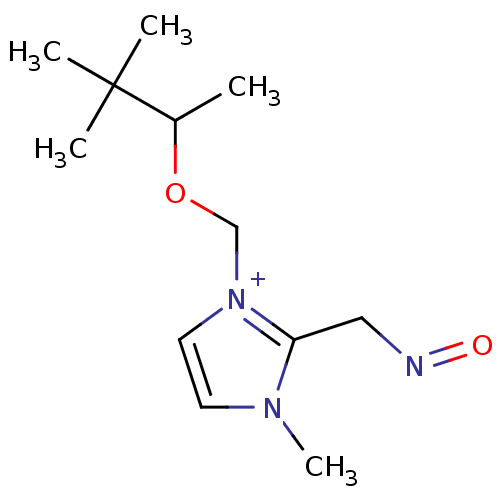 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50280246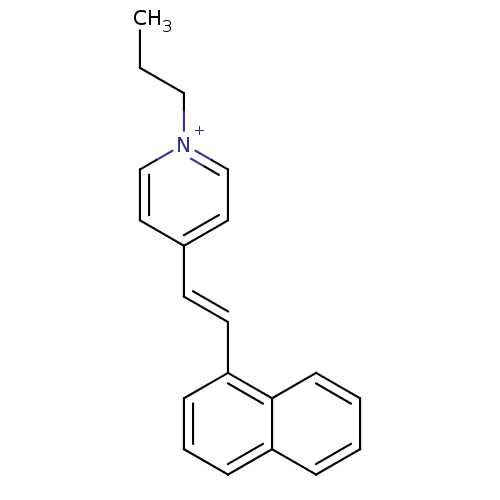 (4-((E)-2-Naphthalen-1-yl-vinyl)-1-propyl-pyridiniu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against rat choline acetyltransferase (ChAT) | Bioorg Med Chem Lett 2: 1493-1496 (1992) Article DOI: 10.1016/S0960-894X(00)80415-1 BindingDB Entry DOI: 10.7270/Q2QN66PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50011780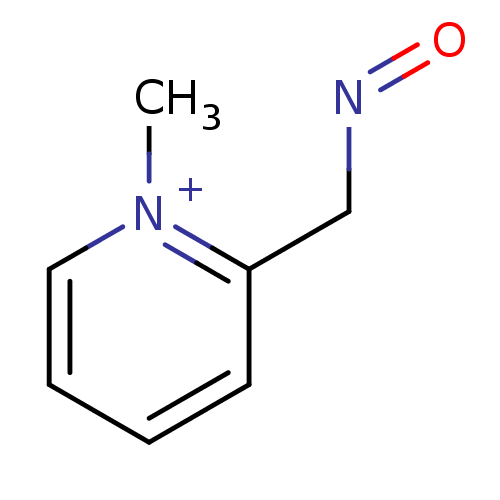 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020514 (2-(Hydroxyimino-methyl)-1-methoxymethyl-3-methyl-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020522 (1-(((4-carbamoylpyridinium-1-yl)methoxy)methyl)-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||