 Found 18 hits Enz. Inhib. hit(s) with Target = 'D(2) dopamine receptor' AND taxid = 9615
Found 18 hits Enz. Inhib. hit(s) with Target = 'D(2) dopamine receptor' AND taxid = 9615 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(CANINE) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM50026957
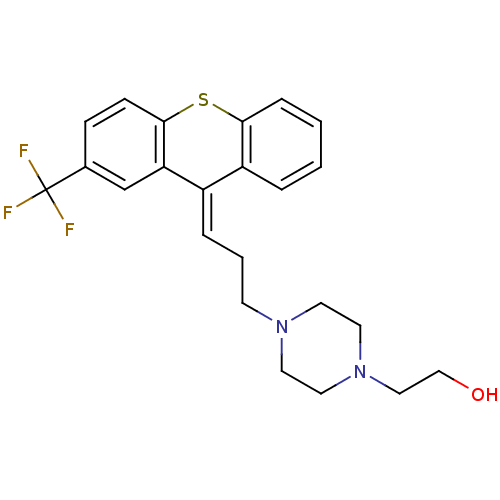
((cis) 2-{4-[3-(2-Trifluoromethyl-thioxanthen-9-yli...)Show SMILES OCCN1CCN(CC\C=C2/c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2/b18-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)-Rattus norvegicus (rat)) | BDBM50104122

(9-Oxo-9H-fluorene-4-carboxylic acid {4-[4-(2,3-dic...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cccc4C(=O)c5ccccc5-c34)CC2)c1Cl Show InChI InChI=1S/C28H27Cl2N3O2/c29-23-11-6-12-24(26(23)30)33-17-15-32(16-18-33)14-4-3-13-31-28(35)22-10-5-9-21-25(22)19-7-1-2-8-20(19)27(21)34/h1-2,5-12H,3-4,13-18H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Inhibition of [3H]YM-09151-2 binding to primate Dopamine D2L receptor expressed in CHO cells |
J Med Chem 48: 3663-79 (2005)
Article DOI: 10.1021/jm040190e
BindingDB Entry DOI: 10.7270/Q2474BNF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM35256
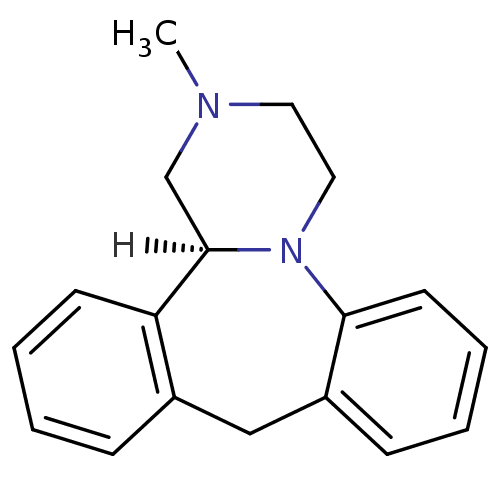
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)-Rattus norvegicus (rat)) | BDBM50071959
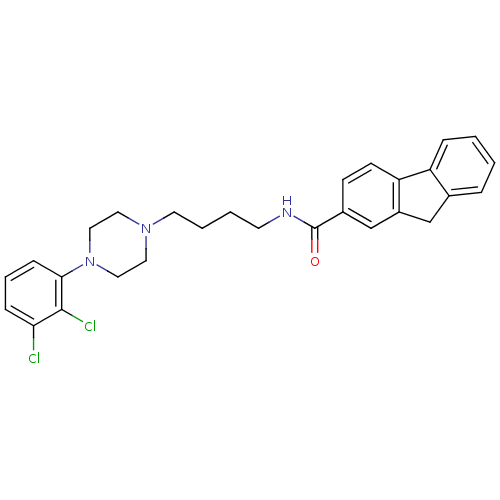
(9H-Fluorene-2-carboxylic acid {4-[4-(2,3-dichloro-...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3ccc-4c(Cc5ccccc-45)c3)CC2)c1Cl Show InChI InChI=1S/C28H29Cl2N3O/c29-25-8-5-9-26(27(25)30)33-16-14-32(15-17-33)13-4-3-12-31-28(34)21-10-11-24-22(19-21)18-20-6-1-2-7-23(20)24/h1-2,5-11,19H,3-4,12-18H2,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program
Curated by ChEMBL
| Assay Description
Inhibition of [3H]YM-09151-2 binding to primate Dopamine D2L receptor expressed in CHO cells |
J Med Chem 48: 3663-79 (2005)
Article DOI: 10.1021/jm040190e
BindingDB Entry DOI: 10.7270/Q2474BNF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 247: 343-8 (1988)
BindingDB Entry DOI: 10.7270/Q24J0CM0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(CANINE) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The IC50 value was reported as apparent, since [3H]NCA was purported to be irreversible (dopamine receptor from Canine striatal membranes). Result in... |
J Med Chem 27: 806-10 (1984)
BindingDB Entry DOI: 10.7270/Q26111HB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463984

(CHEMBL4241844)Show SMILES CCCN(CCc1ccc(NC(=O)CCc2cn(CCCCCCCCN(C)CCCCCNC(=O)COc3cncc(c3)C#Cc3csc(C)n3)nn2)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C53H71N9O4S/c1-4-29-61(48-24-25-50-44(35-48)15-14-16-51(50)63)33-27-42-17-20-45(21-18-42)57-52(64)26-23-46-38-62(59-58-46)32-13-8-6-5-7-11-30-60(3)31-12-9-10-28-55-53(65)39-66-49-34-43(36-54-37-49)19-22-47-40-67-41(2)56-47/h14-18,20-21,34,36-38,40,48,63H,4-13,23-33,35,39H2,1-3H3,(H,55,65)(H,57,64) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as inhibition of L-Glu-induced MAPK p... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463980

(CHEMBL4246881)Show SMILES COc1ccccc1N1CCN(Cc2ccc(OCc3cn(CCCCCCCCN(C)CCCCCNC(=O)COc4cncc(c4)C#Cc4csc(C)n4)nn3)c(OC)c2)CC1 Show InChI InChI=1S/C49H65N9O5S/c1-39-52-42(38-64-39)20-18-40-30-44(33-50-32-40)62-37-49(59)51-22-12-9-14-24-55(2)23-13-7-5-6-8-15-25-58-35-43(53-54-58)36-63-47-21-19-41(31-48(47)61-4)34-56-26-28-57(29-27-56)45-16-10-11-17-46(45)60-3/h10-11,16-17,19,21,30-33,35,38H,5-9,12-15,22-29,34,36-37H2,1-4H3,(H,51,59) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as inhibition of L-Glu-induced MAPK p... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463985
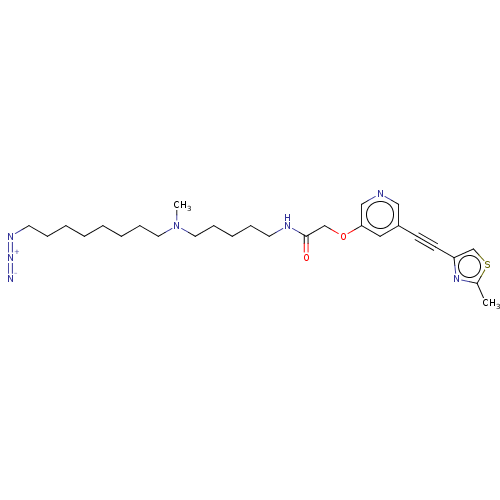
(CHEMBL4244406)Show SMILES CN(CCCCCCCCN=[N+]=[N-])CCCCCNC(=O)COc1cncc(c1)C#Cc1csc(C)n1 Show InChI InChI=1S/C27H39N7O2S/c1-23-32-25(22-37-23)13-12-24-18-26(20-29-19-24)36-21-27(35)30-14-8-7-11-17-34(2)16-10-6-4-3-5-9-15-31-33-28/h18-20,22H,3-11,14-17,21H2,1-2H3,(H,30,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Antagonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as inhibition of L-Glu-induced MAPK p... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463982
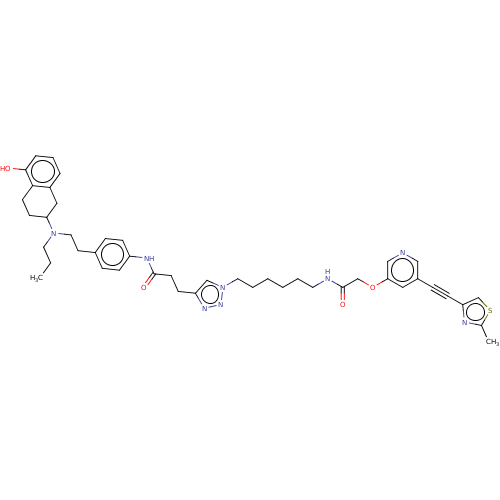
(CHEMBL4239987)Show SMILES CCCN(CCc1ccc(NC(=O)CCc2cn(CCCCCCNC(=O)COc3cncc(c3)C#Cc3csc(C)n3)nn2)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C45H54N8O4S/c1-3-23-52(40-18-19-42-36(27-40)9-8-10-43(42)54)25-21-34-11-14-37(15-12-34)49-44(55)20-17-38-30-53(51-50-38)24-7-5-4-6-22-47-45(56)31-57-41-26-35(28-46-29-41)13-16-39-32-58-33(2)48-39/h8-12,14-15,26,28-30,32,40,54H,3-7,17-25,27,31H2,1-2H3,(H,47,56)(H,49,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP a... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463981
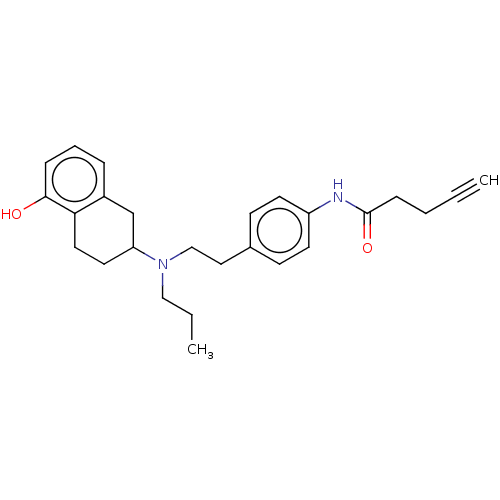
(CHEMBL4242192)Show SMILES CCCN(CCc1ccc(NC(=O)CCC#C)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C26H32N2O2/c1-3-5-9-26(30)27-22-12-10-20(11-13-22)16-18-28(17-4-2)23-14-15-24-21(19-23)7-6-8-25(24)29/h1,6-8,10-13,23,29H,4-5,9,14-19H2,2H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP a... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463986

(CHEMBL4246185)Show SMILES CCCN(CCc1ccc(NC(=O)CCc2cn(CCCCCCCCCCN(C)CCCCCNC(=O)COc3cncc(c3)C#Cc3csc(C)n3)nn2)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C55H75N9O4S/c1-4-31-63(50-26-27-52-46(37-50)17-16-18-53(52)65)35-29-44-19-22-47(23-20-44)59-54(66)28-25-48-40-64(61-60-48)34-15-10-8-6-5-7-9-13-32-62(3)33-14-11-12-30-57-55(67)41-68-51-36-45(38-56-39-51)21-24-49-42-69-43(2)58-49/h16-20,22-23,36,38-40,42,50,65H,4-15,25-35,37,41H2,1-3H3,(H,57,67)(H,59,66) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP a... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50018958
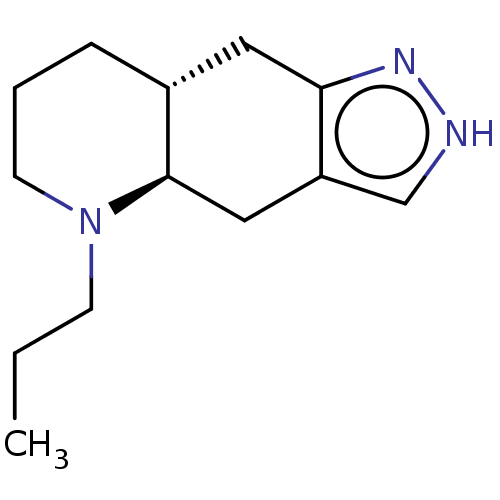
(CHEBI:75401 | QUINPIROLE)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP a... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor/Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50463984

(CHEMBL4241844)Show SMILES CCCN(CCc1ccc(NC(=O)CCc2cn(CCCCCCCCN(C)CCCCCNC(=O)COc3cncc(c3)C#Cc3csc(C)n3)nn2)cc1)C1CCc2c(O)cccc2C1 Show InChI InChI=1S/C53H71N9O4S/c1-4-29-61(48-24-25-50-44(35-48)15-14-16-51(50)63)33-27-42-17-20-45(21-18-42)57-52(64)26-23-46-38-62(59-58-46)32-13-8-6-5-7-11-30-60(3)31-12-9-10-28-55-53(65)39-66-49-34-43(36-54-37-49)19-22-47-40-67-41(2)56-47/h14-18,20-21,34,36-38,40,48,63H,4-13,23-33,35,39H2,1-3H3,(H,55,65)(H,57,64) | PDB
MMDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Ghent University
Curated by ChEMBL
| Assay Description
Agonist activity at human dopamine D2 receptor /mGluR5a (unknown origin) expressed in HEK293T cells assessed as reduction in forskolin-induced cAMP a... |
J Med Chem 61: 8212-8225 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00671
BindingDB Entry DOI: 10.7270/Q2X92DZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data